Impact of Food-Derived Bioactive Compounds on Intestinal Immunity
Abstract
:1. Introduction
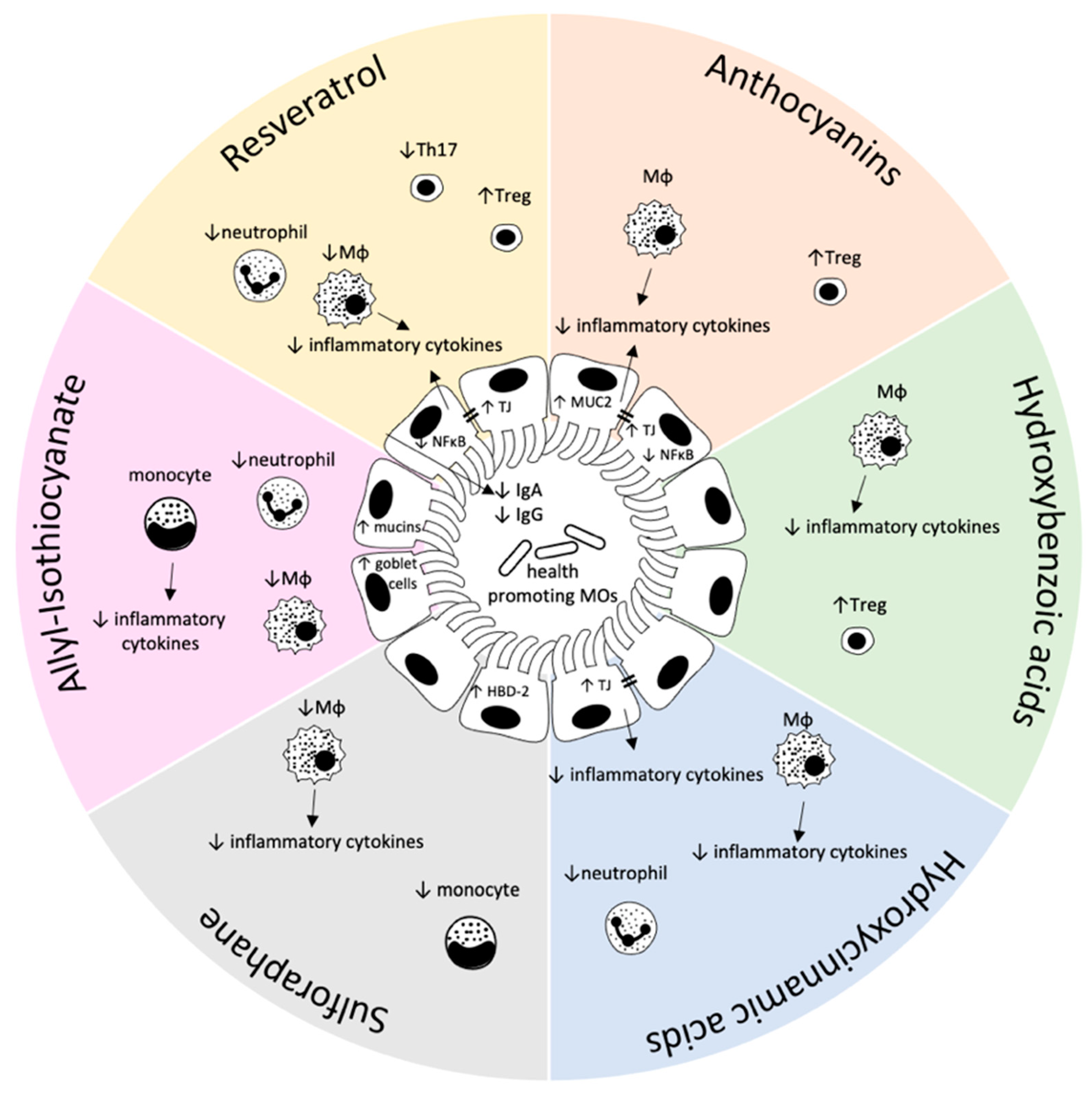
2. Effect of Food-Derived Bioactive Compounds on the Gastrointestinal (Mucosal) Immune System
2.1. Flavonoids
Anthocyanins
2.2. Phenolic Acids
2.2.1. Hydroxybenzoic Acids
2.2.2. Hydroxycinnamic Acids
2.3. Stilbens
2.4. Glucosinolates
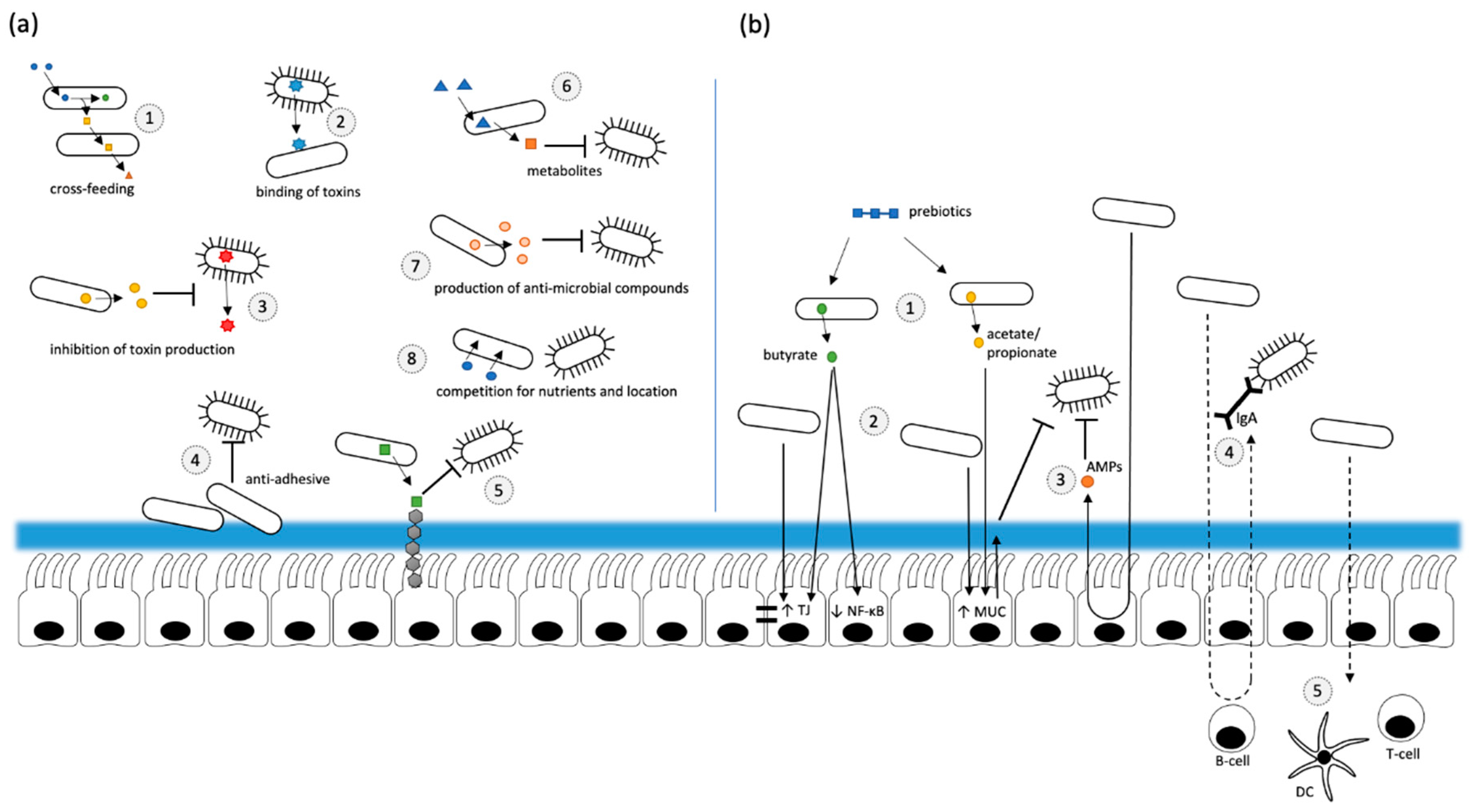
2.5. Probiotics and Prebiotics
2.5.1. Probiotics
2.5.2. Prebiotics
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Monteleone, I.; Fantini, M.C.; Monteleone, G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology 2011, 140, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayram, B.; González-Sarrías, A.; Istas, G.; Garcia-Aloy, M.; Morand, C.; Tuohy, K.; García-Villalba, R.; Mena, P. Breakthroughs in the Health Effects of Plant Food Bioactives: A Perspective on Microbiomics, Nutri(epi)genomics, and Metabolomics. J. Agric. Food Chem. 2018, 66, 10686–10692. [Google Scholar] [CrossRef]
- Sturm, C.; Wagner, A.E. Brassica-Derived Plant Bioactives as Modulators of Chemopreventive and Inflammatory Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, T.; Sequoia, J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Lo, A.W.-Y.; Xiao, Z.; Shin, T.-S.; Chung, G.; Lam, H.-M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Leonov, A.; Arlia-Ciommo, A.; Piano, A.; Svistkova, V.; Lutchman, V.; Medkour, Y.; Titorenko, V.I. Longevity extension by phytochemicals. Molecules 2015, 20, 6544–6572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. Gut immunity: Its development and reasons and opportunities for modulation in monogastric production animals. Anim. Health Res. Rev. 2018, 19, 46–52. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Zhang, H.; Hassan, Y.I.; Renaud, J.; Liu, R.; Yang, C.; Sun, Y.; Tsao, R. Bioaccessibility, bioavailability, and anti-inflammatory effects of anthocyanins from purple root vegetables using mono- and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. [Google Scholar] [CrossRef]
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Busà, R.; Saija, A.; Speciale, A. Cyanidin-3-O-Glucoside Modulates the In Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells. Mediat. Inflamm. 2017, 2017, 3454023. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tian, L.-M.; Liu, Y.; Guo, K.-S.; Lv, M.; Li, Q.-T.; Hao, S.-Y.; Ma, C.-H.; Chen, Y.-X.; Tanaka, M.; et al. Low Dose of Cyanidin-3-O-Glucoside Alleviated Dextran Sulfate Sodium-Induced Colitis, Mediated by CD169+ Macrophage Pathway. Inflamm. Bowel Dis. 2019, 25, 1510–1521. [Google Scholar] [CrossRef]
- Montane, J.; Obach, M.; Alvarez, S.; Bischoff, L.; Dai, D.L.; Soukhatcheva, G.; Priatel, J.J.; Hardenberg, G.; Levings, M.K.; Tan, R.; et al. CCL22 Prevents Rejection of Mouse Islet Allografts and Induces Donor-Specific Tolerance. Cell Transplant. 2015, 24, 2143–2154. [Google Scholar] [CrossRef]
- Misikangas, M.; Pajari, A.-M.; Päivärinta, E.; Oikarinen, S.I.; Rajakangas, J.; Marttinen, M.; Tanayama, H.; Törrönen, R.; Mutanen, M. Three Nordic berries inhibit intestinal tumorigenesis in multiple intestinal neoplasia/+ mice by modulating beta-catenin signaling in the tumor and transcription in the mucosa. J. Nutr. 2007, 137, 2285–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Päivärinta, E.; Niku, M.; Maukonen, J.; Storvik, M.; Heiman-Lindh, A.; Saarela, M.; Pajari, A.-M.; Mutanen, M. Changes in intestinal immunity, gut microbiota, and expression of energy metabolism-related genes explain adenoma growth in bilberry and cloudberry-fed ApcMin mice. Nutr. Res. 2016, 36, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-C.; Kim, S.-J.; Kim, D.-S.; Jeon, Y.-D.; Park, S.J.; Lee, H.S.; Um, J.-Y.; Hong, S.-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Hsiang, C.-Y.; Hseu, Y.-C.; Chang, Y.-C.; Kumar, K.J.S.; Ho, T.-Y.; Yang, H.-L. Toona sinensis and its major bioactive compound gallic acid inhibit LPS-induced inflammation in nuclear factor-κB transgenic mice as evaluated by in vivo bioluminescence imaging. Food Chem. 2013, 136, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.H.; Gil, K.C.; Kim, S.G.; Park, S.-Y.; Hwang, K.W. Delphinidin Chloride and Its Hydrolytic Metabolite Gallic Acid Promote Differentiation of Regulatory T cells and Have an Anti-inflammatory Effect on the Allograft Model. J. Food Sci. 2019, 84, 920–930. [Google Scholar] [CrossRef]
- Szulc-Kielbik, I.; Kielbik, M.; Klink, M. Ferulic acid but not alpha-lipoic acid effectively protects THP-1-derived macrophages from oxidant and pro-inflammatory response to LPS. Immunopharmacol. Immunotoxicol. 2017, 39, 330–337. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Astiazarán-García, H.; González-Aguilar, G.A.; Loarca-Piña, G.; Ezquerra-Brauer, J.-M.; Domínguez Avila, J.A.; Robles-Sánchez, M. Ferulic Acid on Glucose Dysregulation, Dyslipidemia, and Inflammation in Diet-Induced Obese Rats: An Integrated Study. Nutrients 2017, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin--from molecule to biological function. Angew. Chem. Int. Ed. Engl. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Wang, L.; Konrad, A.; Schoeb, T.; Elson, C.O. Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur. J. Immunol. 2009, 39, 3134–3146. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kim, K.-H. Curcumin prevents leptin-induced tight junction dysfunction in intestinal Caco-2 BBe cells. J. Nutr. Biochem. 2014, 25, 26–35. [Google Scholar] [CrossRef]
- Larmonier, C.B.; Uno, J.K.; Lee, K.-M.; Karrasch, T.; Laubitz, D.; Thurston, R.; Midura-Kiela, M.T.; Ghishan, F.K.; Sartor, R.B.; Jobin, C.; et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1079-91. [Google Scholar] [CrossRef] [PubMed]
- McFadden, R.-M.T.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The Role of Curcumin in Modulating Colonic Microbiota during Colitis and Colon Cancer Prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Larmonier, C.B.; Midura-Kiela, M.T.; Ramalingam, R.; Laubitz, D.; Janikashvili, N.; Larmonier, N.; Ghishan, F.K.; Kiela, P.R. Modulation of neutrophil motility by curcumin: Implications for inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Memarzia, A.; Khazdair, M.R.; Behrouz, S.; Gholamnezhad, Z.; Jafarnezhad, M.; Saadat, S.; Boskabady, M.H. Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review. Biofactors 2021, 47, 311–350. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Mayangsari, Y.; Suzuki, T. Resveratrol Ameliorates Intestinal Barrier Defects and Inflammation in Colitic Mice and Intestinal Cells. J. Agric. Food Chem. 2018, 66, 12666–12674. [Google Scholar] [CrossRef]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potoka, D.A.; Nadler, E.P.; Upperman, J.S.; Ford, H.R. Role of nitric oxide and peroxynitrite in gut barrier failure. World J. Surg. 2002, 26, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Romier, B.; van de Walle, J.; During, A.; Larondelle, Y.; Schneider, Y.-J. Modulation of signalling nuclear factor-kappaB activation pathway by polyphenols in human intestinal Caco-2 cells. Br. J. Nutr. 2008, 100, 542–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, U.P.; Singh, N.P.; Singh, B.; Hofseth, L.J.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J. Pharmacol. Exp. Ther. 2010, 332, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.-H.; Zhou, X.-X.; Ma, Y.-Y.; Pan, W.-S.; Zhao, F.; Yu, M.-S.; Liu, J.-Q. Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy. World J. Gastroenterol. 2020, 26, 4945–4959. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wei, C.; Wang, J.-Y.; Zhang, R.; Li, Y.-X.; Wang, L.-S. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J. Gastroenterol. 2015, 21, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de La Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010, 633, 78–84. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Singh, B.; Hofseth, L.J.; Taub, D.D.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(-/-) mice. Brain Behav. Immun. 2012, 26, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Haile, L.A.; von Wasielewski, R.; Gamrekelashvili, J.; Krüger, C.; Bachmann, O.; Westendorf, A.M.; Buer, J.; Liblau, R.; Manns, M.P.; Korangy, F.; et al. Myeloid-derived suppressor cells in inflammatory bowel disease: A new immunoregulatory pathway. Gastroenterology 2008, 135, 871–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsami-Kor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2015, 46, 280–285. [Google Scholar] [CrossRef]
- Voorrips, L.E.; Goldbohm, R.A.; van Poppel, G.; Sturmans, F.; Hermus, R.J.; van den Brandt, P.A. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: The Netherlands Cohort Study on Diet and Cancer. Am. J. Epidemiol. 2000, 152, 1081–1092. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, D.T.; Goldbohm, R.A.; van Poppel, G.; Verhagen, H.; van den Brandt, P.A. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 733–748. [Google Scholar]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef] [PubMed]
- Rouzaud, G.; Rabot, S.; Ratcliffe, B.; Duncan, A.J. Influence of plant and bacterial myrosinase activity on the metabolic fate of glucosinolates in gnotobiotic rats. Br. J. Nutr. 2003, 90, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.-H.; Miller, M.J.; Jeffery, E. Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption. Food Funct. 2010, 1, 161–166. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47 (Suppl. 2), 73–88. [Google Scholar] [CrossRef]
- Getahun, S.M.; Chung, F.L. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol. Biomark. Prev. 1999, 8, 447–451. [Google Scholar]
- Youn, H.S.; Kim, Y.S.; Park, Z.Y.; Kim, S.Y.; Choi, N.Y.; Joung, S.M.; Seo, J.A.; Lim, K.-M.; Kwak, M.-K.; Hwang, D.H.; et al. Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J. Immunol. 2010, 184, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.E.; Will, O.; Sturm, C.; Lipinski, S.; Rosenstiel, P.; Rimbach, G. DSS-induced acute colitis in C57BL/6 mice is mitigated by sulforaphane pre-treatment. J. Nutr. Biochem. 2013, 24, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Stefanson, A.L.; Bakovic, M. Falcarinol Is a Potent Inducer of Heme Oxygenase-1 and Was More Effective than Sulforaphane in Attenuating Intestinal Inflammation at Diet-Achievable Doses. Oxid. Med. Cell. Longev. 2018, 2018, 3153527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, L.; Li, C.; Wu, H.; Ran, D.; Zhang, Z. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Express 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Davaatseren, M.; Hwang, J.-T.; Park, J.H.; Kim, M.-S.; Wang, S.; Sung, M.J. Allyl isothiocyanate ameliorates angiogenesis and inflammation in dextran sulfate sodium-induced acute colitis. PLoS ONE 2014, 9, e102975. [Google Scholar] [CrossRef]
- Kim, M.W.; Choi, S.; Kim, S.Y.; Yoon, Y.S.; Kang, J.-H.; Oh, S.H. Allyl Isothiocyanate Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mouse by Enhancing Tight Junction and Mucin Expression. Int. J. Mol. Sci. 2018, 19, 2025. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Wehkamp, J.; Fellermann, K.; Herrlinger, K.R.; Bevins, C.L.; Stange, E.F. Mechanisms of disease: Defensins in gastrointestinal diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 406–415. [Google Scholar] [CrossRef]
- Wagner, A.E.; Boesch-Saadatmandi, C.; Dose, J.; Schultheiss, G.; Rimbach, G. Anti-inflammatory potential of allyl-isothiocyanate--role of Nrf2, NF-(κ) B and microRNA-155. J. Cell. Mol. Med. 2012, 16, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Kidane, A.I.; Yu, T.-W.; Dashwood, W.-M.; Bisson, W.H.; Löhr, C.V.; Ho, E.; Williams, D.E.; Dashwood, R.H. HDAC turnover, CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates. Epigenetics 2013, 8, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Pröll, M.; Neuhoff, C.; Zhang, R.; Cinar, M.U.; Hossain, M.M.; Tesfaye, D.; Große-Brinkhaus, C.; Salilew-Wondim, D.; Tholen, E.; et al. Sulforaphane epigenetically regulates innate immune responses of porcine monocyte-derived dendritic cells induced with lipopolysaccharide. PLoS ONE 2015, 10, e0121574. [Google Scholar] [CrossRef]
- Ali, M.; Bonay, M.; Vanhee, V.; Vinit, S.; Deramaudt, T.B. Comparative effectiveness of 4 natural and chemical activators of Nrf2 on inflammation, oxidative stress, macrophage polarization, and bactericidal activity in an in vitro macrophage infection model. PLoS ONE 2020, 15, e0234484. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yasmeen, R.; Fukagawa, N.K.; Yu, L.; Kim, Y.S.; Wang, T.T.Y. Dose-Dependent Responses of I3C and DIM on T-Cell Activation in the Human T Lymphocyte Jurkat Cell Line. Int. J. Mol. Sci. 2017, 18, 1409. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggeletopoulou, I.; Konstantakis, C.; Assimakopoulos, S.F.; Triantos, C. The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microb. Pathog. 2019, 137, 103774. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. IJMM 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef] [Green Version]
- Falony, G.; Vlachou, A.; Verbrugghe, K.; de Vuyst, L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl. Environ. Microbiol. 2006, 72, 7835–7841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivière, A.; Gagnon, M.; Weckx, S.; Roy, D.; de Vuyst, L. Mutual Cross-Feeding Interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 Explain the Bifidogenic and Butyrogenic Effects of Arabinoxylan Oligosaccharides. Appl. Environ. Microbiol. 2015, 81, 7767–7781. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.H.; Perini, F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect. Immun. 1988, 56, 2610–2614. [Google Scholar] [CrossRef] [Green Version]
- Guiot, H.F. Role of competition for substrate in bacterial antagonism in the gut. Infect. Immun. 1982, 38, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Tuncil, Y.E.; Xiao, Y.; Porter, N.T.; Reuhs, B.L.; Martens, E.C.; Hamaker, B.R. Reciprocal Prioritization to Dietary Glycans by Gut Bacteria in a Competitive Environment Promotes Stable Coexistence. mBio 2017, 8, e01068-17. [Google Scholar] [CrossRef]
- Rattanaprasert, M.; Roos, S.; Hutkins, R.W.; Walter, J. Quantitative evaluation of synbiotic strategies to improve persistence and metabolic activity of Lactobacillus reuteri DSM 17938 in the human gastrointestinal tract. J. Funct. Food 2014, 10, 85–94. [Google Scholar] [CrossRef]
- Frese, S.A.; Hutkins, R.W.; Walter, J. Comparison of the Colonization Ability of Autochthonous and Allochthonous Strains of Lactobacilli in the Human Gastrointestinal Tract. AiM 2012, 2, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Charbonneau, D.; Gibb, R.D.; Quigley, E.M.M. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes 2013, 4, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Gómez, M.X.; Martínez, I.; Bottacini, F.; O’Callaghan, A.; Ventura, M.; van Sinderen, D.; Hillmann, B.; Vangay, P.; Knights, D.; Hutkins, R.W.; et al. Stable Engraftment of Bifidobacterium longum AH1206 in the Human Gut Depends on Individualized Features of the Resident Microbiome. Cell Host Microbe 2016, 20, 515–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharseeva, G.G.; Mironov, A.Y.; Alieva, A.A. Suppression of Bacterial Adhesion: Modern Approaches, Problems, and Prospects. Biol. Bull. Rev. 2020, 10, 158–165. [Google Scholar] [CrossRef]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Hafez, M.M. Upregulation of Intestinal Mucin Expression by the Probiotic Bacterium E. coli Nissle 1917. Probiotics Antimicrob. Proteins 2012, 4, 67–77. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hashiba, H.; Hirota, T.; Forstner, J.F. Inhibition of the binding of enterotoxigenic Escherichia coli Pb176 to human intestinal epithelial cell line HCT-8 by an extracellular protein fraction containing BIF of Bifidobacterium longum SBT2928: Suggestive evidence of blocking of the binding receptor gangliotetraosylceramide on the cell surface. Int. J. Food Microbiol. 2001, 67, 97–106. [Google Scholar] [CrossRef]
- Wu, X.; Vallance, B.A.; Boyer, L.; Bergstrom, K.S.B.; Walker, J.; Madsen, K.; O’Kusky, J.R.; Buchan, A.M.; Jacobson, K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G295–G306. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, I.; Leplingard, A.; Darfeuille-Michaud, A. Lactobacillus casei DN-114 001 inhibits the ability of adherent-invasive Escherichia coli isolated from Crohn’s disease patients to adhere to and to invade intestinal epithelial cells. Appl. Environ. Microbiol. 2005, 71, 2880–2887. [Google Scholar] [CrossRef] [Green Version]
- Iseppi, R.; Messi, P.; Camellini, S.; Sabia, C. Bacteriocin activity of Lactobacillus brevis and Lactobacillus paracasei ssp. paracasei. J. Med Microbiol. 2019, 68, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Cleusix, V.; Lacroix, C.; Vollenweider, S.; Le Blay, G. Glycerol induces reuterin production and decreases Escherichia coli population in an in vitro model of colonic fermentation with immobilized human feces. FEMS Microbiol. Ecol. 2008, 63, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, Z.; Johnson, M.G. Characterization and antimicrobial spectrum of bifidocin B, a bacteriocin produced by Bifidobacterium bifidum NCFB 1454. J. Food Prot. 1998, 61, 47–51. [Google Scholar] [CrossRef]
- Yildirim, Z.; Winters, D.K.; Johnson, M.G. Purification, amino acid sequence and mode of action of bifidocin B produced by Bifidobacterium bifidum NCFB 1454. J. Appl. Microbiol. 1999, 86, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Corr, S.C.; Li, Y.; Riedel, C.U.; O’Toole, P.W.; Hill, C.; Gahan, C.G.M. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; O’Riordan, M.X.D. Regulation of bacterial pathogenesis by intestinal short-chain Fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahara, T.; Shimizu, K.; Nomoto, K.; Hamabata, T.; Ozawa, A.; Takeda, Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 2004, 72, 2240–2247. [Google Scholar] [CrossRef] [Green Version]
- Carey, C.M.; Kostrzynska, M.; Ojha, S.; Thompson, S. The effect of probiotics and organic acids on Shiga-toxin 2 gene expression in enterohemorrhagic Escherichia coli O157:H7. J. Microbiol. Methods 2008, 73, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Matsui, T.; Itoh, K. Prevention of Escherichia coli O157:H7 infection in gnotobiotic mice associated with Bifidobacterium strains. Antonie Van Leeuwenhoek 2010, 97, 107–117. [Google Scholar] [CrossRef]
- Turner, P.C.; Wu, Q.K.; Piekkola, S.; Gratz, S.; Mykkänen, H.; El-Nezami, H. Lactobacillus rhamnosus strain GG restores alkaline phosphatase activity in differentiating Caco-2 cells dosed with the potent mycotoxin deoxynivalenol. Food Chem. Toxicol. 2008, 46, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zou, Y.; Cho, Y.; Ahn, J. Effects of bile salt deconjugation by probiotic strains on the survival of antibiotic-resistant foodborne pathogens under simulated gastric conditions. J. Food Prot. 2012, 75, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, Y.; Wang, J.; Zhang, H.; Qi, W. Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. PLoS ONE 2012, 7, e29452. [Google Scholar] [CrossRef] [Green Version]
- Ukena, S.N.; Singh, A.; Dringenberg, U.; Engelhardt, R.; Seidler, U.; Hansen, W.; Bleich, A.; Bruder, D.; Franzke, A.; Rogler, G.; et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE 2007, 2, e1308. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Henry, K.C.; Donato, K.A.; Shen-Tu, G.; Gordanpour, M.; Sherman, P.M. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect. Immun. 2008, 76, 1340–1348. [Google Scholar] [CrossRef] [Green Version]
- Parassol, N.; Freitas, M.; Thoreux, K.; Dalmasso, G.; Bourdet-Sicard, R.; Rampal, P. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res. Microbiol. 2005, 156, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sicard, J.-F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef] [PubMed]
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1168-74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, T.; Ikari, N.; Kouchi, T.; Kowatari, Y.; Kubota, Y.; Shimojo, N.; Tsuji, N.M. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci. Rep. 2018, 8, 5065. [Google Scholar] [CrossRef] [PubMed]
- Kotani, Y.; Kunisawa, J.; Suzuki, Y.; Sato, I.; Saito, T.; Toba, M.; Kohda, N.; Kiyono, H. Role of Lactobacillus pentosus Strain b240 and the Toll-like receptor 2 axis in Peyer’s patch dendritic cell-mediated immunoglobulin A enhancement. PLoS ONE 2014, 9, e91857. [Google Scholar] [CrossRef]
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. Biomed. Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef]
- Möndel, M.; Schroeder, B.O.; Zimmermann, K.; Huber, H.; Nuding, S.; Beisner, J.; Fellermann, K.; Stange, E.F.; Wehkamp, J. Probiotic E. coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal. Immunol. 2009, 2, 166–172. [Google Scholar] [CrossRef]
- Schlee, M.; Wehkamp, J.; Altenhoefer, A.; Oelschlaeger, T.A.; Stange, E.F.; Fellermann, K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 2007, 75, 2399–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolillo, R.; Romano Carratelli, C.; Sorrentino, S.; Mazzola, N.; Rizzo, A. Immunomodulatory effects of Lactobacillus plantarum on human colon cancer cells. Int. Immunopharmacol. 2009, 9, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Kingma, S.D.K.; Li, N.; Sun, F.; Valladares, R.B.; Neu, J.; Lorca, G.L. Lactobacillus johnsonii N6.2 stimulates the innate immune response through Toll-like receptor 9 in Caco-2 cells and increases intestinal crypt Paneth cell number in biobreeding diabetes-prone rats. J. Nutr. 2011, 141, 1023–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.G.; Kayama, H.; Ueda, Y.; Takahashi, T.; Asahara, T.; Tsuji, H.; Tsuji, N.M.; Kiyono, H.; Ma, J.S.; Kusu, T.; et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012, 8, e1002714. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kim, S.-H.; Whang, K.-Y.; Kim, Y.-J.; Oh, S. Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J. Microbiol. Biotechnol. 2008, 18, 1278–1285. [Google Scholar]
- Kaisho, T.; Akira, S. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 2002, 1589, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L.K. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264; quiz 1446–1447. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Schild, M.; Kunz, C.; Zimmermann, K.; Kuntz, S. Effects of Live and Heat-Inactivated, E. coli Strains and Their Supernatants on Immune Regulation in HT-29 Cells. Eur. J. Microbiol. Immunol. 2018, 8, 41–46. [Google Scholar] [CrossRef]
- Ren, D.-Y.; Li, C.; Qin, Y.-Q.; Yin, R.-L.; Du, S.-W.; Ye, F.; Liu, H.-F.; Wang, M.-P.; Sun, Y.; Li, X.; et al. Lactobacilli reduce chemokine IL-8 production in response to TNF-α and Salmonella challenge of Caco-2 cells. Biomed. Res. Int. 2013, 2013, 925219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastall, R.A.; Gibson, G.R. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 2015, 32, 42–46. [Google Scholar] [CrossRef]
- Morrison, D.J.; Mackay, W.G.; Edwards, C.A.; Preston, T.; Dodson, B.; Weaver, L.T. Butyrate production from oligofructose fermentation by the human faecal flora: What is the contribution of extracellular acetate and lactate? Br. J. Nutr. 2006, 96, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Egli, I.; Chassard, C.; Lacroix, C.; Hurrell, R. Inulin modifies the bifidobacteria population, fecal lactate concentration, and fecal pH but does not influence iron absorption in women with low iron status. Am. J. Clin. Nutr. 2012, 96, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.-M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Palframan, R.J.; Gibson, G.R.; Rastall, R.A. Effect of pH and dose on the growth of gut bacteria on prebiotic carbohydrates in vitro. Anaerobe 2002, 8, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A. The prebiotic properties of oligofructose at low intake levels. Nutr. Res. 2001, 21, 843–848. [Google Scholar] [CrossRef]
- Seifert, S.; Watzl, B. Inulin and oligofructose: Review of experimental data on immune modulation. J. Nutr. 2007, 137, 2563S–2567S. [Google Scholar] [CrossRef] [PubMed]
| Compound | Model System | Inflammatory Markers | Reference |
|---|---|---|---|
| Flavonoids | |||
| Co-culture cell model | NFκB pathway ↓ | [18,19] |
| Pro-inflammatory cytokines ↓ | |||
| Pro-inflammatory cytokines ↓ | |||
| Mice | Chemokine (CCL2) ↑ | [20] | |
| Phenolic acids | |||
| |||
| Murine peritoneal macrophages | TNFα ↓, IL-6 ↓, COX-2 ↓, PGE2 ↓, NO ↓ | [25] |
| RAW 264.7 cells | NFκB ↓ | [26] |
| Mice | NFκB ↓, IL-6 ↓, pSTAT3Y6 ↑ | [27] | |
| Murine allograft model | Treg ↑, T-cell activation ↓, T-cell number ↓ | [28] | |
| |||
| THP1 cells | IL-1β ↓, IL-6 ↓ | [29] |
| Rats | IL-1β ↓, IL-4 ↓, IL-6 ↓ | [30] | |
| Murine BDMC | Treg ↑ | [33] |
| Caco-2 cells (co-culture) | TNFα ↓, IL-6 ↓ | [34] | |
| Mice (IL-10−/−) | IFNγ ↔ | [35] | |
| Stilbens | |||
| Caco-2 cells | NFκB ↓ | [41,42] |
| SW480 cells | TLR4 ↑, iNOS ↑ | [42] | |
| Caco-2 cells | NFκB ↑ | [44] | |
| Mice | SAA ↓, SIRT1 ↑ | [45] | |
| Pro-inflammatory cytokines ↓, Anti-inflammatory cytokines ↑, Treg/Th17 ↑ | [46,47,48] | ||
| Rats | PGE1 ↓, COX-2 ↓, iNOS ↓ | [48] | |
| PGE1 ↓, COX-2 ↓ | [49] | ||
| Glucosinolates/Isothiocyanates | |||
| Mice | Pro-inflammatory cytokines ↓ | [61] |
| Colitic mice | Weight loss ↓, pro-inflammatory cytokines ↓, monocyte infiltration ↓, miRNA-155 ↓ | [62,63] | |
| Colitic mice | infiltration of immune cells ↓ | [66,67] |
| Caco-2-, HT-29-, SW480 cells | HBD-2 ↑ | [68,69] | |
| Murine macrophages | Inflammatory markers ↓ | [70] |
| Jurkat cells | Inflammatory processes ↑ (high concentrations) | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, C.; Wagner, A.E. Impact of Food-Derived Bioactive Compounds on Intestinal Immunity. Biomolecules 2021, 11, 1901. https://doi.org/10.3390/biom11121901
Zimmermann C, Wagner AE. Impact of Food-Derived Bioactive Compounds on Intestinal Immunity. Biomolecules. 2021; 11(12):1901. https://doi.org/10.3390/biom11121901
Chicago/Turabian StyleZimmermann, Christian, and Anika E. Wagner. 2021. "Impact of Food-Derived Bioactive Compounds on Intestinal Immunity" Biomolecules 11, no. 12: 1901. https://doi.org/10.3390/biom11121901
APA StyleZimmermann, C., & Wagner, A. E. (2021). Impact of Food-Derived Bioactive Compounds on Intestinal Immunity. Biomolecules, 11(12), 1901. https://doi.org/10.3390/biom11121901






