Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence
Abstract
:1. Brief History of Metformin and Its Interaction with Cardiovascular Outcomes
2. Heart Failure Epidemiology in Diabetes
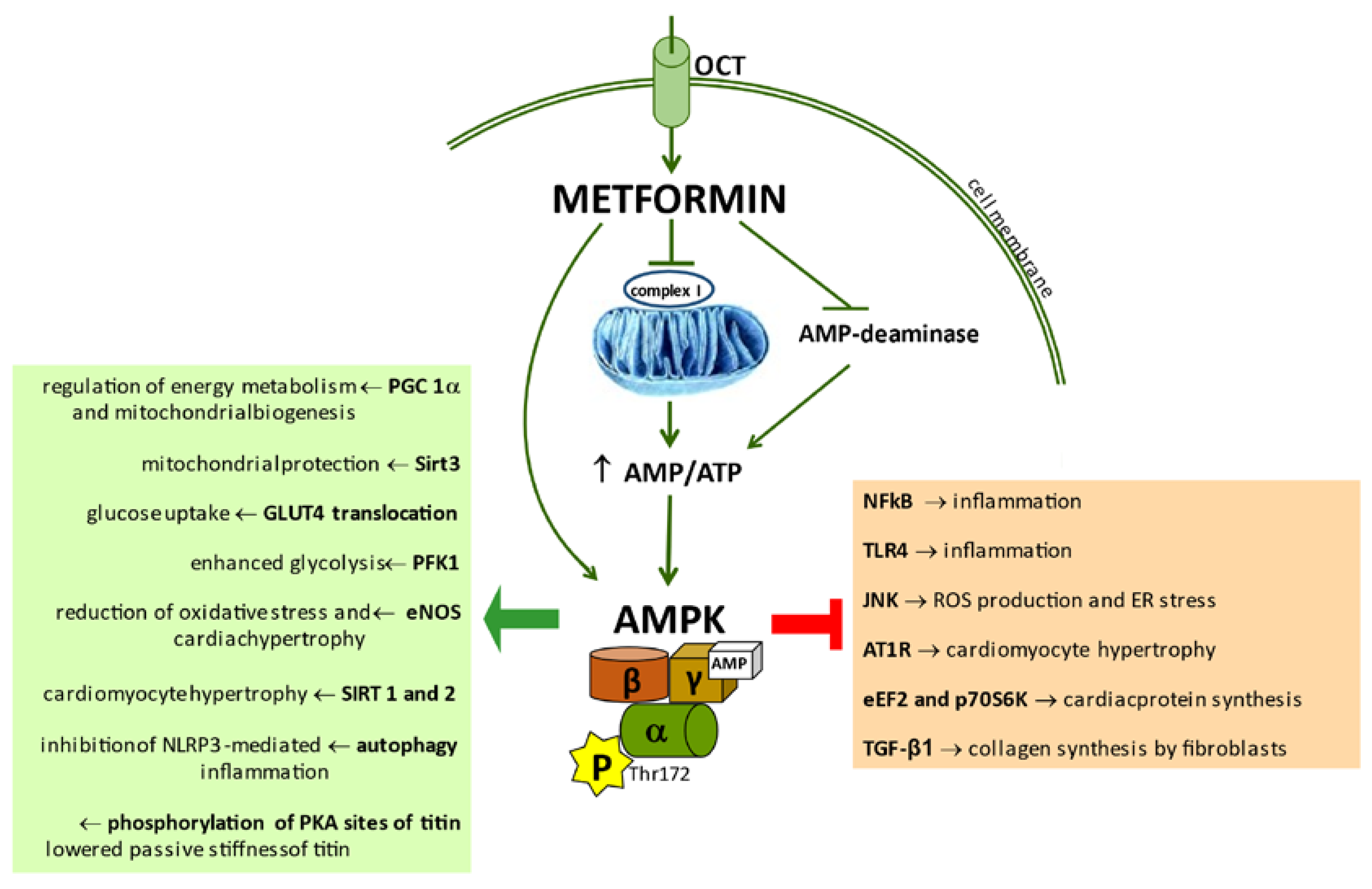
3. Activation of AMPK by Metformin
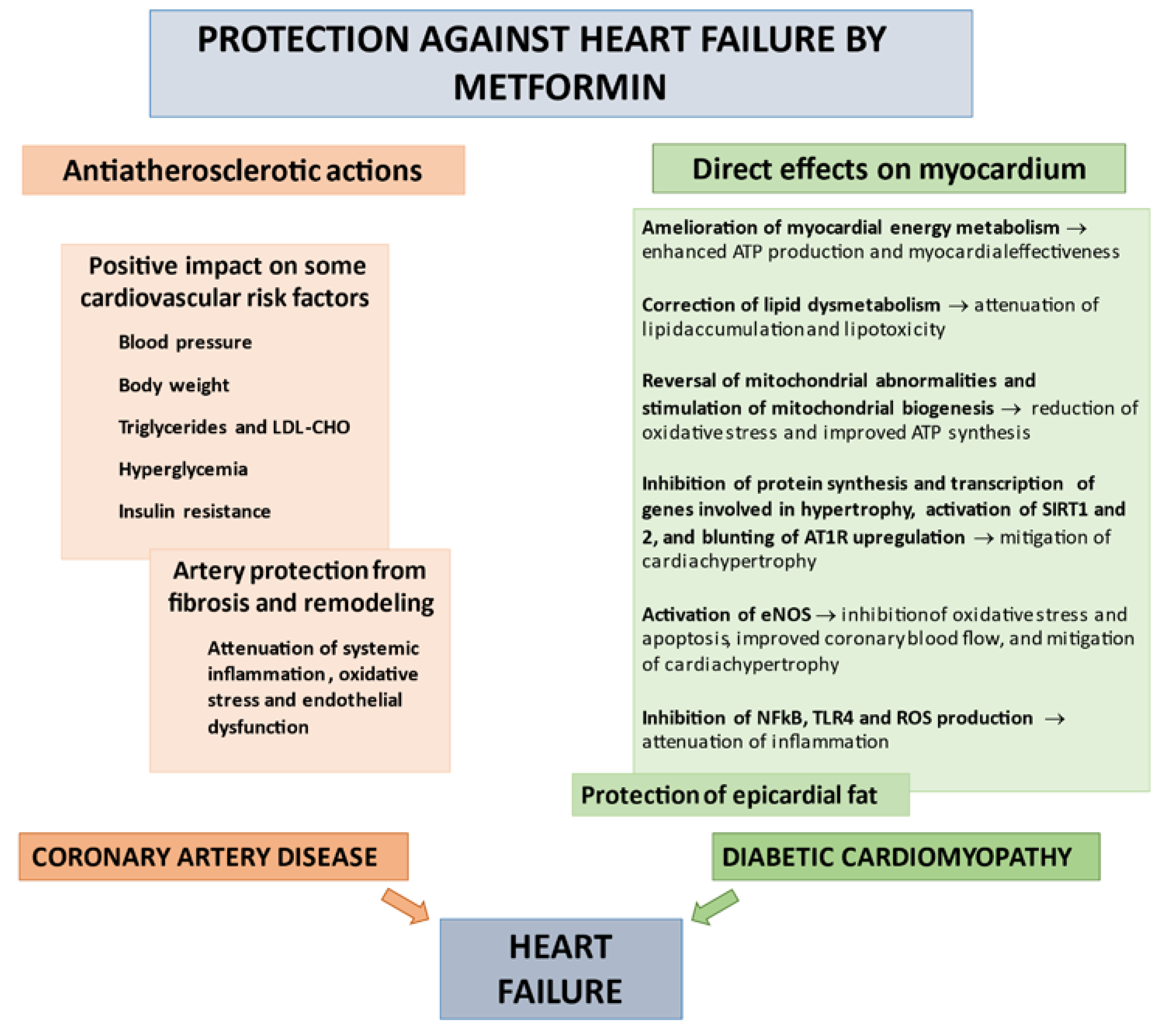
4. Mechanisms of Beneficial Impact of Metformin on Heart Failure
4.1. Correction of CV Risk Factors
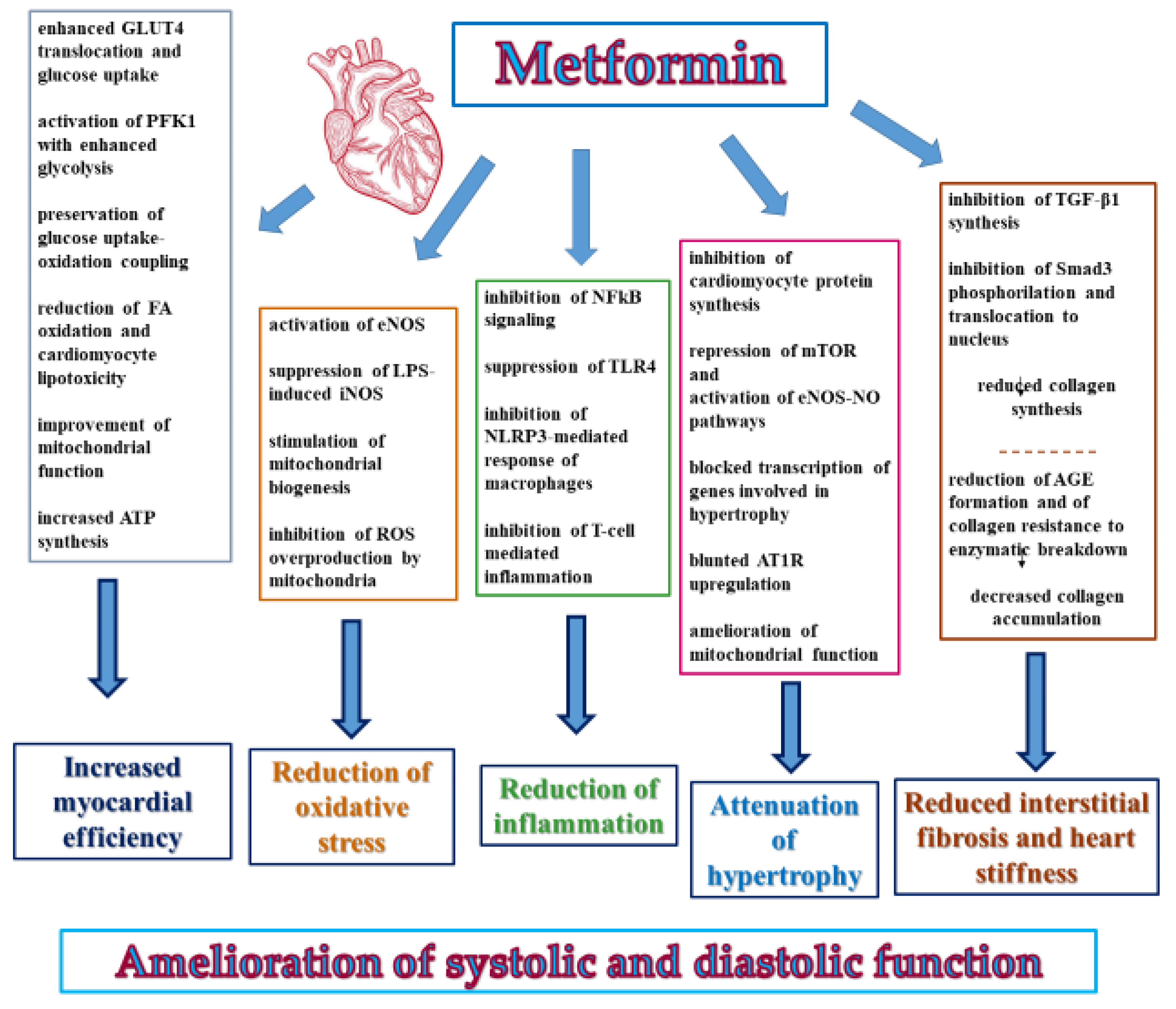
4.2. Direct Beneficial Actions of Metformin on Myocardium
4.2.1. Effects on Myocardial Energy Metabolism and Efficiency
4.2.2. Myocardial Anti-Oxidative and Anti-Inflammatory Effects
4.2.3. Prevention of Remodeling
4.2.4. Amelioration of Left Ventricle Function
5. Effects of Metformin on Adverse Consequences of HF in Clinical Studies
5.1. Effects on Total and HF Mortality
5.2. Risk of Admission for HF
5.3. Risk of New-Onset HF
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Salvatore, T.; Pafundi, P.C.; Marfella, R.; Sardu, C.; Rinaldi, L.; Monaco, L.; Ricozzi, C.; Imbriani, S.; Nevola, R.; Adinolfi, L.E.; et al. Metformin lactic acidosis: Should we still be afraid? Diabetes Res. Clin. Pract. 2019, 157, 107879. [Google Scholar] [CrossRef] [PubMed]
- Witters, L.A. The blooming of the French lilac. J. Clin. Investig. 2001, 108, 1105–1107. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Fonarow, G.C.; Greene, S.J.; DeVore, A.D.; Kavati, A.; Sikirica, S.; Albert, N.M.; Duffy, C.I.; Hill, C.L.; Patterson, J.H.; et al. Contemporary treatment patterns and clinical outcomes of comorbid diabetes mellitus and HFrEF: The CHAMP-HF Registry. JACC Heart Fail. 2020, 8, 469–480. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865, Erratum in 1998, 352, 1558. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [Green Version]
- Scheen, A.J.; Paquot, N. Metformin revisited: A critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab. 2013, 39, 179–190. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can metformin exert as an active drug on endothelial dysfunction in diabetic subjects? Biomedicines 2020, 9, 3. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Heine, R.J.; Holman, R.R.; Sherwin, R.; Zinman, B.; Professional Practice Committee, American Diabetes Association; European Association for the Study of Diabetes. Management of hyperglycaemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Asso-ciation and the European Association for the Study of Diabetes. Diabetologia 2006, 49, 1711–1721, Erratum in 2006, 49, 2816–2818. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S111–S124. [Google Scholar] [CrossRef] [PubMed]
- Misbin, R.I. The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes Care 2004, 27, 1791–1793. [Google Scholar] [CrossRef] [Green Version]
- Holstein, A.; Nahrwold, D.; Hinze, S.; Egberts, E.H. Contra-indications to metformin therapy are largely disregarded. Diabet. Med. 1999, 16, 692–696. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: Systematic review and meta-analysis. Arch. Intern. Med. 2003, 163, 2594–2602. [Google Scholar] [CrossRef] [Green Version]
- Masoudi, F.A.; Inzucchi, S.E.; Wang, Y.; Havranek, E.P.; Foody, J.M.; Krumholz, H.M. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: An observational study. Circulation 2005, 111, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eurich, D.T.; Majumdar, S.R.; McAlister, F.A.; Tsuyuki, R.T.; Johnson, J.A. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care 2005, 28, 2345–2351. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, S.L.; Rø rth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Kø ber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and me-ta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Kluger, A.Y.; Tecson, K.M.; Lee, A.Y.; Lerma, E.V.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc. Diabetol. 2019, 18, 99. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; ESC Scientific Document Group; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Neuen, B.L.; Arnott, C.; Perkovic, V.; Figtree, G.; de Zeeuw, D.; Fulcher, G.; Jun, M.; Jardine, M.J.; Zoungas, S.; Pollock, C.; et al. Sodium-glucose co-transporter-2 inhibitors with and without metformin: A meta-analysis of cardi-ovascular, kidney and mortality outcomes. Diabetes Obes. Metab. 2021, 23, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Tsapas, A.; Karagiannis, T.; Avgerinos, I.; Liakos, A.; Bekiari, E. GLP-1 receptor agonists for cardiovascular outcomes with and without metformin. A systematic review and meta-analysis of cardiovascular outcomes trials. Diabetes Res. Clin. Pract. 2021, 177, 108921. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Fitchett, D.; Jurišić-Eržen, D.; Woo, V.; Hantel, S.; Janista, C.; Kaspers, S.; George, J.T.; Zinman, B. EMPA-REG OUTCOME® Investigators: Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose-lowering therapy? Diabetes Obes. Metab. 2020, 22, 631–639. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Packer, M. Does Metformin Interfere With the Cardiovascular Benefits of SGLT2 Inhibitors? Questions about its role as the cornerstone of diabetes treatment. Am. J. Med. 2020, 133, 781–782. [Google Scholar] [CrossRef] [Green Version]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; NID-2 Study Group Investigators; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef]
- Sasso, F.C.; Salvatore, T.; Tranchino, G.; Cozzolino, D.; Caruso, A.A.; Persico, M.; Gentile, S.; Torella, D.; Torella, R. Cochlear dysfunction in type 2 diabetes: A complication independent of neuropathy and acute hyperglycemia. Metabolism 1999, 48, 1346–1350. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Aglitti, A.; Bucci, T.; Caruso, R.; Salvatore, T.; Sasso, F.C.; Tripodi, M.F.; Persico, M. Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS ONE 2017, 12, e0178473. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. Liver fibrosis by FibroScan® independently of established cardiovascular risk pa-rameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020, 40, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Nichols, G.A.; Hillier, T.A.; Erbey, J.R.; Brown, J.B. Congestive heart failure in type 2 diabetes: Prevalence, incidence, and risk factors. Diabetes Care 2001, 24, 1614–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parving, H.H.; Brenner, B.M.; McMurray, J.J.; de Zeeuw, D.; Haffner, S.M.; Solomon, S.D.; Chaturvedi, N.; Persson, F.; Desai, A.S.; ALTITUDE Investigators; et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 2012, 367, 2204–2213. [Google Scholar] [CrossRef] [Green Version]
- Bertoni, A.G.; Hundley, W.G.; Massing, M.W.; Bonds, D.E.; Burke, G.L.; Goff, D.C., Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004, 27, 699–703. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. 2021, 8, 695792. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.H.; Abraham, W.T.; Albert, N.M.; Chiswell, K.; Clare, R.; Stough, W.G.; Gheorghiade, M.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: A report from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZEHF). Am. Heart J. 2007, 154, 277.e1–277.e8. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Mentz, R.J.; Kwasny, M.J.; Fought, A.J.; Huffman, M.; Subacius, H.; Nodari, S.; Konstam, M.; Swedberg, K.; EVEREST Investigators; et al. Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: Findings from the EVEREST trial. Eur. J. Heart Fail. 2013, 15, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, S.L.; Preiss, D.; Jhund, P.S.; Squire, I.; Cardoso, J.S.; Merkely, B.; Martinez, F.; Starling, R.C.; Desai, A.S.; PARADIGM-HF Investigators and committees; et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: Insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ. Heart Fail. 2016, 9, e002560. [Google Scholar] [CrossRef]
- Zareini, B.; Rørth, R.; Holt, A.; Mogensen, U.M.; Selmer, C.; Gislason, G.; Schou, M.; Køber, L.; Torp-Pedersen, C.; Lamberts, M.; et al. Heart failure and the prognostic impact and incidence of new-onset of diabetes mellitus: A nationwide cohort study. Cardiovasc. Diabetol. 2019, 18, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, S.D.; Rizkala, A.R.; Lefkowitz, M.P.; Shi, V.C.; Gong, J.; Anavekar, N.; Anker, S.D.; Arango, J.L.; Arenas, J.L.; Atar, D.; et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ. Heart Fail. 2018, 11, e004962. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, S.L.; Mogensen, U.M.; Jhund, P.S.; Petrie, M.C.; Preiss, D.; Win, S.; Køber, L.; McKelvie, R.S.; Zile, M.R.; Anand, I.S.; et al. Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: A report from the I-Preserve trial (irbesartan in heart failure with preserved ejection fraction). Circulation 2017, 135, 724–735. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, M.R.; Petrie, M.C.; Varyani, F.; Ostergren, J.; Michelson, E.L.; Young, J.B.; Solomon, S.D.; Granger, C.B.; Swedberg, K.; Yusuf, S.; et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 1377–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swan, J.W.; Anker, S.D.; Walton, C.; Godsland, I.F.; Clark, A.L.; Leyva, F.; Stevenson, J.C.; Coats, A.J. Insulin resistance in chronic heart failure: Relation to severity and etiology of heart failure. J. Am. Coll. Cardiol. 1997, 30, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, I.; Brendorp, B.; Seibaek, M.; Burchardt, H.; Hildebrandt, P.; Køber, L.; Torp-Pedersen, C.; Danish Investigatord of Arrhythmia and Mortality on Dofetilde Study Group. Influence of diabetes and diabetes-gender interaction on the risk of death in patients hospitalized with congestive heart failure. J. Am. Coll. Cardiol. 2004, 43, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Doehner, W.; Rauchhaus, M.; Ponikowski, P.; Godsland, I.F.; von Haehling, S.; Okonko, D.O.; Leyva, F.; Proudler, A.J.; Coats, A.J.; Anker, S.D. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J. Am. Coll. Cardiol. 2005, 46, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Dauriz, M.; Laroche, C.; Temporelli, P.L.; Hassanein, M.; Seferovic, P.M.; Drozdz, J.; Ferrari, R.; Anker, S.; Coats, A.; et al. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: Results from the ESC-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Cavender, M.A.; Steg, P.G.; Smith, S.C., Jr.; Eagle, K.; Ohman, E.M.; Goto, S.; Kuder, J.; Im, K.; Wilson, P.W.; Bhatt, D.L.; et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: Outcomes at 4 years from the reduction of atherothrombosis for continued health (REACH) registry. Circulation 2015, 132, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.G.; Kirk, J.K.; Goff, D.C., Jr.; Wagenknecht, L.E. Excess mortality related to diabetes mellitus in elderly Medicare benefi-ciaries. Ann. Epidemiol. 2004, 14, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. The AMP-activated protein kinase pathway—New players upstream and downstream. J. Cell Sci. 2004, 117, 5479–5487. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G.; Carling, D. The AMP-activated protein kinase—Fuel gauge of the mammalian cell? Eur. J. Biochem. 1997, 246, 259–273. [Google Scholar] [CrossRef]
- Woods, A.; Vertommen, D.; Neumann, D.; Turk, R.; Bayliss, J.; Schlattner, U.; Wallimann, T.; Carling, D.; Rider, M.H. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J. Biol. Chem. 2003, 278, 28434–28442. [Google Scholar] [CrossRef] [Green Version]
- Timm, K.N.; Tyler, D.J. The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc. Drugs Ther. 2020, 34, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Cantó, C.; Auwerx, J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Parakhia, R.A.; Ochs, R.S. Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol Chem. 2011, 286, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Xu, Z.; Zhang, C.; Cai, Z.; Zhang, J. Metformin, beyond an insulin sensitizer, targeting heart and pancreatic β cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1984–1990. [Google Scholar] [CrossRef]
- Petrie, J.R.; Chaturvedi, N.; Ford, I.; Brouwers, M.C.G.J.; Greenlaw, N.; Tillin, T.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; Klein, B.E.K.; et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Petersen, J.S.; Andersen, D.; Muntzel, M.S.; Diemer, N.H.; Holstein-Rathlou, N.H. Intracerebroventricular metformin attenuates salt-induced hypertension in spontaneously hypertensive rats. Am. J. Hypertens. 2001, 14, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Liu, H.; Wen, X.; Peng, Y.; Tian, Y.; Zhao, L. Effects of metformin on blood pressure in nondiabetic patients: A meta-analysis of randomized controlled trials. J. Hypertens. 2017, 35, 18–26. [Google Scholar] [CrossRef]
- Mäkimattila, S.; Nikkilä, K.; Yki-Järvinen, H. Causes of weight gain during insulin therapy with and without metformin in patients with Type II diabetes mellitus. Diabetologia 1999, 42, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Anabtawi, A.; Miles, J.M. Metformin: Nonglycemic effects and potential novel indications. Endocr Pract. 2016, 22, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Wulffelé, M.G.; Kooy, A.; de Zeeuw, D.; Stehouwer, C.D.; Gansevoort, R.T. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: A systematic review. J. Intern. Med. 2004, 256, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Sasso, F.C.; Cacciapuoti, F.; Portoghese, M.; Rizzo, M.R.; Siniscalchi, M.; Carbonara, O.; Ferraraccio, F.; Torella, M.; Petrella, A.; et al. Tight glycemic control may increase regenerative potential of myocardium during acute infarction. J. Clin. Endocrinol. Metab. 2012, 97, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Sasso, F.C.; Rinaldi, L.; Lascar, N.; Marrone, A.; Pafundi, P.C.; Adinolfi, L.E.; Marfella, R. Role of tight glycemic control during acute coronary syndrome on cv outcome in type 2 diabetes. J. Diabetes Res. 2018, 2018, 3106056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caturano, A.; Galiero, R.; Pafundi, P.C.; Cesaro, A.; Vetrano, E.; Palmiero, G.; Rinaldi, L.; Salvatore, T.; Marfella, R.; Sardu, C.; et al. Does a strict glycemic control during acute coronary syndrome play a cardioprotective effect? Pathophysiology and clinical evidence. Diabetes Res. Clin. Pract. 2021, 178, 108959. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of compli-cations in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853, Erratum in 1999, 354, 602. [Google Scholar] [CrossRef]
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.J.; Bahn, G.D.; Moritz, T.E.; Kaufman, D.; Abraira, C.; Duckworth, W.; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care 2011, 34, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Zilov, A.V.; Abdelaziz, S.I.; AlShammary, A.; Al Zahrani, A.; Amir, A.; Assaad Khalil, S.H.; Brand, K.; Elkafrawy, N.; Hassoun, A.A.K.; Jahed, A.; et al. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab. Res. Rev. 2019, 35, e3173. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Das, A.; Chen, J.; Wu, P.; Li, X.; Fang, Z. Metformin in patients with and without diabetes: A paradigm shift in cardiovascular disease management. Cardiovasc. Diabetol. 2019, 18, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, F.; Zhang, J.; Li, Z.; Zhang, J.; Yin, Y.; Wang, Y.; Marin, T.L.; Gongol, B.; Xiao, H.; Zhang, Y.Y.; et al. Cardiovascular protective effect of metformin and telmisartan: Reduction of parp1 activity via the ampk-parp1 cascade. PLoS ONE 2016, 11, e0151845. [Google Scholar] [CrossRef] [Green Version]
- Torella, D.; Iaconetti, C.; Tarallo, R.; Marino, F.; Giurato, G.; Veneziano, C.; Aquila, I.; Scalise, M.; Mancuso, T.; Cianflone, E.; et al. Mirna regulation of the hyperproliferative phenotype of vascular smooth muscle cells in diabetes. Diabetes 2018, 67, 2554–2568. [Google Scholar] [CrossRef] [Green Version]
- Lozano, E.; Briz, O.; Macias, R.I.R.; Serrano, M.A.; Marin, J.J.G.; Herraez, E. Genetic heterogeneity of slc22 family of transporters in drug disposition. J. Pers. Med. 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.T.; Dyck, J.R. Is AMPK the savior of the failing heart? Trends Endocrinol. Metab. 2015, 26, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Lu, Q.; Ren, D.; Sun, X.; Rousselle, T.; Tan, Y.; Li, J. AMPK: A therapeutic target of heart failure-not only metabolism regulation. Biosci. Rep. 2019, 39, BSR20181767. [Google Scholar] [CrossRef]
- Varjabedian, L.; Bourji, M.; Pourafkari, L.; Nader, N.D. Cardioprotection by metformin: Beneficial effects beyond glucose reduction. Am. J. Cardiovasc. Drugs 2018, 18, 181–193. [Google Scholar] [CrossRef]
- Gundewar, S.; Calvert, J.W.; Jha, S.; Toedt-Pingel, I.; Ji, S.Y.; Nunez, D.; Ramachandran, A.; Anaya-Cisneros, M.; Tian, R.; Lefer, D.J. Acti-vation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ. Res. 2009, 104, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Pascual, F.; Coleman, R.A. Fuel availability and fate in cardiac metabolism: A tale of two substrates. Biochim. Biophys. Acta 2016, 1861, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Taegtmeyer, H.; Young, M.E.; Lopaschuk, G.D.; Abel, E.D.; Brunengraber, H.; Darley-Usmar, V.; Des Rosiers, C.; Gerszten, R.; Glatz, J.F.; Griffin, J.L.; et al. American heart association council on basic cardiovascular sciences. assessing cardiac metabolism: A scientific statement from the american heart association. Circ. Res. 2016, 118, 1659–1701. [Google Scholar] [CrossRef]
- Neubauer, S. The failing heart—An engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Yang, F. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem. Biophys. Res. Commun. 2017, 486, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Dziubak, A.; Wójcicka, G.; Wojtak, A.; Bełtowski, J. Metabolic effects of metformin in the failing heart. Int. J. Mol. Sci. 2018, 19, 2869. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, L.; Ginion, A.; Beauloye, C.; Hebert, A.D.; Guigas, B.; Hue, L.; Vanoverschelde, J.L. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H239–H250. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Chen, Y.; Xie, Y.; Li, Y.; Li, Z. Metformin protects against insulin resistance induced by high uric acid in cardiomyocytes via AMPK signalling pathways in vitro and in vivo. J. Cell Mol. Med. 2021, 25, 6733–6745. [Google Scholar] [CrossRef]
- Nascimben, L.; Ingwall, J.S.; Lorell, B.H.; Pinz, I.; Schultz, V.; Tornheim, K.; Tian, R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension 2004, 44, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; Kundu, B.K.; Wu, H.C.; Hashmi, S.S.; Guthrie, P.; Locke, L.W.; Roy, R.J.; Matherne, G.P.; Berr, S.S.; Terwelp, M.; et al. Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J. Am. Heart Assoc. 2013, 2, e004796. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, I.J.; Trent, C.M.; Schulze, P.C. Lipid metabolism and toxicity in the heart. Cell Metab. 2012, 15, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Miklankova, D.; Markova, I.; Hüttl, M.; Zapletalova, I.; Poruba, M.; Malinska, H. Metformin affects cardiac arachidonic acid metabolism and cardiac lipid metabolite storage in a prediabetic rat model. Int. J. Mol. Sci. 2021, 22, 7680. [Google Scholar] [CrossRef]
- Marfella, R.; Amarelli, C.; Cacciatore, F.; Balestrieri, M.L.; Mansueto, G.; D’Onofrio, N.; Esposito, S.; Mattucci, I.; Salerno, G.; De Feo, M.; et al. Lipid accumulation in hearts transplanted from nondiabetic donors to diabetic recipients. J. Am. Coll. Cardiol. 2020, 75, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Schaper, J.; Froede, R.; Hein, S.; Buck, A.; Hashizume, H.; Speiser, B.; Friedl, A.; Bleese, N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation 1991, 83, 504–514. [Google Scholar] [CrossRef] [Green Version]
- Sabbah, H.N.; Sharov, V.; Riddle, J.M.; Kono, T.; Lesch, M.; Goldstein, S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J. Mol. Cell Cardiol. 1992, 24, 1333–1347. [Google Scholar] [CrossRef]
- Psotka, M.A.; Gottlieb, S.S.; Francis, G.S.; Allen, L.A.; Teerlink, J.R.; Adams, K.F., Jr.; Rosano, G.M.C.; Lancellotti, P. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 73, 2345–2353. [Google Scholar] [CrossRef]
- Larsen, A.H.; Jessen, N.; Nørrelund, H.; Tolbod, L.P.; Harms, H.J.; Feddersen, S.; Nielsen, F.; Brøsen, K.; Hansson, N.H.; Frøkiaer, J.; et al. A randomised, double-blind, placebo-controlled trial of metformin on myocardial efficiency in insulin-resistant chronic heart failure patients without diabetes. Eur. J. Heart Fail. 2020, 22, 1628–1637. [Google Scholar] [CrossRef]
- Pillai, V.B.; Bindu, S.; Sharp, W.; Fang, Y.H.; Kim, G.; Gupta, M.; Samant, S.; Gupta, M.P. Sirt3 protects mitochondrial DNA damage and blocks the development of doxorubicin-induced cardiomyopathy in mice. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H962–H972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Hazen, B.C.; Russell, A.P.; Kralli, A. Peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1)- and estro-gen-related receptor (ERR)-induced regulator in muscle 1 (Perm1) is a tissue-specific regulator of oxidative capacity in skeletal muscle cells. J. Biol. Chem. 2013, 288, 25207–25218. [Google Scholar] [CrossRef] [Green Version]
- Beanlands, R.S.; Nahmias, C.; Gordon, E.; Coates, G.; deKemp, R.; Firnau, G.; Fallen, E. The effects of beta(1)-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction: A double-blind, pla-cebo-controlled, positron-emission tomography study. Circulation 2000, 102, 2070–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Kleinert, H. Nitric oxide synthase: Expression and expressional control of the three isoforms. Naunyn Schmiedebergs Arch. Pharmacol. 1995, 352, 351–364. [Google Scholar] [CrossRef]
- Yang, B.; Larson, D.F.; Watson, R.R. Modulation of iNOS activity in age-related cardiac dysfunction. Life Sci. 2004, 75, 655–667. [Google Scholar] [CrossRef]
- Davis, B.J.; Xie, Z.; Viollet, B.; Zou, M.-H. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 2006, 55, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Lefer, A.M. Attenuation of myocardial ischemia-reperfusion injury with nitric oxide replacement therapy. Ann. Thorac. Surg. 1995, 60, 847–851. [Google Scholar] [CrossRef]
- Cittadini, A.; Napoli, R.; Monti, M.G.; Rea, D.; Longobardi, S.; Netti, P.A.; Walser, M.; Samà, M.; Aimaretti, G.; Isgaard, J.; et al. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes 2012, 61, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Wang, Q.; Sun, S.; Xu, G.; Wu, Q.; Qi, M.; Bai, F.; Yu, J. Astragaloside IV promotes the eNOS/NO/cGMP pathway and improves left ventricular diastolic function in rats with metabolic syndrome. J. Int. Med. Res. 2020, 48, 300060519826848. [Google Scholar] [CrossRef] [Green Version]
- Tsujino, M.; Hirata, Y.; Imai, T.; Kanno, K.; Eguchi, S.; Ito, H.; Marumo, F. Induction of nitric oxide synthase gene by interleukin-1 beta in cultured rat cardiocytes. Circulation 1994, 90, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.W.; Lai, E.H.; Yang, C.N.; Lin, S.K.; Hong, C.Y.; Yang, H.; Chang, J.Z.; Kok, S.H. Intracanal metformin promotes healing of apical periodontitis via suppressing inducible nitric oxide synthase expression and monocyte recruitment. J. Endod. 2020, 46, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Liu, X.D.; Li, Y.G.; Wang, G.Y.; Bi, Y.G.; Zhao, Y.; Yan, M.L.; Liu, X.; Wei, M.; Wan, L.L.; Zhang, Q.Y. Metformin protects high glucose cultured cardiomyocytes from oxidative stress by promoting NDUFA13 expression and mitochondrial biogenesis via the AMPK signaling pathway. Mol. Med. Rep. 2020, 22, 5262–5270. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, I.K.; Beis, I.; Gaitanaki, C. ERKs and JNKs mediate hydrogen peroxide-induced Egr-1 expression and nuclear accumulation in H9c2 cells. Physiol. Res. 2010, 59, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ye, P.; Liao, H.; Chen, M.; Yang, F. Metformin protects H9C2 cardiomyocytes from high-glucose and hypoxia/reoxygenation injury via inhibition of reactive oxygen species generation and inflammatory responses: Role of AMPK and JNK. J. Diabetes Res. 2016, 2016, 2961954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhao, D.; Ren, J.; Yang, J. Endoplasmic reticulum stress and protein quality control in diabetic cardiomyopathy. Biochim. Biophys. Acta 2015, 1852, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Thompson, J.; Hu, Y.; Das, A.; Lesnefsky, E.J. Metformin attenuates ER stress-induced mitochondrial dysfunction. Transl. Res. 2017, 190, 40–50. [Google Scholar] [CrossRef]
- Li, H.; Chen, C.; Wang, D.W. Inflammatory cytokines, immune cells, and organ interactions in heart failure. Front. Physiol. 2021, 12, 695047. [Google Scholar] [CrossRef]
- Bai, B.; Chen, H. Metformin: A novel weapon against inflammation. Front. Pharmacol. 2021, 12, 622262. [Google Scholar] [CrossRef]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.; Savinko, T.; Wong, A.K.; et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Gjeloshi, K.; Masini, F.; Acierno, C.; Di Martino, A.; Albanese, G.; Alfano, M.; Rinaldi, L.; et al. Metformin: A potential therapeutic tool for rheumatologists. Pharmaceuticals 2020, 13, 234. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.M.; Li, H.; Zhang, X.; Lei, F.; Qin, J.J.; Chen, Z.; Deng, K.Q.; Lin, L.; Chen, M.M.; et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020, 32, 537–547.e3. [Google Scholar] [CrossRef]
- Isoda, K.; Young, J.L.; Zirlik, A.; MacFarlane, L.A.; Tsuboi, N.; Gerdes, N.; Schönbeck, U.; Libby, P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arter. Thromb. Vasc. Biol. 2006, 26, 611–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saisho, Y. Metformin and inflammation: Its potential beyond glucose-lowering effect. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 196–205. [Google Scholar] [CrossRef]
- Soraya, H.; Farajnia, S.; Khani, S.; Rameshrad, M.; Khorrami, A.; Banani, A.; Maleki-Dizaji, N.; Garjani, A. Short-term treatment with metformin suppresses toll like receptors (TLRs) activity in isoproterenol-induced myocardial infarction in rat: Are AMPK and TLRs connected? Int. Immunopharmacol. 2012, 14, 785–791. [Google Scholar] [CrossRef]
- Soraya, H.; Clanachan, A.S.; Rameshrad, M.; Maleki-Dizaji, N.; Ghazi-Khansari, M.; Garjani, A. Chronic treatment with metformin suppresses toll-like receptor 4 signaling and attenuates left ventricular dysfunction following myocardial infarction. Eur. J. Pharmacol. 2014, 737, 77–84. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, J.; Jiang, S.; Ma, Z.; Wang, D.; Hu, W.; Deng, C.; Fan, C.; Di, S.; Sun, Y.; et al. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016, 7, e2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, Q.; Ma, H.; Zou, J.; Wang, W.; Zhu, L.; Deng, H.; Meng, M.; Tan, S.; Zhang, H.; Xiao, X.; et al. Metformin protects against ischaemic myocardial injury by alleviating autophagy-ROS-NLRP3-mediated inflammatory response in macrophages. J. Mol. Cell Cardiol. 2020, 145, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qin, Y.; Wang, Y.; Meng, S.; Xian, H.; Che, H.; Lv, J.; Li, Y.; Yu, Y.; Bai, Y.; et al. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int. J. Biol. Sci. 2019, 15, 1010–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Lau, K.; Eby, B.; Lozano, P.; He, C.; Pennington, B.; Li, H.; Rathi, S.; Dong, Y.; Tian, R.; et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 2011, 60, 1770–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, K.H.; de Jong, A.; Jukema, J.W.; de Vries, M.R.; Arens, R.; Quax, P.H.A. T cell co-stimulation and co-inhibition in cardiovascular disease: A double-edged sword. Nat. Rev. Cardiol. 2019, 16, 325–343. [Google Scholar] [CrossRef]
- Nyambuya, T.M.; Dludla, P.V.; Mxinwa, V.; Mokgalaboni, K.; Ngcobo, S.R.; Tiano, L.; Nkambule, B.B. The impact of metformin and aspirin on T-cell mediated inflammation: A systematic review of in vitro and in vivo findings. Life Sci. 2020, 255, 117854. [Google Scholar] [CrossRef]
- Li, B.; Po, S.S.; Zhang, B.; Bai, F.; Li, J.; Qin, F.; Liu, N.; Sun, C.; Xiao, Y.; Tu, T.; et al. Metformin regulates adiponectin signalling in epicardial adipose tissue and reduces atrial fibrillation vulnerability. J. Cell Mol. Med. 2020, 24, 7751–7766. [Google Scholar] [CrossRef] [PubMed]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev. Port. Cardiol. 2019, 38, 419–423. [Google Scholar] [CrossRef]
- Packer, M. Drugs that ameliorate epicardial adipose tissue inflammation may have discordant effects in heart failure with a preserved ejection fraction as compared with a reduced ejection fraction. J. Card. Fail. 2019, 25, 986–1003. [Google Scholar] [CrossRef]
- Sasaki, H.; Asanuma, H.; Fujita, M.; Takahama, H.; Wakeno, M.; Ito, S.; Ogai, A.; Asakura, M.; Kim, J.; Minamino, T.; et al. Metformin prevents progression of heart failure in dogs: Role of AMP-activated protein kinase. Circulation 2009, 119, 2568–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasumarthi, K.B.; Field, L.J. Cardiomyocyte cell cycle regulation. Circ. Res. 2002, 90, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, P.; Li, D.; Li, Z.; Liao, Y.; Wang, Y.; Zhou, B.; Wang, L. Single-cell reconstruction of progression trajectory reveals inter-vention principles in pathological cardiac hypertrophy. Circulation 2020, 141, 1704–1719. [Google Scholar] [CrossRef]
- Ritterhoff, J.; Young, S.; Villet, O.; Shao, D.; Neto, F.C.; Bettcher, L.F.; Hsu, Y.A.; Kolwicz, S.C., Jr.; Raftery, D.; Tian, R. Metabolic remodeling promotes cardiac hypertrophy by directing glucose to aspartate biosynthesis. Circ. Res. 2020, 126, 182–196. [Google Scholar] [CrossRef]
- Zarrinpashneh, E.; Beauloye, C.; Ginion, A.; Pouleur, A.C.; Havaux, X.; Hue, L.; Viollet, B.; Vanoverschelde, J.L.; Bertrand, L. AMPKalpha2 counteracts the development of cardiac hypertrophy induced by isoproterenol. Biochem. Biophys. Res. Commun. 2008, 376, 677–681. [Google Scholar] [CrossRef]
- Blair, E.; Redwood, C.; Ashrafian, H.; Oliveira, M.; Broxholme, J.; Kerr, B.; Salmon, A.; Ostman-Smith, I.; Watkins, H. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: Evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 2001, 10, 1215–1220. [Google Scholar] [CrossRef]
- Bolster, D.R.; Crozier, S.J.; Kimball, S.R.; Jefferson, L.S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J. Biol. Chem. 2002, 277, 23977–23980. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.Y.; Soltys, C.L.; Young, M.E.; Proud, C.G.; Dyck, J.R. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 2004, 279, 32771–32779. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.X.; Pan, S.N.; Meng, R.S.; Peng, C.Q.; Xiong, Z.J.; Chen, B.L.; Chen, G.Q.; Yao, F.J.; Chen, Y.L.; Ma, Y.D.; et al. Metformin attenuates ventricular hypertrophy by activating the AMP-activated protein kinase-endothelial nitric oxide synthase pathway in rats. Clin. Exp. Pharmacol. Physiol. 2011, 38, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.S.; Barreto-Torres, G.; Kuznetsov, A.V.; Khuchua, Z.; Javadov, S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: The role of mitochondria. J. Cell Mol. Med. 2014, 18, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, X.F.; Wang, N.Y.; Wang, X.M.; Liang, S.T.; Zheng, W.; Lu, Y.B.; Zhao, X.; Hao, D.L.; Zhang, Z.Q.; et al. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: Implications for understanding the effects of current and future treatments for heart failure. Eur. Heart J. 2020, 41, 3856–3861. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Kong, L.; Shi, H.; Tian, X.; Gao, L.; Liu, Y.; Wu, L.; Du, B.; Huang, Z.; et al. Aldolase promotes the development of cardiac hypertrophy by targeting AMPK signaling. Exp. Cell Res. 2018, 370, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, Z.; Fassett, J.; Zhang, P.; Hu, X.; Liu, X.; Kwak, D.; Li, J.; Zhu, G.; Tao, Y.; et al. Metformin protects against systolic overload-induced heart failure independent of AMP-activated protein kinase α2. Hypertension 2014, 63, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunagawa, Y.; Shimizu, K.; Katayama, A.; Funamoto, M.; Shimizu, K.; Nurmila, S.; Shimizu, S.; Miyazaki, Y.; Katanasaka, Y.; Hasegawa, K.; et al. Metformin suppresses phenylephrine-induced hypertrophic responses by inhibiting p300-HAT activity in cardiomyocytes. J. Pharmacol. Sci. 2021, 147, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Yanazume, T.; Morimoto, T.; Wada, H.; Kawamura, T.; Hasegawa, K. Biological role of p300 in cardiac myocytes. Mol. Cell Biochem. 2003, 248, 115e119. [Google Scholar] [CrossRef]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Ma, X.; Feng, W.; Fu, Y.; Lu, Z.; Xu, M.; Shen, Q.; Zhu, Y.; Zhang, Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc. Res. 2010, 87, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.F.; Zhang, J.Y.; Li, L.; Zhao, X.Y. Beneficial effects of metformin on primary cardiomyocytes via activation of adenosine monophosphate-activated protein kinase. Chin. Med. J. 2011, 124, 1876–1884. [Google Scholar]
- Noppe, G.; Dufeys, C.; Buchlin, P.; Marquet, N.; Castanares-Zapatero, D.; Balteau, M.; Hermida, N.; Bouzin, C.; Esfahani, H.; Viollet, B.; et al. Reduced scar maturation and contractility lead to ex-aggerated left ventricular dilation after myocardial infarction in mice lacking AMPKα1. J. Mol. Cell Cardiol. 2014, 74, 32–43. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, N.; Hua, Y.; Wang, B.; Ling, L.; Ferro, A.; Xu, B. Metformin inhibits angiotensin II-induced differentiation of cardiac fibroblasts into myofibroblasts. PLoS ONE 2013, 8, e72120. [Google Scholar] [CrossRef] [Green Version]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyothirmayi, G.N.; Soni, B.J.; Masurekar, M.; Lyons, M.; Regan, T.J. Effects of metformin on collagen glycation and diastolic dys-function in diabetic myocardium. J. Cardiovasc. Pharmacol. Ther. 1998, 3, 319–326. [Google Scholar] [CrossRef]
- Kuethe, F.; Sigusch, H.H.; Bornstein, S.R.; Hilbig, K.; Kamvissi, V.; Figulla, H.R. Apoptosis in patients with dilated cardiomyopathy and diabetes: A feature of diabetic cardiomyopathy? Horm. Metab. Res. 2007, 39, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Elmadhun, N.Y.; Sabe, A.A.; Lassaletta, A.D.; Chu, L.M.; Sellke, F.W. Metformin mitigates apoptosis in ischemic myocardium. J. Surg. Res. 2014, 192, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; McNeill, J.H. Metformin improves cardiac function in isolated streptozotocin-diabetic rat hearts. Am. J. Physiol. 1994, 266, H714–H719. [Google Scholar] [CrossRef]
- Benes, J.; Kazdova, L.; Drahota, Z.; Houstek, J.; Medrikova, D.; Kopecky, J.; Kovarova, N.; Vrbacky, M.; Sedmera, D.; Strnad, H.; et al. Effect of metformin therapy on cardiac function and survival in a volume-overload model of heart failure in rats. Clin. Sci. 2011, 121, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.F.; Zhang, J.Y.; Li, L.; Zhao, X.Y.; Tao, H.L.; Zhang, L. Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin. Exp. Pharmacol. Physiol. 2011, 38, 94–101. [Google Scholar] [CrossRef]
- Yin, M.; van der Horst, I.C.; van Melle, J.P.; Qian, C.; van Gilst, W.H.; Silljé, H.H.; de Boer, R.A. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H459–H468. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.C.; Tabima, D.M.; Dube, J.J.; Hughan, K.S.; Vanderpool, R.R.; Goncharov, D.A.; St Croix, C.M.; Garcia-Ocaña, A.; Goncharova, E.A.; Tofovic, S.P.; et al. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hy-perglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation 2016, 133, 717–731. [Google Scholar] [CrossRef]
- Slater, R.E.; Strom, J.G.; Methawasin, M.; Liss, M.; Gotthardt, M.; Sweitzer, N.; Granzier, H.L. Metformin improves diastolic function in an HFpEF-like mouse model by increasing titin compliance. J. Gen. Physiol. 2019, 151, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.; van der Velden, J.; Papp, Z.; Bronzwaer, J.G.; Edes, I.; Stienen, G.J.; Paulus, W.J. Cardiomyocyte stiffness in diastolic heart failure. Circulation 2005, 111, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.Y.; Schull-Meade, R.; Downey, M.; Prins, J.; Marwick, T.H. Determinants of subclinical diabetic heart disease. Diabetologia 2005, 48, 394–402. [Google Scholar] [CrossRef]
- Giorda, C.B.; Cioffi, G.; de Simone, G.; Di Lenarda, A.; Faggiano, P.; Latini, R.; Lucci, D.; Maggioni, A.P.; Tarantini, L.; Velussi, M.; et al. Predictors of early-stage left ventricular dysfunction in type 2 diabetes: Results of DYDA study. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Søgaard, P.; Hoffmann, S.; Hansen, P.R.; Vaag, A.; Major-Pedersen, A.; Hansen, T.F.; Bech, J.; Køber, L.; Torp-Pedersen, C.; et al. Metformin is associated with improved left ventricular diastolic function measured by tissue Doppler imaging in patients with diabetes. Eur. J. Endocrinol. 2010, 163, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Leung, M.; Wong, V.W.; Hudson, M.; Leung, D.Y. Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ. Cardiovasc. Imaging 2016, 9, e003643. [Google Scholar] [CrossRef] [Green Version]
- Shah, D.D.; Fonarow, G.C.; Horwich, T.B. Metformin therapy and outcomes in patients with advanced systolic heart failure and diabetes. J. Card. Fail. 2010, 16, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Yin, Z.F.; Zhang, J.F.; Wang, C.Q. Association between long-term prescription of metformin and the progression of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and hypertension. Int. J. Cardiol. 2020, 306, 140–145. [Google Scholar] [CrossRef]
- Wong, A.K.; Symon, R.; AlZadjali, M.A.; Ang, D.S.; Ogston, S.; Choy, A.; Petrie, J.R.; Struthers, A.D.; Lang, C.C. The effect of metformin on insulin resistance and exercise parameters in patients with heart failure. Eur. J. Heart Fail. 2012, 14, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Lexis, C.P.; van der Horst, I.C.; Lipsic, E.; Wieringa, W.G.; de Boer, R.A.; van den Heuvel, A.F.; van der Werf, H.W.; Schurer, R.A.; Pundziute, G.; Tan, E.S.; et al. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: The GIPS-III randomized clinical trial. JAMA 2014, 311, 1526–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Ali, L.; Hartman, M.T.; Lexis, C.P.; Hummel, Y.M.; Lipsic, E.; van Melle, J.P.; van Veldhuisen, D.J.; Voors, A.A.; van der Horst, I.C.; van der Harst, P. The effect of metformin on diastolic function in patients presenting with ST-elevation myocardial infarction. PLoS ONE 2016, 11, e0168340. [Google Scholar] [CrossRef]
- Hartman, M.H.T.; Prins, J.K.B.; Schurer, R.A.J.; Lipsic, E.; Lexis, C.P.H.; van der Horst- Schrivers, A.N.A.; van Veldhuisen, D.J.; van der Horst, I.C.C.; van der Harst, P. Two-year follow-up of 4 months metformin treatment vs. placebo in ST-elevation myocardial infarction: Data from the GIPS-III RCT. Clin. Res. Cardiol. 2017, 106, 939–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dludla, P.V.; Nyambuya, T.M.; Johnson, R.; Silvestri, S.; Orlando, P.; Mazibuko- Mbeje, S.E.; Gabuza, K.B.; Mxinwa, V.; Mokgalaboni, K.; Tiano, L.; et al. Metformin and heart failure-related outcomes in patients with or without diabetes: A systematic review of randomized controlled trials. Heart Fail. Rev. 2021, 26, 1437–1445. [Google Scholar] [CrossRef]
- Ladeiras-Lopes, R.; Sampaio, F.; Leite, S.; Santos-Ferreira, D.; Vilela, E.; Leite-Moreira, A.; Bettencourt, N.; Gama, V.; Braga, P.; Fon-tes-Carvalho, R. Metformin in non-diabetic patients with metabolic syndrome and diastolic dysfunction: The MET-DIME randomized trial. Endocrine 2021, 72, 699–710. [Google Scholar] [CrossRef]
- Evans, J.M.; Doney, A.S.; AlZadjali, M.A.; Ogston, S.A.; Petrie, J.R.; Morris, A.D.; Struthers, A.D.; Wong, A.K.; Lang, C.C. Effect of Metformin on mortality in patients with heart failure and type 2 diabetes mellitus. Am. J. Cardiol. 2010, 106, 1006–1010. [Google Scholar] [CrossRef]
- Andersson, C.; Olesen, J.B.; Hansen, P.R.; Weeke, P.; Norgaard, M.L.; Jø rgensen, C.H.; Lange, T.; Abildstrø m, S.Z.; Schramm, T.K.; Vaag, A.; et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: A retrospective nationwide cohort study. Diabetologia 2010, 53, 2546–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumie, C.L.; Min, J.Y.; D’Agostino McGowan, L.; Presley, C.; Grijalva, C.G.; Hackstadt, A.J.; Hung, A.M.; Greevy, R.A.; Elasy, T.; Griffin, M.R. Comparative Safety of Sulfonylurea and Metformin Monotherapy on the Risk of Heart Failure: A Cohort Study. J. Am. Heart Assoc. 2017, 6, e005379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, M.R.; Eurich, D.T.; Majumdar, S.R.; Lewsey, J.D.; Bhagra, S.; Jhund, P.S.; Petrie, M.C.; McMurray, J.J.; Petrie, J.R.; McAlister, F.A. Treatment of type 2 diabetes and outcomes in patients with heart failure: A nested case-control study from the U.K. General Practice Research Database. Diabetes Care 2010, 33, 1213–1218. [Google Scholar] [CrossRef] [Green Version]
- Roussel, R.; Travert, F.; Pasquet, B.; Wilson, P.W.; Smith, S.C., Jr.; Goto, S.; Ravaud, P.; Marre, M.; Porath, A.; Bhatt, D.L.; et al. Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch. Intern. Med. 2010, 170, 1892–1899. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, D.; Chan, W.; Bozkurt, B.; Ramasubbu, K.; Deswal, A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ. Heart Fail. 2011, 4, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Fung, C.S.; Wan, E.Y.; Wong, C.K.; Jiao, F.; Chan, A.K. Effect of metformin monotherapy on cardiovascular diseases and mortality: A retrospective cohort study on Chinese type 2 diabetes mellitus patients. Cardiovasc. Diabetol. 2015, 14, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera Comoglio, R.; Vidal Guitart, X. Cardiovascular events and mortality among type 2 diabetes mellitus patients newly prescribed first-line blood glucose-lowering drugs monotherapies: A population-based cohort study in the Catalan electronic medical record database, SIDIAP, 2010–2015. Prim. Care Diabetes 2021, 15, 323–331. [Google Scholar] [CrossRef]
- He, S.; Qian, X.; Chen, Y.; Shen, X.; Zhang, B.; Chen, X.; Xu, X.; Li, G. Risk of Death and Heart Failure among Patients with Type 2 Diabetes Treated by Metformin and Nonmetformin Monotherapy: A Real-World Study. J. Diabetes Res. 2021, 2021, 5534387. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.P.; Andrey, J.L.; Garcia-Egido, A.; Escobar, M.A.; Perez, V.; Corzo, R.; Garcia- Domiguez, G.J.; Gomez, F. Metformin therapy and prognosis of patients with heart failure and new-onset diabetes mellitus. A propensity-matched study in the community. Int. J. Cardiol. 2013, 166, 404–412. [Google Scholar] [CrossRef]
- Lamanna, C.; Monami, M.; Marchionni, N.; Mannucci, E. Effect of metformin on cardiovascular events and mortality: A me-ta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2011, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Bolen, S.; Tseng, E.; Hutfless, S.; Segal, J.B.; Suarez-Cuervo, C.; Berger, Z.; Wilson, L.M.; Chu, Y.; Iyoha, E.; Maruthur, N.M. Diabetes Medications for Adults with Type 2 Diabetes: An Update; 2016 Apr. Report No.: 16-EHC013-EF; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2016.
- Crowley, M.J.; Diamantidis, C.J.; McDuffie, J.R.; Cameron, C.B.; Stanifer, J.W.; Mock, C.K.; Wang, X.; Tang, S.; Nagi, A.; Kosinski, A.S.; et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: A systematic review. Ann. Intern. Med. 2017, 166, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef] [Green Version]
- Fácila, L.; Fabregat-Andrés, Ó.; Bertomeu, V.; Navarro, J.P.; Miñana, G.; García-Blas, S.; Valero, E.; Morell, S.; Sanchis, J.; Núñez, J. Met-formin and risk of long-term mortality following an admission for acute heart failure. J. Cardiovasc. Med. 2017, 18, 69–73. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Min, X.; Yuan, T.; Wei, J.; Cai, Z. The Association Between Metformin Treatment and Outcomes in Type 2 Diabetes Mellitus Patients With Heart Failure With Preserved Ejection Fraction: A Retrospective Study. Front. Cardiovasc. Med. 2021, 8, 648212. [Google Scholar] [CrossRef]
- Halabi, A.; Sen, J.; Huynh, Q.; Marwick, T.H. Metformin treatment in heart failure with preserved ejection fraction: A systematic review and meta-regression analysis. Cardiovasc. Diabetol. 2020, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Eurich, D.T.; Weir, D.L.; Majumdar, S.R.; Tsuyuki, R.T.; Johnson, J.A.; Tjosvold, L.; Vanderloo, S.E.; McAlister, F.A. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: Systematic review of observational studies involving 34,000 patients. Circ. Heart Fail. 2013, 6, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Giordano, M.; Ciarambino, T.; Castellino, P.; Malatino, L.; Cataliotti, A.; Rinaldi, L.; Paolisso, G. Adinolfi LE Seasonal variations of hyponatremia in the emergency department: Age-related changes. Am. J. Emerg. Med. 2017, 35, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Boussageon, R.; Supper, I.; Bejan-Angoulvant, T.; Kellou, N.; Cucherat, M.; Boissel, J.P.; Kassai, B.; Moreau, A.; Gueyffier, F.; Cornu, C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: A meta-analysis of randomised controlled trials. PLoS Med. 2012, 9, e1001204. [Google Scholar] [CrossRef] [Green Version]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.C.; Mavridis, D.; Nicolucci, A.; Johnson, D.W.; Tonelli, M.; Craig, J.C.; Maggo, J.; Gray, V.; De Berardis, G.; Ruospo, M.; et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: A meta-analysis. JAMA 2016, 316, 313–324. [Google Scholar] [CrossRef]
- Rao, A.D.; Kuhadiya, N.; Reynolds, K.; Fonseca, V.A. Is the combination of sulfonylureas and metformin associated with an in-creased risk of cardiovascular disease or all-cause mortality? A meta-analysis of observational studies. Diabetes Care 2008, 31, 1672–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariyanto, T.I.; Kurniawan, A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020, 19, 100290. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Qiu, L.; Liu, Y.; Liu, X.L.; Zheng, J.L.; Xue, H.Y.; Liu, W.H.; Liu, D.; Li, J. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 2020, 103, 69–72. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis. J. Med. Virol. 2021, 93, 695–697. [Google Scholar] [CrossRef]
- Richardson, T.L., Jr.; Hackstadt, A.J.; Hung, A.M.; Greevy, R.A.; Grijalva, C.G.; Griffin, M.R.; Elasy, T.A.; Roumie, C.L. Hospitalization for heart failure among patients with diabetes mellitus and reduced kidney function treated with metformin versus sulfonylureas: A retrospective cohort study. J. Am. Heart Assoc. 2021, 10, e019211. [Google Scholar] [CrossRef]
- Weir, D.L.; Abrahamowicz, M.; Beauchamp, M.E.; Eurich, D.T. Acute vs cumulative benefits of metformin use in patients with type 2 diabetes and heart failure. Diabetes Obes. Metab. 2018, 20, 2653–2660. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H. Metformin use is associated with a lower risk of hospitalization for heart failure in patients with type 2 diabetes mellitus: A retrospective cohort analysis. J. Am. Heart Assoc. 2019, 8, e011640. [Google Scholar] [CrossRef]
- Nichols, G.A.; Koro, C.E.; Gullion, C.M.; Ephross, S.A.; Brown, J.B. The incidence of congestive heart failure associated with antidi-abetic therapies. Diabetes Metab. Res. Rev. 2005, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, K.M.; Kattan, M.W.; Yu, C.; Wells, B.J.; Arrigain, S.; Jain, A.; Atreja, A.; Zimmerman, R.S. The risk of developing coronary artery disease or congestive heart failure, and overall mortality, in type 2 diabetic patients receiving rosiglitazone, pioglitazone, metformin, or sulfonylureas: A retrospective analysis. Acta Diabetol. 2009, 46, 145–154. [Google Scholar] [CrossRef]
- McAlister, F.A.; Eurich, D.T.; Majumdar, S.R.; Johnson, J.A. The risk of heart failure in patients with type 2 diabetes treated with oral agent monotherapy. Eur. J. Heart Fail. 2008, 10, 703–708. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, X.; Zheng, Y.; Li, J.; Wang, Y.; Lin, Y.; He, Z.; Zhao, W.; Chen, C.; Qiu, K.; et al. Association of glucose-lowering medications with cardiovascular outcomes: An umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Fralick, M.; Schneeweiss, S.; Redelmeier, D.A.; Razak, F.; Gomes, T.; Patorno, E. Comparative effectiveness and safety of sodi-um-glucose cotransporter-2 inhibitors versus metformin in patients with type 2 diabetes: An observational study using data from routine care. Diabetes Obes. Metab. 2021, 23, 2320–2328. [Google Scholar] [CrossRef]
- Rosenstock, J.; Chuck, L.; González-Ortiz, M.; Merton, K.; Craig, J.; Capuano, G.; Qiu, R. Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-naïve type 2 diabetes. Diabetes Care 2016, 39, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Hedrington, M.S.; Davis, S.N. The role of empagliflozin in the management of type 2 diabetes by patient profile. Clin. Risk Manag. 2015, 11, 739–749. [Google Scholar]
- Salvatore, T.; Pafundi, P.C.; Morgillo, F.; Di Liello, R.; Galiero, R.; Nevola, R.; Marfella, R.; Monaco, L.; Rinaldi, L.; Adinolfi, L.E.; et al. Metformin: An old drug against old age and associated morbidities. Diabetes Res. Clin. Pract. 2020, 160, 108025. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, F.; Sasso, F.C.; Della Corte, C.M.; Festino, L.; Manzo, A.; Martinelli, E.; Troiani, T.; Capuano, A.; Ciardiello, F. Metformin in lung cancer: Rationale for a combination therapy. Expert Opin. Investig. Drugs. 2013, 22, 1401–1409. [Google Scholar] [CrossRef]
- Morgillo, F.; Fasano, M.; Della Corte, C.M.; Sasso, F.C.; Papaccio, F.; Viscardi, G.; Esposito, G.; Di Liello, R.; Normanno, N.; Capuano, A.; et al. Results of the safety run-in part of the METAL (METformin in Advanced Lung cancer) study: A multicentre, open-label phase I-II study of metformin with erlotinib in second-line therapy of patients with stage IV non-small-cell lung cancer. ESMO Open 2017, 2, e000132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiggers, H.; Køber, L.; Gislason, G.; Schou, M.; Poulsen, M.K.; Vraa, S.; Nielsen, O.W.; Bruun, N.E.; Nørrelund, H.; Hollingdal, M.; et al. The DANish randomized, double-blind, placebo controlled trial in patients with chronic HEART failure (DANHEART): A 2 × 2 factorial trial of hydralazine-isosorbide dinitrate in patients with chronic heart failure (H-HeFT) and metformin in patients with chronic heart failure and diabetes or prediabetes (Met-HeFT). Am. Heart J. 2021, 231, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.J.; Bethel, M.A.; Holman, R.R.; Khunti, K.; Wareham, N.; Brierley, G.; Davies, M.; Dymond, A.; Eichenberger, R.; Evans, P.; et al. Metformin in non-diabetic hyperglycaemia: The GLINT feasibility RCT. Health Technol. Assess. 2018, 22, 1–64. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules 2021, 11, 1834. https://doi.org/10.3390/biom11121834
Salvatore T, Galiero R, Caturano A, Vetrano E, Rinaldi L, Coviello F, Di Martino A, Albanese G, Marfella R, Sardu C, et al. Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules. 2021; 11(12):1834. https://doi.org/10.3390/biom11121834
Chicago/Turabian StyleSalvatore, Teresa, Raffaele Galiero, Alfredo Caturano, Erica Vetrano, Luca Rinaldi, Francesca Coviello, Anna Di Martino, Gaetana Albanese, Raffaele Marfella, Celestino Sardu, and et al. 2021. "Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence" Biomolecules 11, no. 12: 1834. https://doi.org/10.3390/biom11121834
APA StyleSalvatore, T., Galiero, R., Caturano, A., Vetrano, E., Rinaldi, L., Coviello, F., Di Martino, A., Albanese, G., Marfella, R., Sardu, C., & Sasso, F. C. (2021). Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules, 11(12), 1834. https://doi.org/10.3390/biom11121834








