Association of Circulating miR-145-5p and miR-let7c and Atherosclerotic Plaques in Hypertensive Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Characteristics
2.3. Carotid Ultrasound
2.4. RNA Isolation and Quantitative Real Time PCR (qRT-PCR)
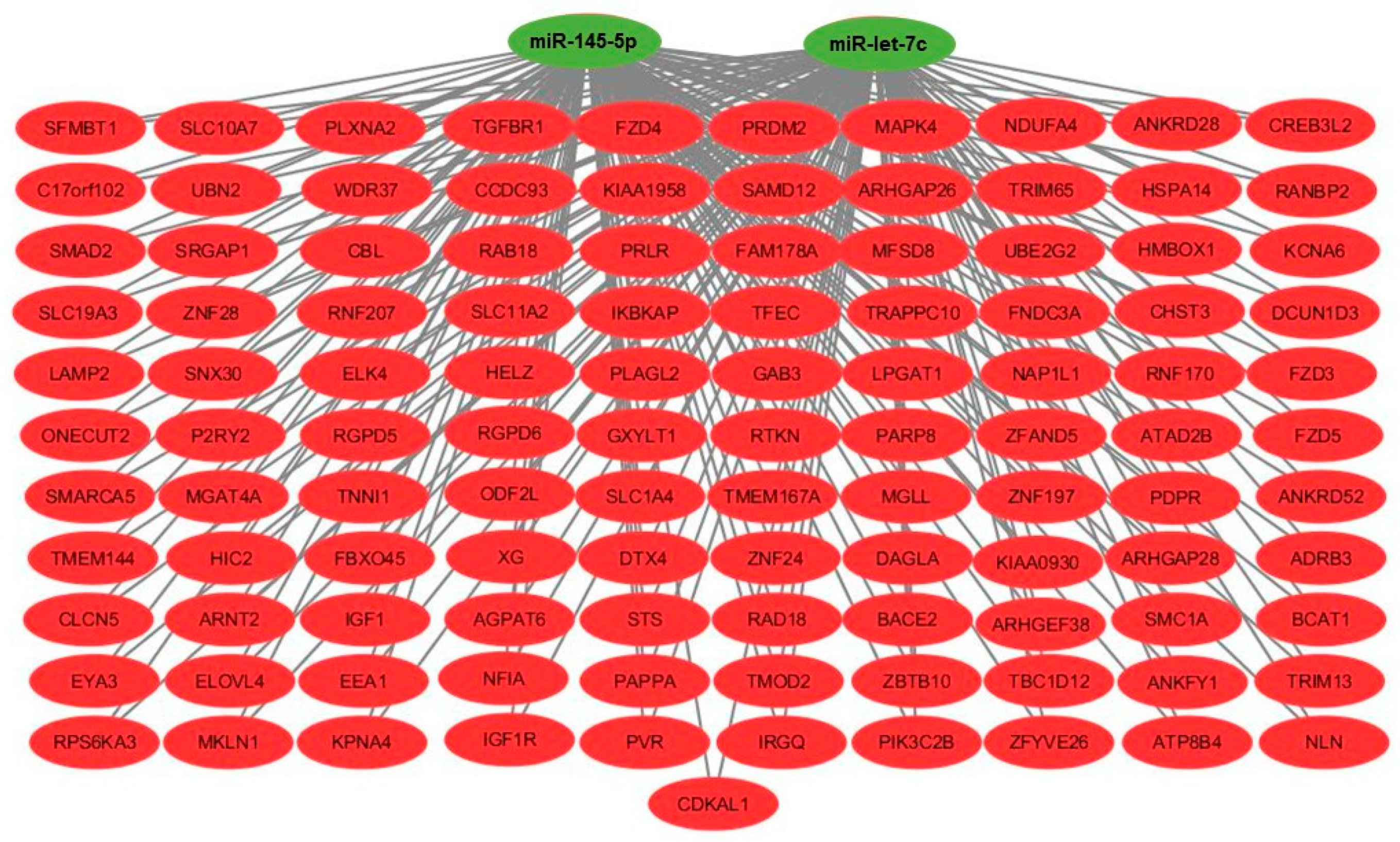
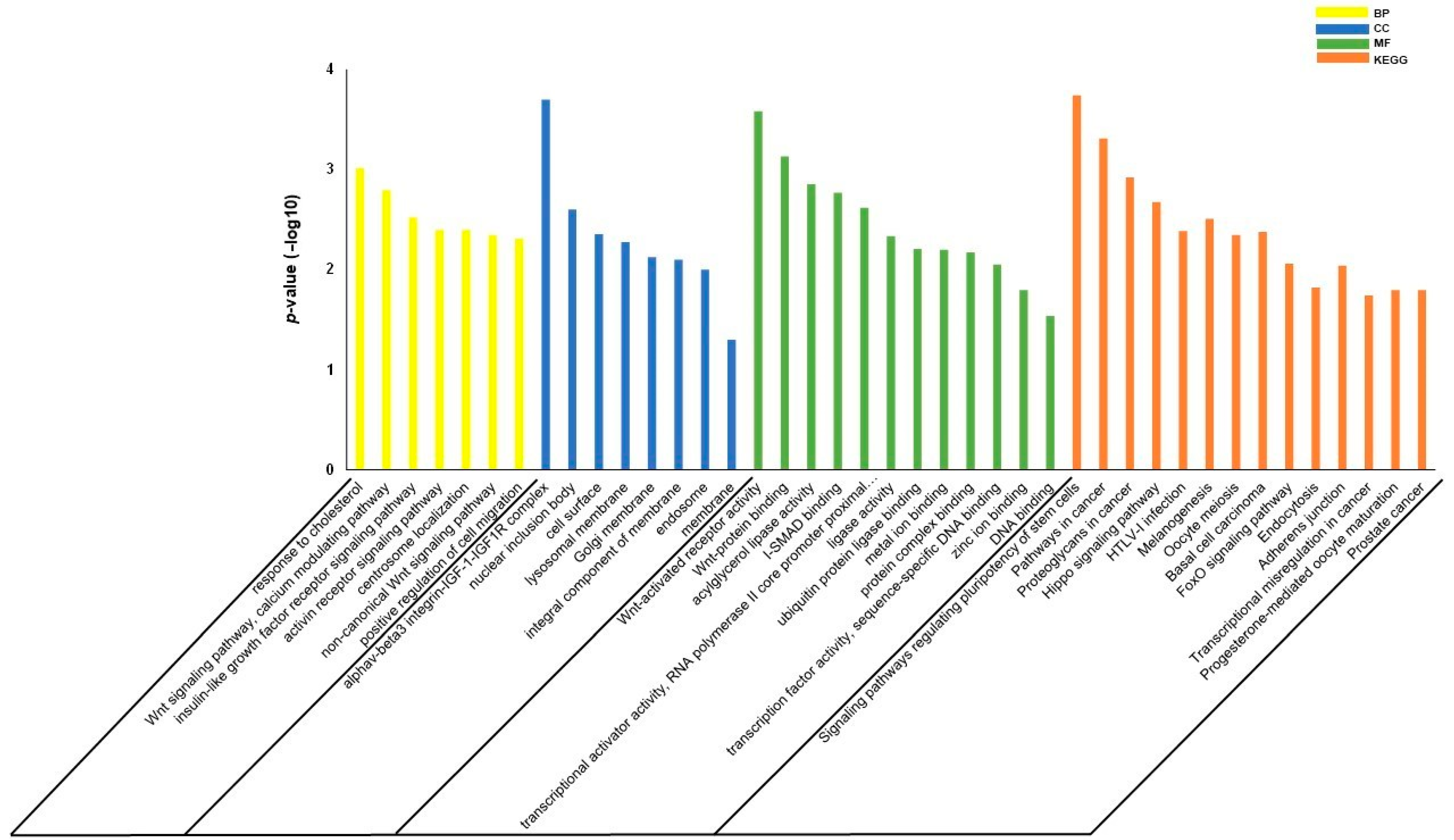
2.5. Gene Set Enrichment Analysis
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Participants
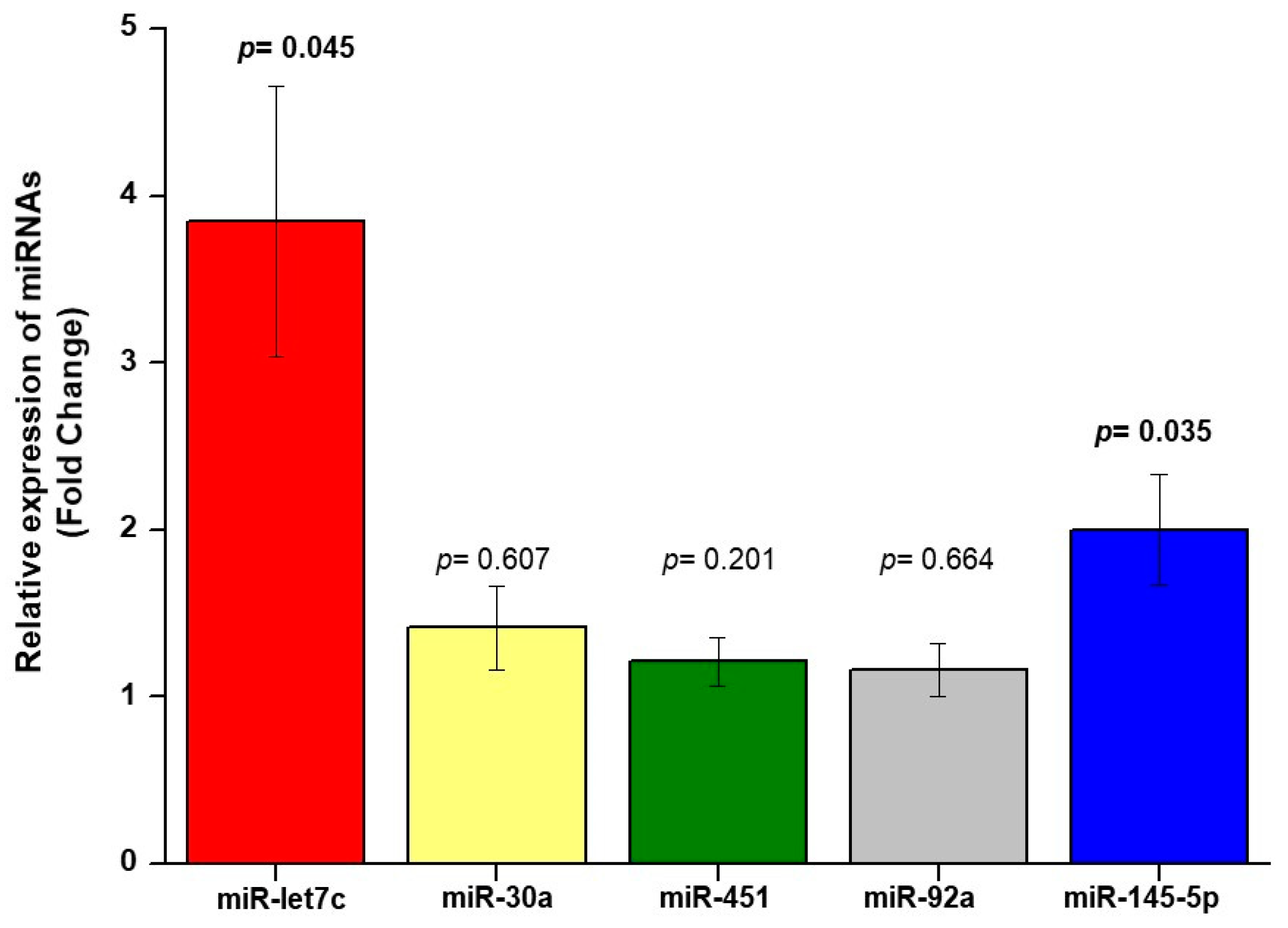
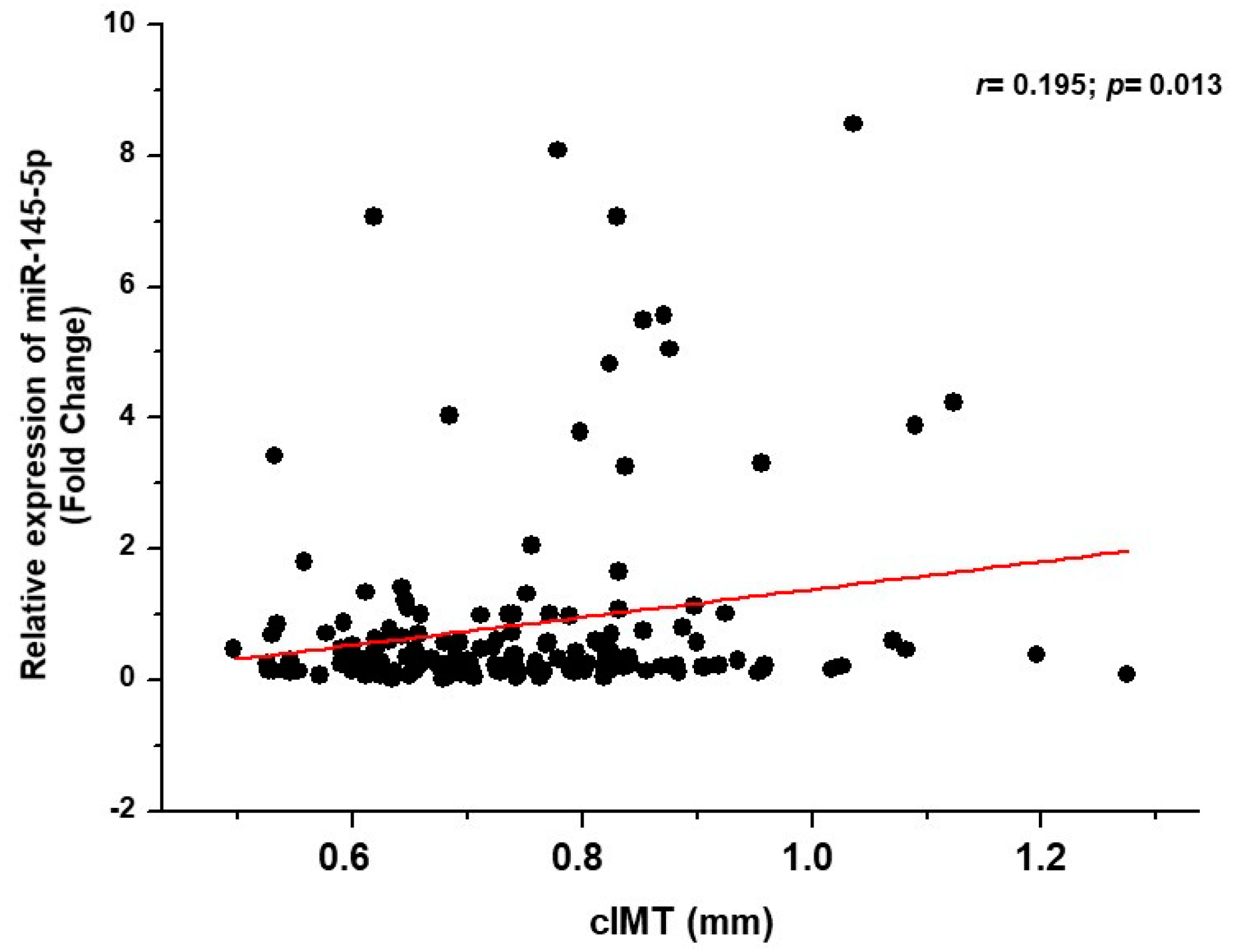
3.2. Relationship between miRNA and Carotid Plaques and cIMT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grandhi, G.; Mszar, R.; Vahidy, F.; Valero-Elizondo, J.; Blankstein, R.; Blaha, M.J.; Virani, S.S.; Andrieni, J.D.; Omer, S.B.; Nasir, K. Sociodemographic disparities in influenza vaccination among adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2020, 1, 87–91. [Google Scholar] [CrossRef]
- Van den Oord, S.; Sijbrands, E.; Kate, G.L.T.; van Klaveren, D.; van Domburg, R.T.; van der Steen, A.F.; Schinkel, A.F. Carotid intima-media thickness for cardiovascular risk assessment: Systematic review and meta-analysis. Atherosclerosis 2013, 228, 1–11. [Google Scholar] [CrossRef]
- Nadruz, W., Jr.; Claggett, B.; Henglin, M.; Shah, A.M.; Skali, H.; Rosamond, W.D.; Folsom, A.R.; Solomon, S.D.; Cheng, S. Racial disparities in risks of stroke. N. Engl. J. Med. 2017, 376, 2089–2090. [Google Scholar] [CrossRef] [Green Version]
- Nadruz, W., Jr.; Claggett, B.; Henglin, M.; Shah, A.M.; Skali, H.; Rosamond, W.D.; Folsom, A.R.; Solomon, S.D.; Cheng, S. Widening racial differences in risks for coronary heart disease. Circulation 2018, 137, 1195–1197. [Google Scholar] [CrossRef]
- Zanchetti, A.; Hennig, M.; Hollweck, R.; Bond, G.; Tang, R.; Cuspidi, C. Baseline values but not treatment-induced changes in carotid intima-media thickness predict incident cardiovascular events in treated hypertensive patients: Findings in the European Lacidipine Study on Atherosclerosis (ELSA). Circulation 2009, 120, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Ohishi, M.; Takeya, Y.; Onishi, M.; Ito, N.; Oguro, R.; Yamamoto, K.; Kamide, K.; Rakugi, H. Carotid plaque score and intima media thickness as predictors of stroke and mortality in hypertensive patients. Hypertens. Res. 2013, 36, 902–909. [Google Scholar] [CrossRef]
- Cuspidi, C.; Sala, C.; Tadic, M.; Gherbesi, E.; Grassi, G.; Mancia, G. Pre-hypertension and subclinical carotid damage: A meta-analysis. J. Hum. Hypertens. 2019, 33, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Berezin, A.E.; Berezin, A. Extracellular endothelial cell-derived vesicles: Emerging role in cardiac and vascular remodeling in heart failure. Front. Cardiovasc. Med. 2020, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santovito, D.; Mandolini, C.; Marcantonio, P.; De Nardis, V.; Bucci, M.; Paganelli, C.; Magnacca, F.; Ucchino, S.; Mastroiacovo, D.; Desideri, G.; et al. Overexpression of microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert Opin. Ther. Targets 2013, 17, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Huang, C.; Chen, J.-Y.; Li, J.; Feng, Y.-Q. Plasma expression level of miRNA let-7 is positively correlated with carotid intima-media thickness in patients with essential hypertension. J. Hum. Hypertens. 2017, 31, 843–847. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, N.; Han, X.; Ren, J.; Zhou, P.; Ding, P. MicroRNA-451 inhibits vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via Ywhaz/p38 MAPK pathway. Exp. Cell Res. 2019, 379, 214–224. [Google Scholar] [CrossRef]
- Long, G.; Wang, F.; Li, H.; Yin, Z.; Sandip, C.; Lou, Y.; Wang, Y.; Chen, C.; Wang, D.W. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013, 13, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.R.; Sharma, G.; Bhattacharya, M.; Lee, S.-S.; Chakraborty, C. Circulating miRNA in atherosclerosis: A clinical biomarker and early diagnostic tool. Curr. Mol. Med. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Sardeli, A.V.; Gáspari, A.F.; de Rossi, G.; de Souza, G.V.; de Souza, T.M.F.; Cavaglieri, C.R.; Matos-Souza, J.R.; Nadruz, W., Jr.; Chacon-Mikahilet, M.P.T. Carotid intima-media thickness is associated with media rather than intima thickness. Atherosclerosis 2017, 261, 169–171. [Google Scholar] [CrossRef]
- Carvalho-Romano, L.F.; Bonafé, R.P.; Paim, L.R.; Marques, E.R.; Vegian, C.F.; Pio-Magalhães, J.A.; Mello, D.S.; Schreiber, R.; Sposito, A.C.; Matos-Souza, J.R.; et al. Association of left ventricular strain and E/e’ ratio with carotid wall layers. Atherosclerosis 2020, 310, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kowdley, K.V. Method for microRNA isolation from clinical serum samples. Anal. Biochem. 2012, 431, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Dweep, H.; Sticht, C.; Pandey, P.; Gretz, N. miRWalk—Database: Prediction of possible miRNA binding sites by “walking” the genes of three genomes. J. Biomed. Inform. 2011, 44, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; Duvekot, M.H.; Kate, G.-J.R.T.; Verhoeven, A.J.; Mulder, M.T.; Schinkel, A.F.; Nieman, K.; Watts, G.F.; Sijbrands, E.J.; van Lennep, J.E.R. Carotid artery plaques and intima medial thickness in familial hypercholesteraemic patients on long-term statin therapy: A case control study. Atherosclerosis 2017, 256, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Andreou, I.; Sun, X.; Stone, P.H.; Edelman, E.R.; Feinberg, M.W. miRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends Mol. Med. 2015, 21, 307–318. [Google Scholar] [CrossRef]
- Hergenreider, E.; Heydt, S.; Tréguer, K.; Boettger, T.; Horrevoets, A.J.G.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Knoka, E.; Trusinskis, K.; Mazule, M.; Briede, I.; Crawford, W.; Jegere, S.; Kumsars, I.; Narbute, I.; Sondore, D.; Lejnieks, A.; et al. Circulating plasma microRNA-126, microRNA-145, and microRNA-155 and their association with atherosclerotic plaque characteristics. J. Clin. Transl. Res. 2020, 5, 60–67. [Google Scholar]
- Reilly, N.A.; Lutgens, E.; Kuiper, J.; Heijmans, B.T.; Jukema, J.W. Effects of fatty acids on T cell function: Role in atherosclerosis. Nat. Rev. Cardiol. 2021, 18, 824–837. [Google Scholar] [CrossRef]
- Frostegård, J.; Zhang, Y.; Sun, J.; Yan, K.; Liu, A. Oxidized low-density lipoprotein (OxLDL)—Treated dendritic cells promote activation of T cells in human atherosclerotic plaque and blood, which is repressed by statins: MicroRNA let-7c is integral to the effect. J. Am. Hearth Assoc. 2016, 5, 003976. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Frostegård, J. PCSK9 plays a novel immunological role in oxidized LDL-induced dendritic cell maturation and activation of T cells from human blood and atherosclerotic plaque. J. Intern. Med. 2018, 284, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, T.Z.; Lee, M.-S. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc. Imaging 2014, 7, 1025–1038. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-Q.; Li, Y.-F.; Wang, Z.-H. Effects of β3-adrenoceptor on scavenger receptor class B type 1 and its signal transduction pathway in apolipoprotein E knockout mice. Eur. J. Pharmacol. 2013, 714, 295–302. [Google Scholar] [CrossRef]
- Seijkens, T.; Poels, K.; Meiler, S.; Van Tiel, C.M.; Kusters, P.J.H.; Reiche, M.; Atzler, D.; Winkels, H.; Tjwa, M.; Poelman, H.; et al. Deficiency of the T cell regulator Casitas B-cell lymphoma-B aggravates atherosclerosis by inducing CD8+ T cell-mediated macrophage death. Eur. Hearth J. 2019, 40, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Sukhanov, S.; Shai, S.-Y.; Danchuk, S.; Snarski, P.; Li, Z.; Hou, X.; Hamblin, M.H.; Woods, T.C.; Wang, M.; et al. Endothelial deficiency of insulin-like growth factor-1 receptor reduces endothelial barrier function and promotes atherosclerosis in Apoe-deficient mice. Am. J. Physiol. Circ. Physiol. 2020, 319, H730–H743. [Google Scholar] [CrossRef]
- Vecerova, L.; Strasky, Z.; Rathouska, J.; Slanarova, M.; Brcakova, E.; Micuda, S.; Nachtigal, P. Activation of TGF-β receptors and smad proteins by atorvastatin is related to reduced atherogenesis in ApoE/LDLR double knockout mice. J. Atheroscler. Thromb. 2012, 19, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Hu, L.; Zhu, X.; Wang, Y.; Li, Q.; Ma, J.; Li, H. MALAT1 overexpression attenuates AS by inhibiting ox-LDL-stimulated dendritic cell maturation via miR-155-5p/NFIA axis. Cell Cycle 2020, 19, 2472–2485. [Google Scholar] [CrossRef] [PubMed]
- Brauner, S.; Jiang, X.; Thorlacius, G.E.; Lundberg, A.M.; Östberg, T.; Yan, Z.-Q.; Kuchroo, V.K.; Hansson, G.K.; Wahren-Herlenius, M. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc. Res. 2018, 114, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Bao, Q.; Yan, M.; Liang, J.; Zhu, Y.; Wang, C.; Ai, D. The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. Br. J. Pharmacol. 2017, 175, 1354–1361. [Google Scholar] [CrossRef]
- Menghini, R.; Casagrande, V.; Cardellini, M.; Ballanti, M.; Davato, F.; Cardolini, I.; Stoehr, R.; Fabrizi, M.; Morelli, M.; Anemona, L.; et al. FoxO1 regulates asymmetric dimethylarginine via downregulation of dimethylaminohydrolase 1 in human endothelial cells and subjects with atherosclerosis. Atherosclerosis 2015, 242, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, L.-M.; Hu, B.; Wang, J.-L.; Lu, Y.-B.; Zhang, R.-Y.; He, X.; Shi, C.; Wu, L.-M.; Wu, C.-M.; et al. TM4SF19 aggravates LPS-induced attenuation of vascular endothelial cell adherens junctions by suppressing VE-cadherin expression. Biochem. Biophys. Res. Commun. 2020, 533, 1204–1211. [Google Scholar] [CrossRef]
- Badimon, L.; Borrell, M. Wnt signaling in the vessel wall. Curr. Opin. Hematol. 2017, 24, 230–239. [Google Scholar] [CrossRef]
- Mandraffino, G.; Gullo, A.L.; Cinquegrani, M.; D’Ascola, A.; Sinicropi, D.; Imbalzano, E.; Blando, G.; Campo, G.; Morace, C.; Giuffrida, C.; et al. Expression and change of miRs 145, 221 and 222 in hypertensive subjects treated with enalapril, losartan or olmesartan. Biomedicines 2021, 9, 860. [Google Scholar] [CrossRef]
| Variables | Plaques–No | Plaques–Yes | p-Value |
|---|---|---|---|
| n = 72 (40%) | n = 105 (59%) | ||
| Clinical | |||
| Age, years | 55.0 ± 11.2 | 64.3 ± 8.6 | <0.001 |
| Male sex, % | 42 | 45 | 0.80 |
| Systolic blood pressure, mmHg | 150.7 ± 25.7 | 152.4 ± 25.6 | 0.67 |
| Diastolic blood pressure, mmHg | 88.1 ± 16.4 | 81.5 ± 14.9 | 0.007 |
| Pulse pressure, mmHg | 62.7 ± 17.3 | 70.7 ± 20.3 | 0.006 |
| Body mass index, kg/m2 | 30.3 ± 6.1 | 29.9 ± 5.4 | 0.68 |
| Diabetes mellitus, % | 48 | 57 | 0.43 |
| Current smoking, % | 7 | 7 | 0.96 |
| Coronary artery disease, % | 19 | 27 | 0.79 |
| Previous stroke, % | 12 | 19 | 0.74 |
| Diuretics, % | 71 | 69 | 0.87 |
| ACEI or ARB, % | 85 | 80 | 0.55 |
| Beta-blocker, % | 58 | 63 | 0.65 |
| Calcium-channel blocker, % | 64 | 59 | 0.62 |
| Statin, % | 65 | 74 | 0.26 |
| Glucose, mg/dL | 99 [87, 108] | 103 [92, 133] | 0.07 |
| Triglycerides, mg/dL | 124 [90, 173] | 154 [103, 204] | 0.26 |
| HDL-C, mg/dL | 46.4 ± 13.4 | 44.1 ± 12.5 | 0.25 |
| LDL-C, mg/dL | 96.9 ± 28.5 | 90.4 ± 35.5 | 0.19 |
| Creatinine, mg/dL | 0.9 [0.7, 1.1] | 1.1 [0.8, 1.3] | 0.039 |
| cIMT, mm | 0.669 ± 0.103 | 0.799 ± 0.138 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minin, E.O.Z.; Paim, L.R.; Lopes, E.C.P.; Bueno, L.C.M.; Carvalho-Romano, L.F.R.S.; Marques, E.R.; Vegian, C.F.L.; Pio-Magalhães, J.A.; Coelho-Filho, O.R.; Sposito, A.C.; et al. Association of Circulating miR-145-5p and miR-let7c and Atherosclerotic Plaques in Hypertensive Patients. Biomolecules 2021, 11, 1840. https://doi.org/10.3390/biom11121840
Minin EOZ, Paim LR, Lopes ECP, Bueno LCM, Carvalho-Romano LFRS, Marques ER, Vegian CFL, Pio-Magalhães JA, Coelho-Filho OR, Sposito AC, et al. Association of Circulating miR-145-5p and miR-let7c and Atherosclerotic Plaques in Hypertensive Patients. Biomolecules. 2021; 11(12):1840. https://doi.org/10.3390/biom11121840
Chicago/Turabian StyleMinin, Eduarda O. Z., Layde R. Paim, Elisangela C. P. Lopes, Larissa C. M. Bueno, Luís F. R. S. Carvalho-Romano, Edmilson R. Marques, Camila F. L. Vegian, José A. Pio-Magalhães, Otavio R. Coelho-Filho, Andrei C. Sposito, and et al. 2021. "Association of Circulating miR-145-5p and miR-let7c and Atherosclerotic Plaques in Hypertensive Patients" Biomolecules 11, no. 12: 1840. https://doi.org/10.3390/biom11121840
APA StyleMinin, E. O. Z., Paim, L. R., Lopes, E. C. P., Bueno, L. C. M., Carvalho-Romano, L. F. R. S., Marques, E. R., Vegian, C. F. L., Pio-Magalhães, J. A., Coelho-Filho, O. R., Sposito, A. C., Matos-Souza, J. R., Nadruz, W., & Schreiber, R. (2021). Association of Circulating miR-145-5p and miR-let7c and Atherosclerotic Plaques in Hypertensive Patients. Biomolecules, 11(12), 1840. https://doi.org/10.3390/biom11121840






