The Role of Pumilio RNA Binding Protein in Plants
Abstract
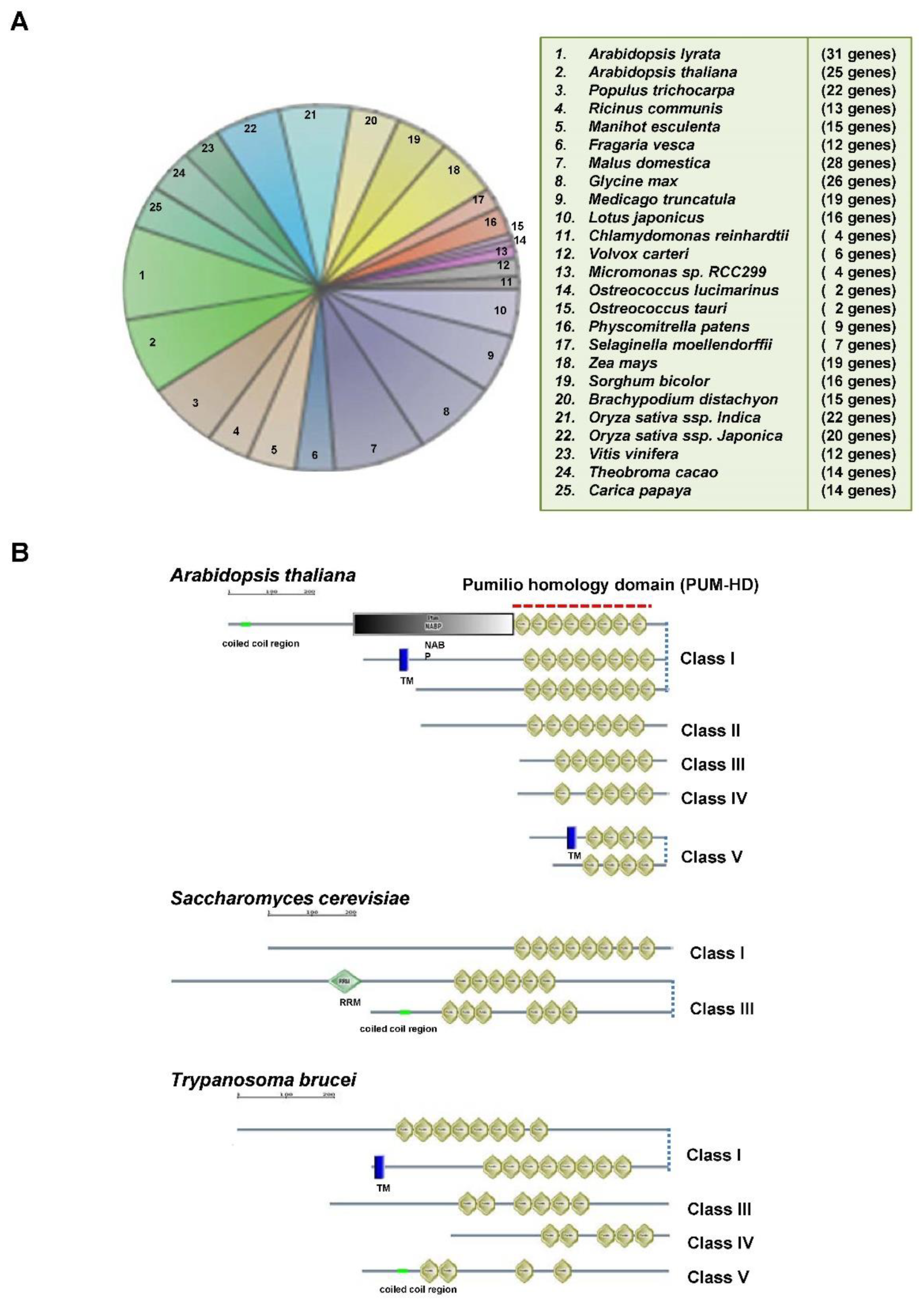
1. Evolutionally Conserved Pumilio RNA Binding Proteins
2. RNA-Binding Specificity of Pumilio-Homology Domain
3. Protein–Protein Interaction in Plant Pum
4. Dynamic Subcellular Localization of Plant Pum
5. The Role of Pum in Plant Development
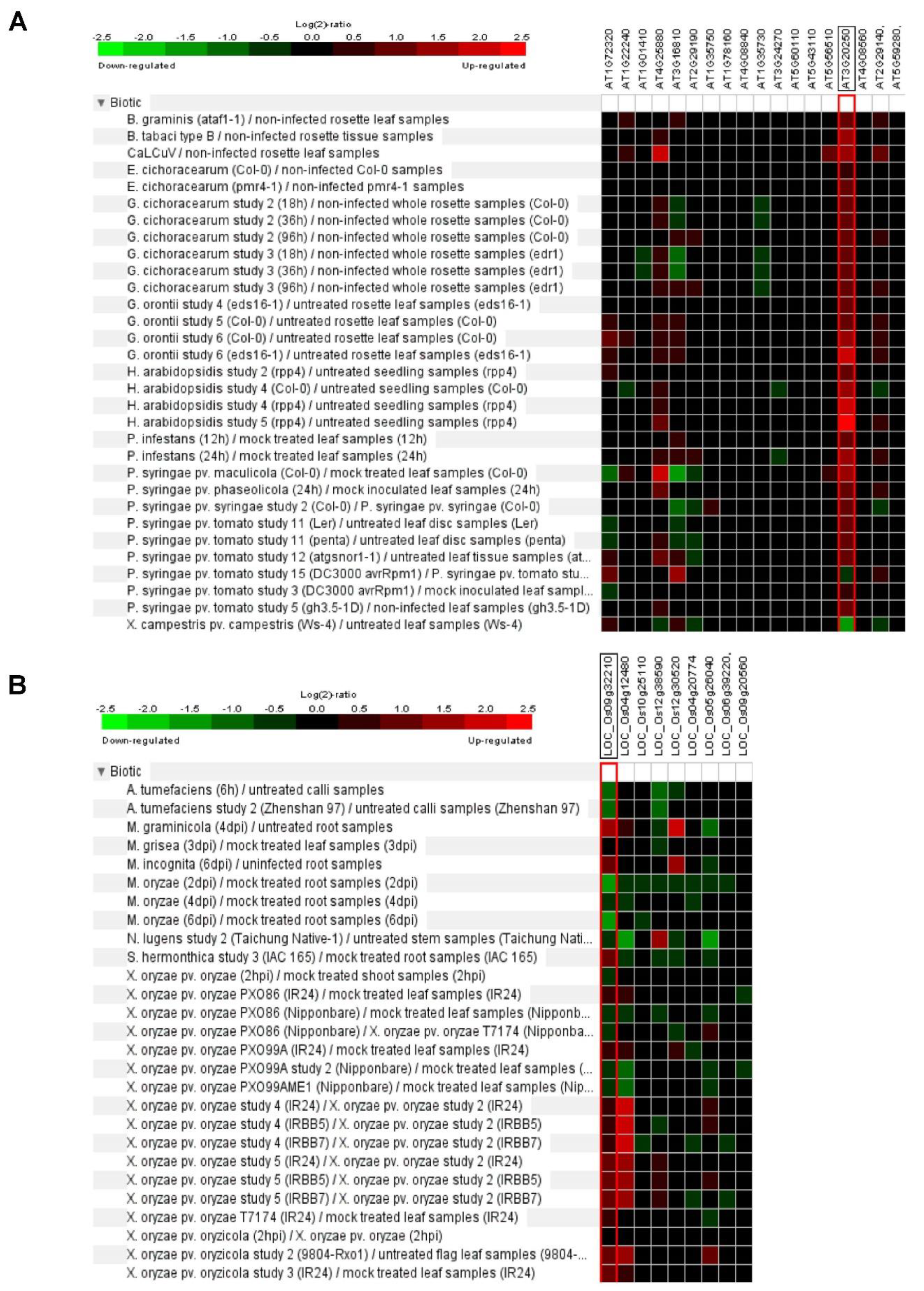
6. Plant Pum Function in Biotic Stress
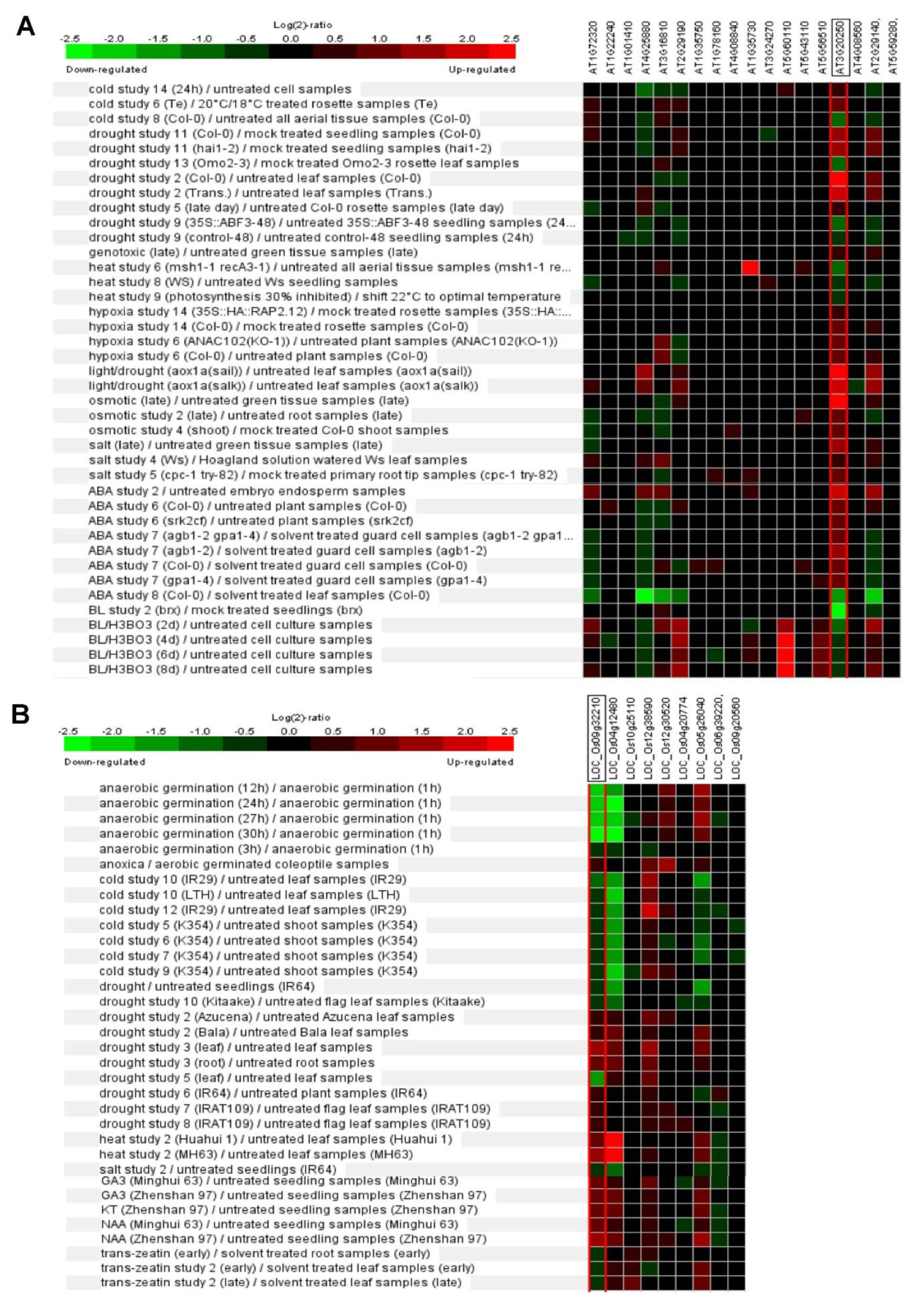
7. Plant Pum Function in Abiotic Stress
8. The Multifunctional Plant Pum Protein and Crop Engineering
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.H.; Wu, X.; Zhu, Y. RNA-binding protein PUM2 regulates mesenchymal stem cell fate via repression of JAK2 and RUNX2 mRNAs. J. Cell Physiol. 2020, 235, 3874–3885. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, Z.; Pan, X.; Shen, H.B.; Choi, K.S.; Wang, L.; Wang, S.; Wu, J. RNA-binding protein recognition based on multi-view deep feature and multi-label learning. Brief. Bioinform. 2020, 22, bbaa174. [Google Scholar] [CrossRef] [PubMed]
- Goldstrohm, A.C.; Hall, T.M.T.; McKenney, K.M. Post-transcriptional Regulatory Functions of Mammalian Pumilio Proteins. Trends Genet. 2018, 34, 972–990. [Google Scholar] [CrossRef] [PubMed]
- Nishanth, M.J.; Simon, B. Functions, mechanisms and regulation of Pumilio/Puf family RNA binding proteins: A comprehensive review. Mol. Biol. Rep. 2020, 47, 785–807. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Yao, P.; Arif, A.; Fox, P.L. Regulation and dysregulation of 3’UTR-mediated translational control. Curr. Opin. Genet. Dev. 2013, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Dutcher, R.C.; Porter, D.F.; Arava, Y.; Wickens, M.; Hall, T.M.T. Distinct RNA-binding modules in a single PUF protein cooperate to determine RNA specificity. Nucleic Acids Res. 2019, 47, 8770–8784. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Fan, Q.; Parker, D.; Li, X.; Li, J.; Cui, L. Puf mediates translation repression of transmission-blocking vaccine candidates in malaria parasites. PLoS Pathog. 2013, 9, e1003268. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ham, B.-K.; Chong, Y.H.; Yeh, S.-D.; Lucas, W.J. A Plant SMALL RNA-BINDING PROTEIN 1 Family Mediates Cell-to-Cell Trafficking of RNAi Signals. Mol. Plant 2020, 13, 321–335. [Google Scholar] [CrossRef]
- Galgano, A.; Forrer, M.; Jaskiewicz, L.; Kanitz, A.; Zavolan, M.; Gerber, A.P. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS ONE 2008, 3, e3164. [Google Scholar] [CrossRef]
- Joshna, C.R.; Saha, P.; Atugala, D.; Chua, G.; Muench, D.G. Plant PUF RNA-binding proteins: A wealth of diversity for post-transcriptional gene regulation. Plant Sci. 2020, 297, 110505. [Google Scholar] [CrossRef] [PubMed]
- Spassov, D.S.; Jurecic, R. The PUF family of RNA-binding proteins: Does evolutionarily conserved structure equal conserved function? IUBMB Life 2003, 55, 359–366. [Google Scholar] [CrossRef]
- Spassov, D.S.; Jurecic, R. Cloning and comparative sequence analysis of PUM1 and PUM2 genes, human members of the Pumilio family of RNA-binding proteins. Gene 2002, 299, 195–204. [Google Scholar] [CrossRef]
- Spassov, D.S.; Jurecic, R. Mouse Pum1 and Pum2 genes, members of the Pumilio family of RNA-binding proteins, show differential expression in fetal and adult hematopoietic stem cells and progenitors. Blood Cells Mol. Dis. 2003, 30, 55–69. [Google Scholar] [CrossRef]
- Gerber, A.P.; Herschlag, D.; Brown, P.O. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2004, 2, E79. [Google Scholar] [CrossRef]
- Stumpf, C.R.; Kimble, J.; Wickens, M. A Caenorhabditis elegans PUF protein family with distinct RNA binding specificity. RNA 2008, 14, 1550–1557. [Google Scholar] [CrossRef]
- Caro, F.; Bercovich, N.; Atorrasagasti, C.; Levin, M.J.; Vazquez, M.P. Trypanosoma cruzi: Analysis of the complete PUF RNA-binding protein family. Exp. Parasitol. 2006, 113, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Fan, Q.; Li, J. The malaria parasite Plasmodium falciparum encodes members of the Puf RNA-binding protein family with conserved RNA binding activity. Nucleic Acids Res. 2002, 30, 4607–4617. [Google Scholar] [CrossRef] [PubMed]
- Schweers, B.A.; Walters, K.J.; Stern, M. The Drosophila melanogaster translational repressor pumilio regulates neuronal excitability. Genetics 2002, 161, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.P.; Luschnig, S.; Krasnow, M.A.; Brown, P.O.; Herschlag, D. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2006, 103, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Van Bel, M.; Proost, S.; Wischnitzki, E.; Movahedi, S.; Scheerlinck, C.; Van de Peer, Y.; Vandepoele, K. Dissecting plant genomes with the PLAZA comparative genomics platform. Plant Physiol. 2012, 158, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef]
- Wang, X.; McLachlan, J.; Zamore, P.D.; Hall, T.M. Modular recognition of RNA by a human pumilio-homology domain. Cell 2002, 110, 501–512. [Google Scholar] [CrossRef]
- Wharton, R.P.; Sonoda, J.; Lee, T.; Patterson, M.; Murata, Y. The Pumilio RNA-binding domain is also a translational regulator. Mol. Cell 1998, 1, 863–872. [Google Scholar] [CrossRef]
- Najdrova, V.; Stairs, C.W.; Vinopalova, M.; Voleman, L.; Dolezal, P. The evolution of the Puf superfamily of proteins across the tree of eukaryotes. BMC Biol. 2020, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.T.; Higgin, J.J.; Hall, T.M. Basis of altered RNA-binding specificity by PUF proteins revealed by crystal structures of yeast Puf4p. Nat. Struct. Mol. Biol. 2008, 15, 397–402. [Google Scholar] [CrossRef]
- Ryder, S.P. Pumilio RNA recognition: The consequence of promiscuity. Structure 2011, 19, 277–279. [Google Scholar] [CrossRef]
- Cooke, A.; Prigge, A.; Opperman, L.; Wickens, M. Targeted translational regulation using the PUF protein family scaffold. Proc. Natl. Acad. Sci. USA 2011, 108, 15870–15875. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.G.; Hall, T.M. Engineering RNA sequence specificity of Pumilio repeats. Proc. Natl. Acad. Sci. USA 2006, 103, 13635–13639. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.; Tsai, Y.S.; Dominguez, D.; Wang, Y.; Wang, Z. Engineering RNA endonucleases with customized sequence specificities. Nat. Commun. 2012, 3, 1147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Hall, T.M. Engineered proteins with PUF scaffold to manipulate RNA metabolism. FEBS J. 2013, 280, 3755–3767. [Google Scholar] [CrossRef] [PubMed]
- Saint-Georges, Y.; Garcia, M.; Delaveau, T.; Jourdren, L.; Le Crom, S.; Lemoine, S.; Tanty, V.; Devaux, F.; Jacq, C. Yeast mitochondrial biogenesis: A role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS ONE 2008, 3, e2293. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.U.; Kim, M.J.; Paek, K.H. Arabidopsis Pumilio protein APUM5 suppresses Cucumber mosaic virus infection via direct binding of viral RNAs. Proc. Natl. Acad. Sci. USA 2013, 110, 779–784. [Google Scholar] [CrossRef]
- Sonoda, J.; Wharton, R.P. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev. 1999, 13, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Francischini, C.W.; Quaggio, R.B. Molecular characterization of Arabidopsis thaliana PUF proteins-binding specificity and target candidates. FEBS J. 2009, 276, 5456–5470. [Google Scholar] [CrossRef] [PubMed]
- Haramati, O.; Brodov, A.; Yelin, I.; Atir-Lande, A.; Samra, N.; Arava, Y. Identification and characterization of roles for Puf1 and Puf2 proteins in the yeast response to high calcium. Sci. Rep. 2017, 7, 3037. [Google Scholar] [CrossRef]
- Gu, W.; Deng, Y.; Zenklusen, D.; Singer, R.H. A new yeast PUF family protein, Puf6p, represses ASH1 mRNA translation and is required for its localization. Genes Dev. 2004, 18, 1452–1465. [Google Scholar] [CrossRef]
- Abbasi, N.; Kim, H.-B.; Park, N.-I.; Kim, H.-S.; Kim, Y.-K.; Park, Y.-I.; Choi, S.-B. APUM23, a nucleolar Puf domain protein, is involved in pre-ribosomal RNA processing and normal growth patterning in Arabidopsis. Plant J. 2010, 64, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Beak, J.Y.; Kim, Y.S.; Petrovich, R.M.; Collins, J.B.; Grissom, S.F.; Jetten, A.M. NABP1, a novel RORgamma-regulated gene encoding a single-stranded nucleic-acid-binding protein. Biochem. J. 2006, 397, 89–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lorkovic, Z.J.; Barta, A. Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res. 2002, 30, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.U.; Paek, K.H. Role of Arabidopsis Pumilio RNA binding protein 5 in virus infection. Plant Signal. Behav. 2013, 8, e23975. [Google Scholar] [CrossRef]
- Wu, J.; Campbell, Z.T.; Menichelli, E.; Wickens, M.; Williamson, J.R. A protein.protein interaction platform involved in recruitment of GLD-3 to the FBF.fem-3 mRNA complex. J. Mol. Biol. 2013, 425, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Goldstrohm, A.C.; Hook, B.A.; Seay, D.J.; Wickens, M. PUF proteins bind Pop2p to regulate messenger RNAs. Nat. Struct. Mol. Biol. 2006, 13, 533–539. [Google Scholar] [CrossRef]
- Van Etten, J.; Schagat, T.L.; Hrit, J.; Weidmann, C.A.; Brumbaugh, J.; Coon, J.J.; Goldstrohm, A.C. Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs. J. Biol. Chem. 2012, 287, 36370–36383. [Google Scholar] [CrossRef] [PubMed]
- Goldstrohm, A.C.; Seay, D.J.; Hook, B.A.; Wickens, M. PUF protein-mediated deadenylation is catalyzed by Ccr4p. J. Biol. Chem. 2007, 282, 109–114. [Google Scholar] [CrossRef]
- Arae, T.; Morita, K.; Imahori, R.; Suzuki, Y.; Yasuda, S.; Sato, T.; Yamaguchi, J.; Chiba, Y. Identification of Arabidopsis CCR4-NOT Complexes with Pumilio RNA-Binding Proteins, APUM5 and APUM2. Plant Cell Physiol. 2019, 60, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Traven, A.; Lo, T.L.; Lithgow, T.; Heierhorst, J. The yeast PUF protein Puf5 has Pop2-independent roles in response to DNA replication stress. PLoS ONE 2010, 5, e10651. [Google Scholar] [CrossRef]
- Friend, K.; Campbell, Z.T.; Cooke, A.; Kroll-Conner, P.; Wickens, M.P.; Kimble, J. A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nat. Struct. Mol. Biol. 2012, 19, 176–183. [Google Scholar] [CrossRef]
- Tam, P.P.; Barrette-Ng, I.H.; Simon, D.M.; Tam, M.W.; Ang, A.L.; Muench, D.G. The Puf family of RNA-binding proteins in plants: Phylogeny, structural modeling, activity and subcellular localization. BMC Plant Biol. 2010, 10, 44. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, H.-S.; Kalita, P.J.; Choi, S.-B. Structural and functional similarities and differences in nucleolar Pumilio RNA-binding proteins between Arabidopsis and the charophyte Chara corallina. BMC Plant Biol. 2020, 20, 230. [Google Scholar] [CrossRef]
- Wreden, C.; Verrotti, A.C.; Schisa, J.A.; Lieberfarb, M.E.; Strickland, S. Nanos and pumilio establish embryonic polarity in Drosophila by promoting posterior deadenylation of hunchback mRNA. Development 1997, 124, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Bouchabke-Coussa, O.; Obellianne, M.; Linderme, D.; Montes, E.; Maia-Grondard, A.; Vilaine, F.; Pannetier, C. Wuschel overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutum L.) tissues cultured in vitro. Plant Cell Rep. 2013, 32, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.L.; De Smet, I. Development: CLAVATA1 joins the club of root stem cell regulators. Curr. Biol. 2013, 23, R245–R247. [Google Scholar] [CrossRef]
- Moore, F.L.; Jaruzelska, J.; Fox, M.S.; Urano, J.; Firpo, M.T.; Turek, P.J.; Dorfman, D.M.; Pera, R.A.R. Human Pumilio-2 is expressed in embryonic stem cells and germ cells and interacts with DAZ (Deleted in AZoospermia) and DAZ-like proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Qiang, W.; Zhu, M.; Ding, Y.; Shi, Q.; Chen, X.; Zsiros, E.; Wang, K.; Yang, X.; Kurita, T.; et al. Mammalian Pum1 and Pum2 Control Body Size via Translational Regulation of the Cell Cycle Inhibitor Cdkn1b. Cell Rep. 2019, 26, 2434–2450.e6. [Google Scholar] [CrossRef]
- Racher, H.; Hansen, D. PUF-8, a Pumilio homolog, inhibits the proliferative fate in the Caenorhabditis elegans germline. G3 Genes Genomes Genet. 2012, 2, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Bernoux, M.; Timmers, T.; Jauneau, A.; Briere, C.; de Wit, P.J.; Marco, Y.; Deslandes, L. RD19, an Arabidopsis cysteine protease required for RRS1-R-mediated resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 effector. Plant Cell 2008, 20, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N. Abscisic Acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-C.; Lin, W.-C.; Cheng, W.-H. Salt hypersensitive mutant 9, a nucleolar APUM23 protein, is essential for salt sensitivity in association with the ABA signaling pathway in Arabidopsis. BMC Plant Biol. 2018, 18, 40. [Google Scholar] [CrossRef]
- Huh, S.U.; Paek, K.H. APUM5, encoding a Pumilio RNA binding protein, negatively regulates abiotic stress responsive gene expression. BMC Plant Biol. 2014, 14, 75. [Google Scholar] [CrossRef]
- Nyiko, T.; Auber, A.; Bucher, E. Functional and molecular characterization of the conserved Arabidopsis PUMILIO protein, APUM9. Plant Mol. Biol. 2019, 100, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.-L.; Luo, L. Effects of engineered Sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress. Appl. Environ. Microbiol. 2012, 78, 8056–8061. [Google Scholar] [CrossRef] [PubMed]
- Popko, J.; Hansch, R.; Mendel, R.-R.; Polle, A.; Teichmann, T. The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 2010, 12, 242–258. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, S.U. The Role of Pumilio RNA Binding Protein in Plants. Biomolecules 2021, 11, 1851. https://doi.org/10.3390/biom11121851
Huh SU. The Role of Pumilio RNA Binding Protein in Plants. Biomolecules. 2021; 11(12):1851. https://doi.org/10.3390/biom11121851
Chicago/Turabian StyleHuh, Sung Un. 2021. "The Role of Pumilio RNA Binding Protein in Plants" Biomolecules 11, no. 12: 1851. https://doi.org/10.3390/biom11121851
APA StyleHuh, S. U. (2021). The Role of Pumilio RNA Binding Protein in Plants. Biomolecules, 11(12), 1851. https://doi.org/10.3390/biom11121851





