Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
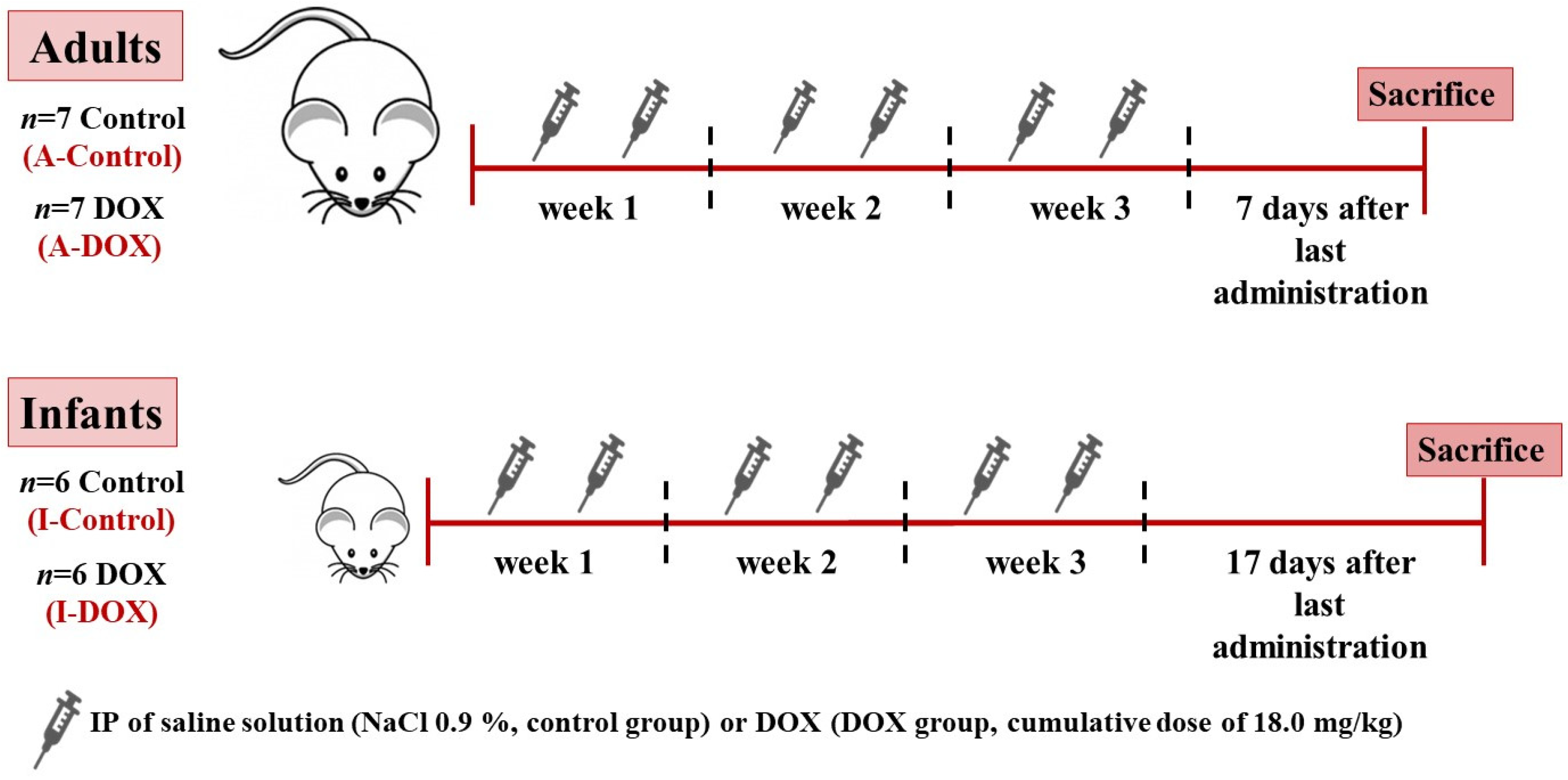
2.2. Experimental Protocol
2.3. DOX Administration Schedule
2.4. Blood Collection and Measurement of Plasma Biomarkers
2.5. Tissue Collection
2.6. Histological Analysis of Cardiac Tissue
2.7. Measurement of ATP Levels
2.8. Measurement of tGSH, GSH, and GSSG
2.9. Protein Carbonylation Evaluation
2.10. Immunohistochemistry
2.11. Western Blotting Analysis
2.12. Statistical Analysis
3. Results
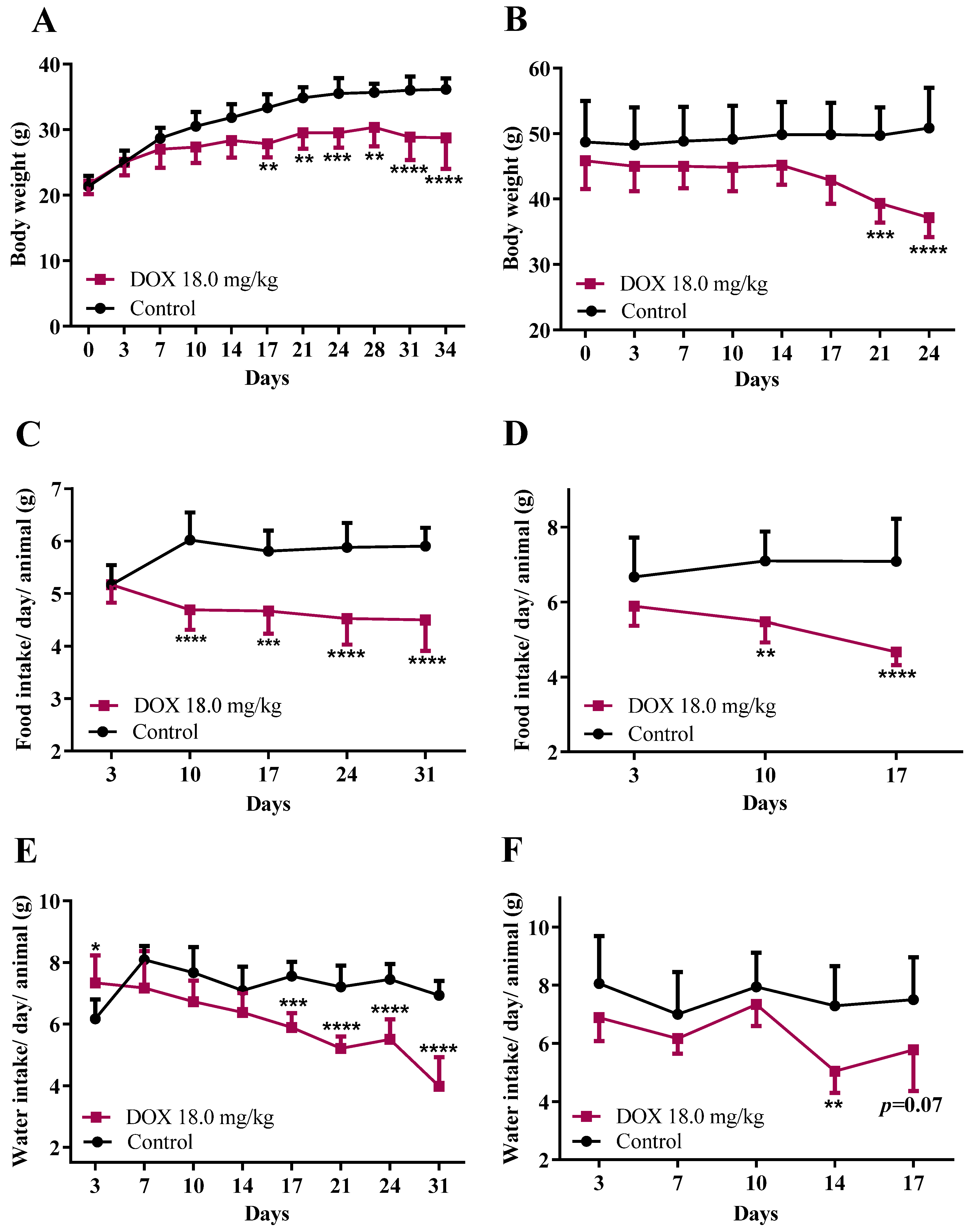
3.1. DOX Treatment Affected Mice Body Weight, Food/Water Consumption, and the Heart Weight-to-Brain Weight Ratio
3.2. Plasma Aspartate Aminotransferase Was Found to Be Increased in Both Groups, While Creatine Kinase-MB and Total Creatine-Kinase Only Increased in Adult Mice
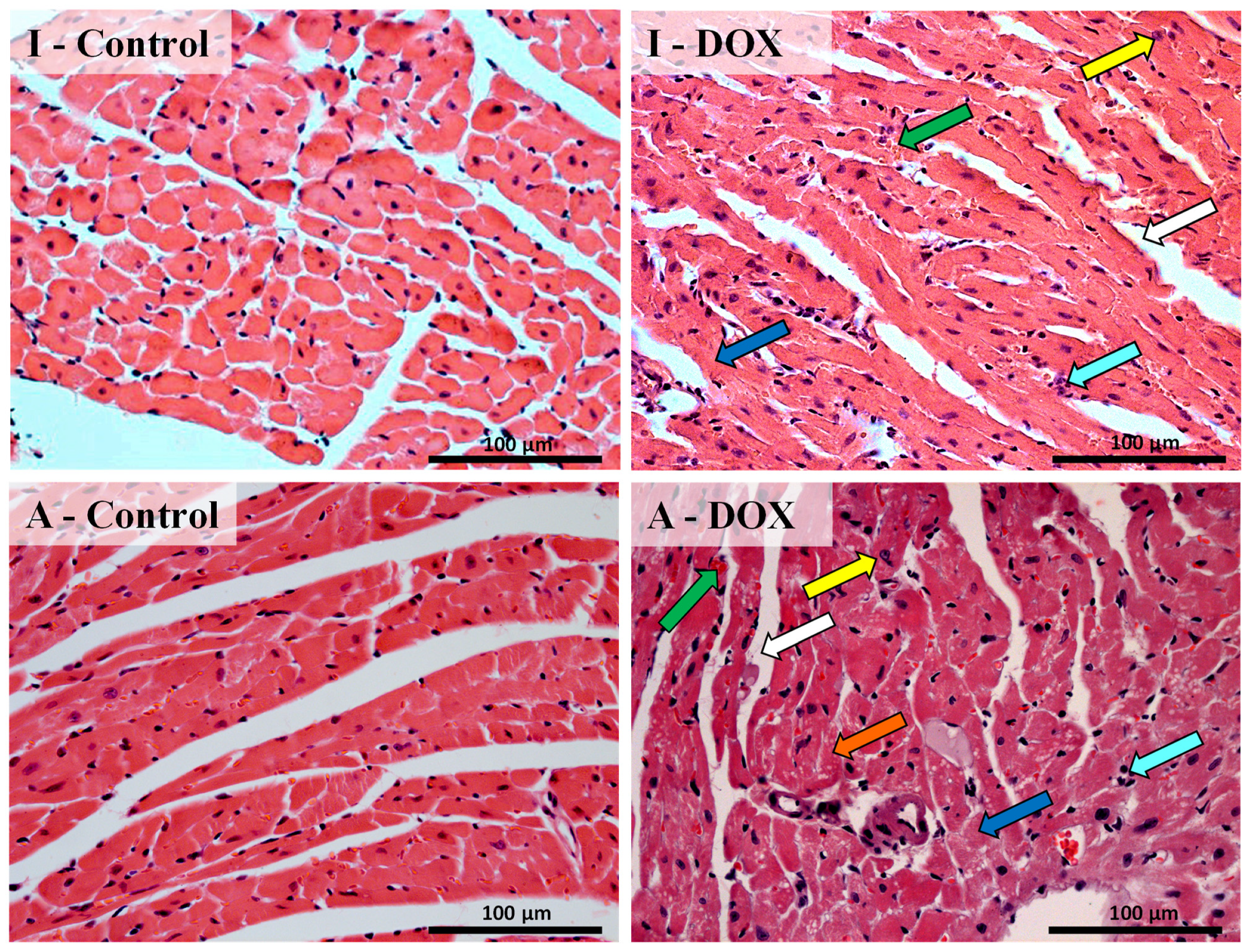
3.3. Histological Changes Were Seen in the Cardiac Structure of Both Mice Groups after DOX Treatment
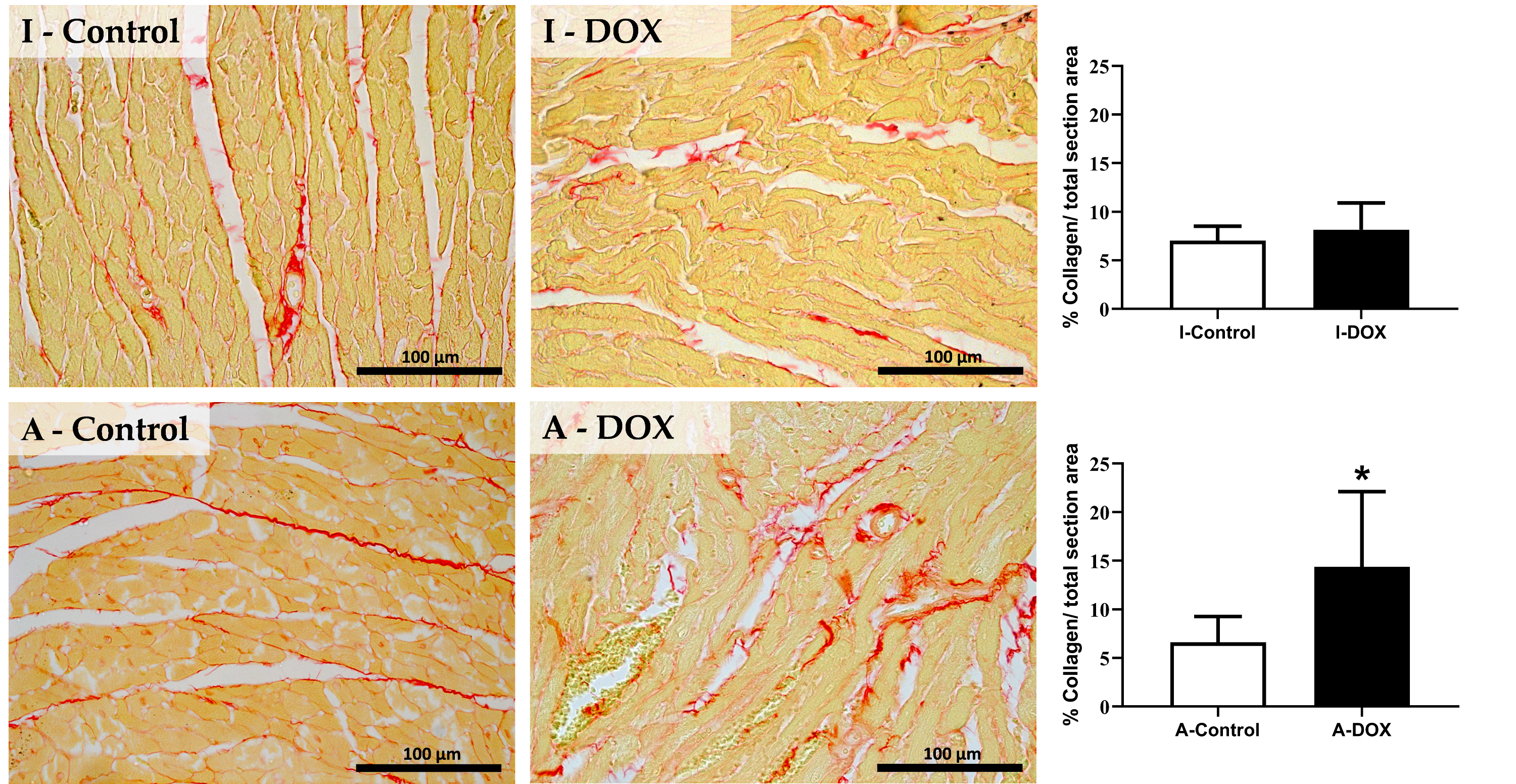
3.4. DOX Treatment Induced Myocardial Fibrosis in Adult Mice
3.5. No Significant Changes in the Cardiac Levels of ATP or Glutathione-Related Measurements Were Observed in DOX-Treated Mice of Both Groups
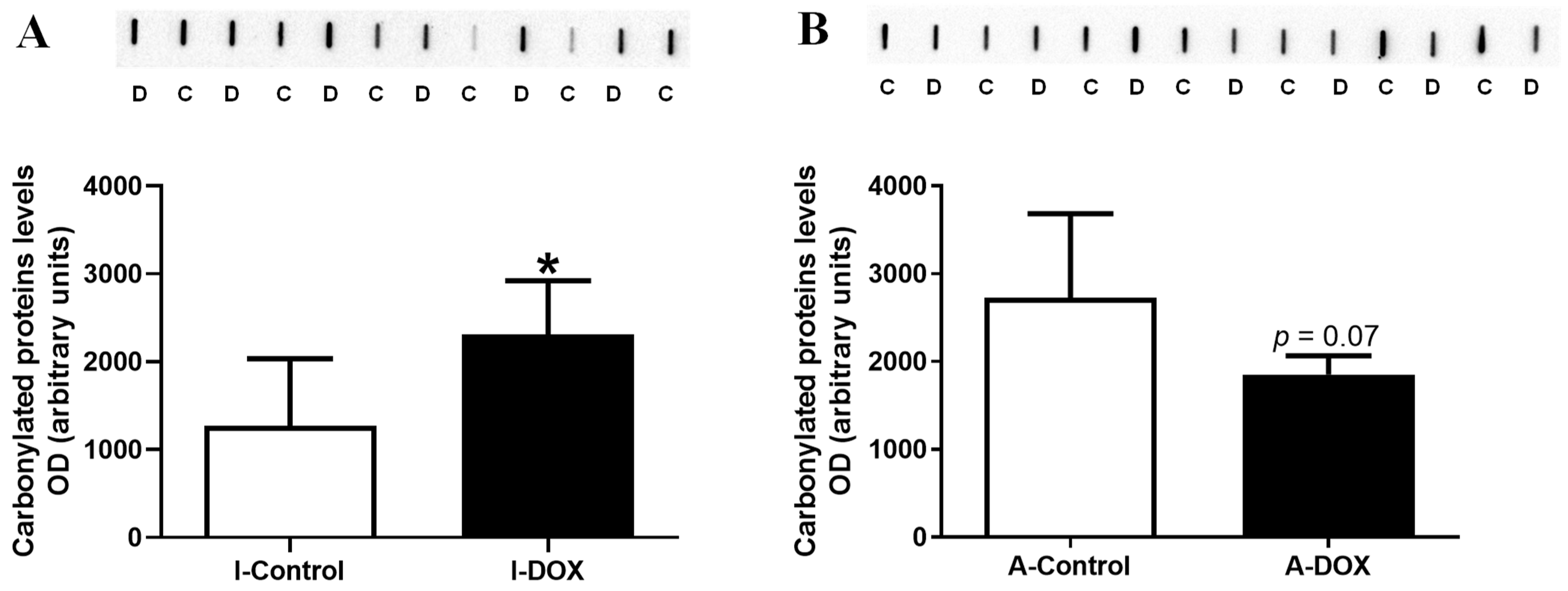
3.6. Protein Carbonylation in Cardiac Lysates Increased in DOX-Treated Infant Mice and Decreased in DOX-Treated Adult Mice
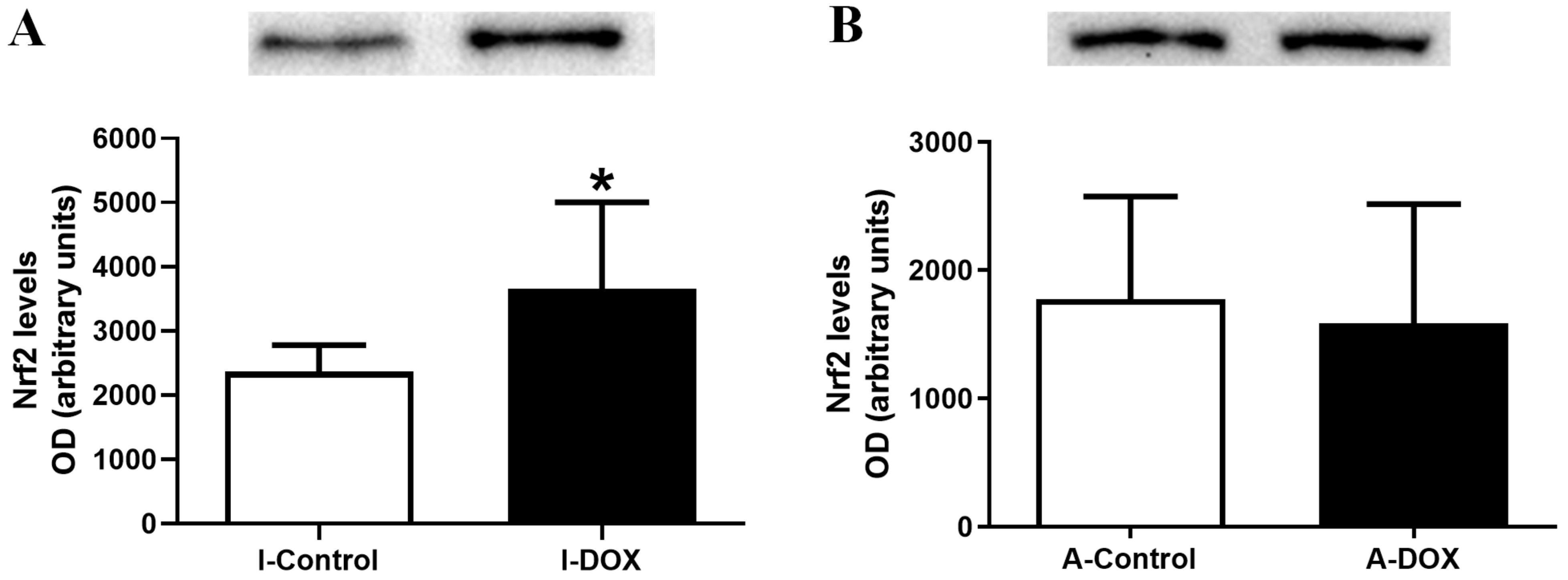
3.7. DOX-Treated Infant Mice Showed a Significant Increase in the Heart Levels of Nuclear Factor Erythroid-2 Related Factor 2
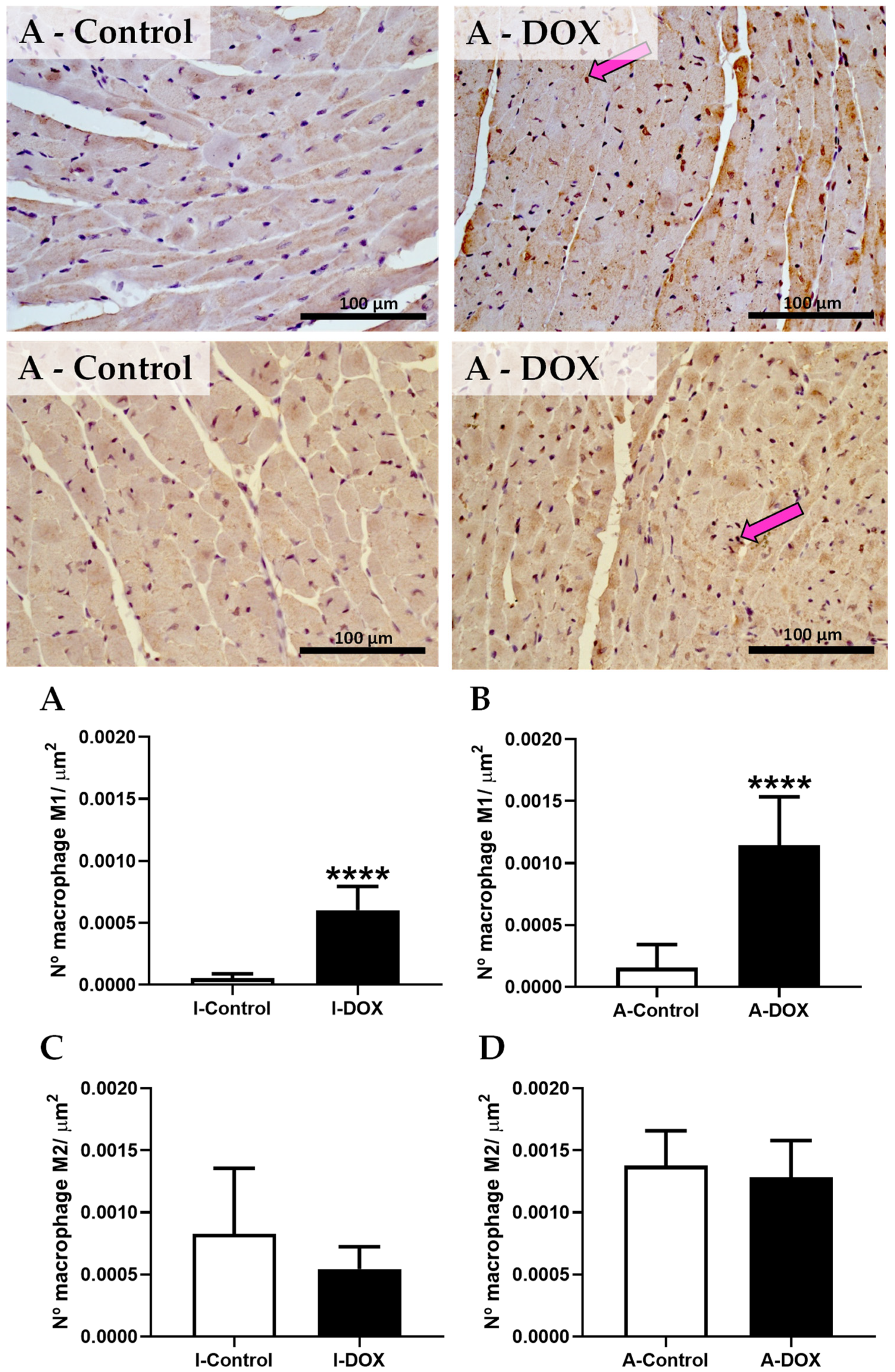
3.8. DOX-Treated Mice Showed a Higher Density of Infiltrating M1 Macrophages in the Cardiac Tissue
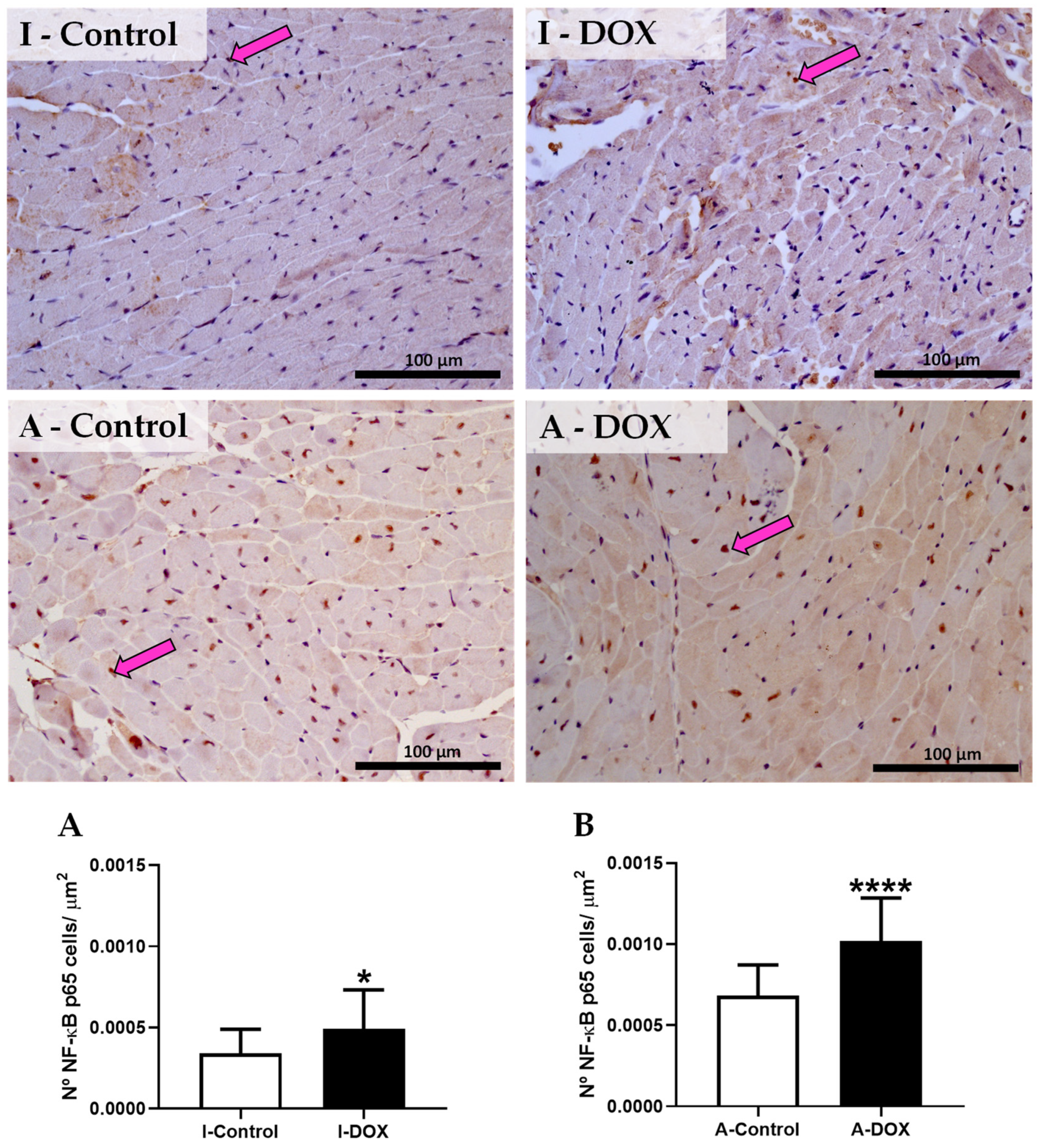
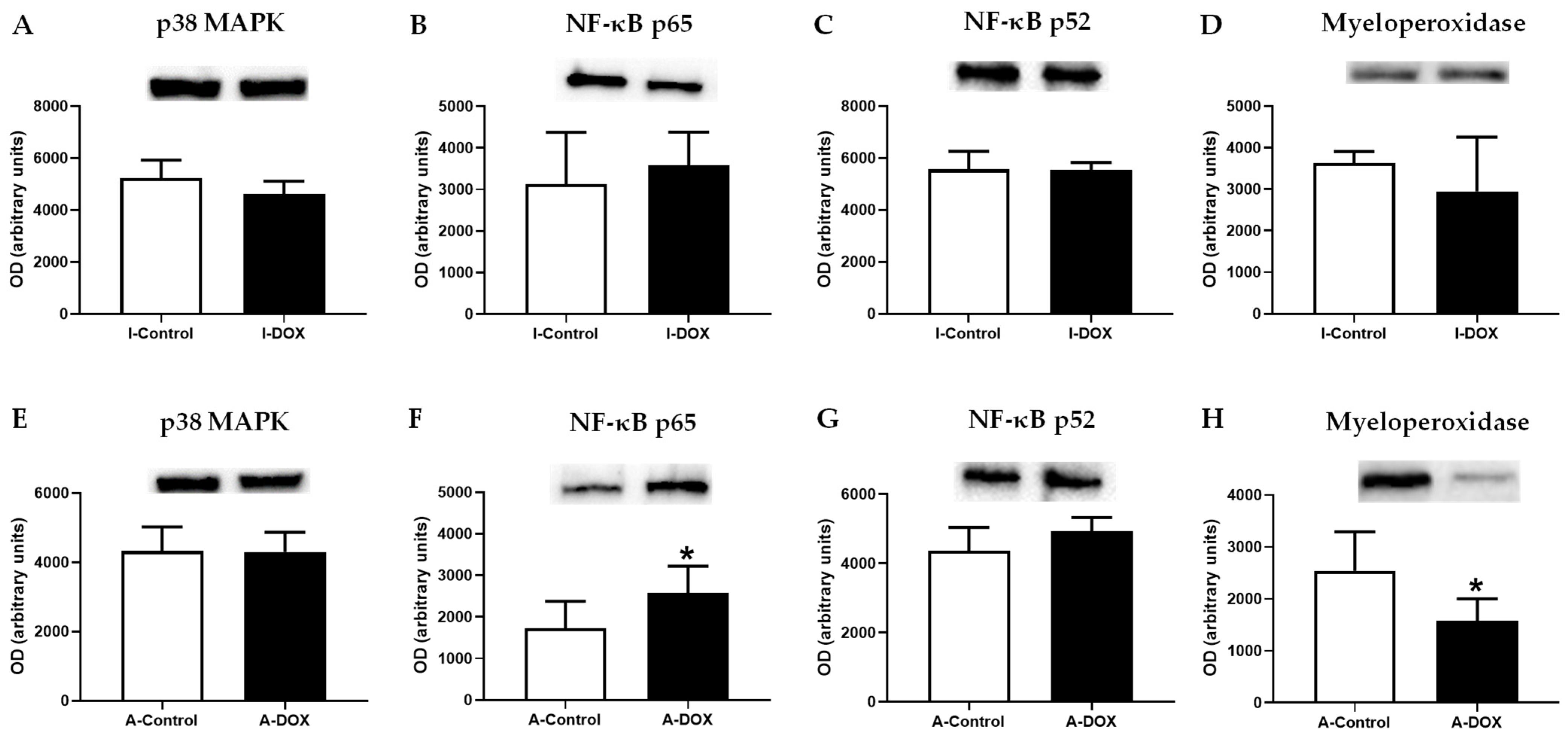
3.9. DOX-Treated Mice Showed a Significant Increase in the Nuclear Factor-ĸB p65 Subunit
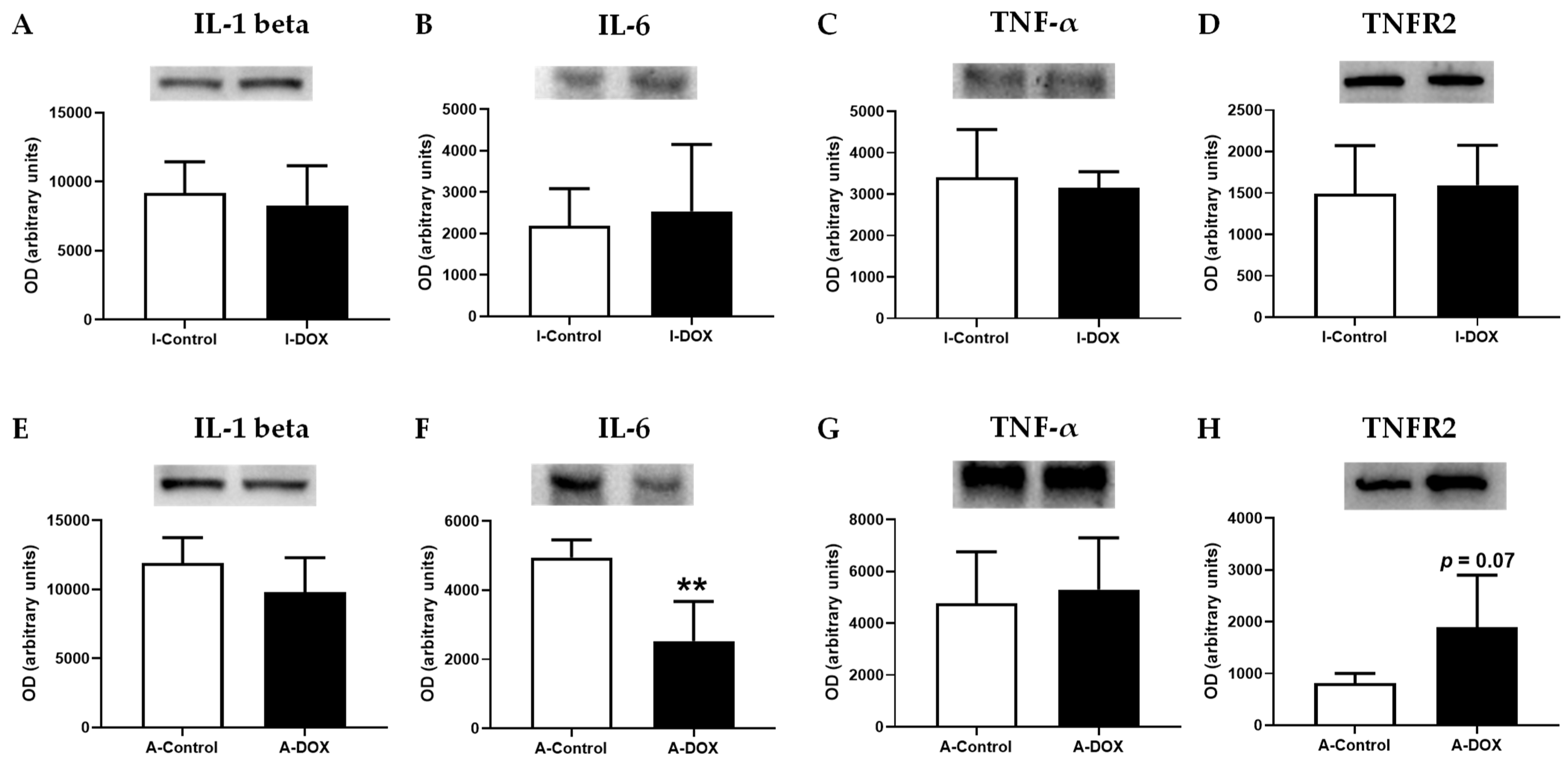
3.10. DOX Decreased IL-6 Cardiac Levels, with No Impact on Other Evaluated Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seiter, K. Toxicity of the topoisomerase II inhibitors. Expert Opin. Drug Saf. 2005, 4, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.F.; Sousa, E.; Bastos, M.D.L.; Costa, V.M. The Role of the metabolism of anticancer drugs in their induced-cardiotoxicity. Curr. Drug Metab. 2015, 17, 75–90. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; DAVIS, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective anal-ysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, E.C.; van der Pal, H.J.; Kok, W.E.; Caron, H.N.; Kremer, L.C. Clinical heart failure in a cohort of children treated with anthracyclines: A long-term follow-up study. Eur. J. Cancer 2006, 42, 3191–3198. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Yeazel, M.W.; Leisenring, W.M.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009, 339, b4606. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, T.R.; Iskra, B.; Aune, G.J. Doxorubicin-Induced Cardiomyopathy in Children. Compr. Physiol. 2019, 9, 905–931. [Google Scholar] [CrossRef]
- Damiani, R.M.; Moura, D.J.; Viau, C.M.; Caceres, R.A.; Henriques, J.A.P.; Saffi, J. Pathways of cardiac toxicity: Comparison between chemotherapeutic drugs doxorubicin and mitoxan-trone. Arch. Toxicol. 2016, 90, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, J.; Zhang, L.; Du, R.; Xiang, D.; Wu, M.; Zhang, R.; Han, W. Interleukin-1 signaling mediates acute doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2011, 65, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.T.S.; Chetty, M.C.; Kavimani, S. Antioxidants and tumor necrosis factor alpha-inhibiting activity of sesame oil against doxorubicin-induced cardiotoxicity. Ther. Adv. Cardiovasc. Dis. 2014, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, Y.W.; Yu, W.Y.; Yao, X.; Ma, X.; Sheng, G.; Ma, Q. Vitexin attenuates acute doxorubicin cardiotoxicity in rats via the suppression of oxidative stress, inflammation and apoptosis and the activation of FOXO3a. Exp. Ther. Med. 2016, 12, 1879–1884. [Google Scholar] [CrossRef]
- Prathumsap, N.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. Effects of doxorubicin on the heart: From molecular mechanisms to intervention strategies. Eur. J. Pharmacol. 2020, 866, 172818. [Google Scholar] [CrossRef]
- Guo, R.M.; Xu, W.M.; Lin, J.C.; Mo, L.Q.; Hua, X.X.; Chen, P.X.; Wu, K.; Zheng, D.D.; Feng, J.Q. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cyto-toxicity in H9c2 cardiac cells. Mol. Med. Rep. 2013, 8, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kotamraju, S.; Konorev, E.; Kalivendi, S.; Joseph, J.; Kalyanaraman, B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myo-cytes is pro-apoptotic: The role of hydrogen peroxide. Biochem. J. 2002, 367, 729–740. [Google Scholar] [CrossRef]
- Benzer, F.; Kandemir, F.M.; Ozkaraca, M.; Kucukler, S.; Caglayan, C. Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J. Biochem. Mol. Toxicol. 2018, 32, e22030. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in heart failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establish-ment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.; Dores-Sousa, J.; Bastos, M.; Costa, V.; Padrão, A.; Duarte-Araújo, M.; Duarte, J.; Seabra, V.; Gonçalves-Monteiro, S.; Remião, F.; et al. Inflammation as a Possible Trigger for Mitoxantrone-Induced Cardiotoxicity: An In Vivo Study in Adult and Infant Mice. Pharmaceuticals 2021, 14, 510. [Google Scholar] [CrossRef]
- Curry, S.H.; DeCory, H.H.; Gabrielsson, J. Phase I: The First Opportunity for Extrapolation from Animal Data to Human Exposure, in Principles and Practice of Pharmaceutical Medicine; Edwards, L.D., Fox, A.W., Stonier, P.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 84–106. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- McEvoy, G.K. American Hospital Formulary Service—Drug Information; American Society of Hospital Pharmacists, Cop.: Bethesda, MD, USA, 1995. [Google Scholar]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal route of drug administration: Should it be used in experimental animal studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Marcus, C.; Blumenschein, G.R.; Smith, T.L. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer 1985, 55, 2761–2765. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dores-Sousa, J.L.; Duarte, J.A.; Seabra, V.; Bastos, M.D.L.; Carvalho, F.; Costa, V.M. The age factor for mitoxantrone´s cardiotoxicity: Multiple doses render the adult mouse heart more susceptible to injury. Toxicology 2015, 329, 106–119. [Google Scholar] [CrossRef]
- Carvalho, M.; Milhazes, N.; Remião, F.; Borges, F.; Fernandes, E.; Amado, F.; Monks, T.J.; Carvalho, F.; Bastos, M.L. Hepatotoxicity of 3,4-methylenedioxyamphetamine and alpha-methyldopamine in isolated rat hepatocytes: Formation of glutathione conjugates. Arch. Toxicol. 2004, 78, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Padrão, A.I.; Oliveira, P.; Vitorino, R.; Colaço, B.J.A.; Pires, M.J.; Marquez, M.; Castellanos, E.; Neuparth, M.J.; Texeira, C.; Costa, C.; et al. Bladder cancer-induced skeletal muscle wasting: Disclosing the role of mitochondria plasticity. Int. J. Biochem. Cell Biol. 2013, 45, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Deng, J.; Sunkara, M.; Morris, A.J.; Wang, C.; Clair, D.S.; Vore, M. Loss of multidrug resistance-associated protein 1 potentiates chronic doxorubicin-induced cardiac dysfunc-tion in mice. J. Pharmacol. Exp. Ther. 2015, 355, 280–287. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.; Stojanovska, V.; Abalo, R.; Bornstein, J.; Nurgali, K. Chemotherapy-Induced Constipation and Diarrhea: Pathophysiology, Current and Emerging Treatments. Front. Pharmacol. 2016, 7, 414. [Google Scholar] [CrossRef]
- Machado, N.G.; Baldeiras, I.; Pereira, G.C.; Pereira, S.P.; Oliveira, P.J. Sub-chronic administration of doxorubicin to Wistar rats results in oxidative stress and unaltered apoptotic signaling in the lung. Chem.-Biol. Interact. 2010, 188, 478–486. [Google Scholar] [CrossRef]
- Song, S.; Chu, L.; Liang, H.; Chen, J.; Liang, J.; Huang, Z.; Zhang, B.; Chen, X. Protective effects of dioscin against doxorubicin-induced hepatotoxicity via regulation of Sirt1/FOXO1/NF-κb Signal. Front. Pharmacol. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, Y.; Li, L.; Xu, L.; Tang, Z.; Qi, Y.; Yin, L.; Peng, J. MicroRNA-128-3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting Sirtuin-1. Pharmacol. Res. 2019, 146, 104276. [Google Scholar] [CrossRef]
- Zhang, S.; You, Z.Q.; Yang, L.; Li, L.L.; Wu, Y.P.; Gu, L.Q.; Xin, Y.F. Protective effect of Shenmai injection on doxorubicin-induced cardiotoxicity via regulation of inflammatory mediators. BMC Complement. Altern. Med. 2019, 19, 317. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Silva, J.; Miranda, S.E.M.; Leite, E.A.; de Paula Sabino, A.; Borges, K.B.G.; Cardoso, V.N.; Cassali, G.D.; Guimarães, A.G.; Oliveira, M.C.; de Barros, A.L.B. Toxicological study of a new doxorubicin-loaded pH-sensitive liposome: A preclinical approach. Toxicol. Appl. Pharmacol. 2018, 352, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Warpe, V.S.; Mali, V.R.; S, A.; Bodhankar, S.; Mahadik, K.R. Cardioprotective effect of ellagic acid on doxorubicin induced cardiotoxicity in Wistar rats. J. Acute Med. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Bjork, J.A.; Santos, M.S.; Leino, R.L.; Froberg, M.K.; Moreno, A.J.; Wallace, K.B. Carvedilol-mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol. Appl. Pharmacol. 2004, 200, 159–168. [Google Scholar] [CrossRef]
- Berthiaume, J.M.; Oliveira, P.J.; Fariss, M.W.; Wallace, K.B. Dietary vitamin E decreases doxorubicin-induced oxidative stress without preventing mitochondrial dysfunction. Cardiovasc. Toxicol. 2005, 5, 257–267. [Google Scholar] [CrossRef]
- Iwasaki, T.; Suzuki, T. Ultrastructural alterations of the myocardium induced by doxorubicin. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1991, 60, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, L.; Theophilidis, G.; Thomopoulos, G.N.; Tsiftsoglou, A.S. Structural and functional impairment of mitochondria in adriamycin-induced cardiomyopathy in mice: Suppression of cytochrome c oxidase II gene expression. Biochem. Pharmacol. 1999, 57, 481–489. [Google Scholar] [CrossRef]
- Shaker, R.A.; Abboud, S.H.; Assad, H.C.; Hadi, N. Enoxaparin attenuates doxorubicin induced cardiotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. BMC Pharmacol. Toxicol. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.A.; Mohamed, R.H.; Pasha, H.F.; Abdel-Aziz, H.R. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamy-cin-treated rats. Clin. Exp. Med. 2012, 12, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Ahmed, M.M.; Elwey, H.M.; Amin, A. Melissa officinalis protects against doxorubicin-induced cardiotoxicity in rats and potentiates its anti-cancer activity on MCF-7 Cells. PLoS ONE 2016, 11, e0167049. [Google Scholar] [CrossRef]
- Quagliariello, V.; Coppola, C.; Mita, D.G.; Piscopo, G.; Iaffaioli, R.V.; Botti, G.; Maurea, N. Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environ. Toxicol. Pharmacol. 2019, 69, 1–8. [Google Scholar] [CrossRef]
- Chaikijurajai, T.; Tang, W.H.W. Reappraisal of inflammatory biomarkers in heart failure. Curr. Hear. Fail. Rep. 2020, 17, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Al-Salam, S.; Hashmi, S. Myocardial ischemia reperfusion injury: Apoptotic, inflammatory and oxidative stress role of galectin-3. Cell. Physiol. Biochem. 2018, 50, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Inoue, T.; Yoshimaru, T.; Ra, C. Galectin-3 but not galectin-1 induces mast cell death by oxidative stress and mitochondrial permeability transition. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 924–934. [Google Scholar] [CrossRef]
- Yorulmaz, H.; Ozkok, E.; Kaptan, E.; Ates, G.; Tamer, S. Therapeutic effects of simvastatin on Galectin-3 and oxidative stress parameters in endotoxemic lung tissue. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Tian, Y.; Lv, W.; Lu, C.; Jiang, Y.; Yang, X.; Song, M. Galectin-3 inhibition attenuates doxorubicin-induced cardiac dysfunction by upregulating the expression of peroxiredoxin-4. Can. J. Physiol. Pharmacol. 2020, 98, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.O.; Shimpo, M.; Hurwitz, S.; Tominaga, S.I.; Rouleau, J.L.; Lee, R.T. Identification of serum soluble ST2 receptor as a nov-el heart failure biomarker. Circulation 2003, 107, 721–726. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Sanada, S.; Kudinova, A.Y.; Steinhauser, M.L.; Handa, V.; Gannon, J.; Lee, R.T. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ. Hear. Fail. 2009, 2, 684–691. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, R.; Ying, C.; Zhang, G.; Rui, T.; Tao, A. Interleukin-33 attenuates doxorubicin-induced cardiomyocyte apoptosis through suppression of ASK1/JNK signaling pathway. Biochem. Biophys. Res. Commun. 2017, 493, 1288–1295. [Google Scholar] [CrossRef]
- Meldrum, D.R. Tumor necrosis factor in the heart. Am. J. Physiol. Integr. Comp. Physiol. 1998, 274, R577–R595. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, E.A.; Benincasa, G.; Di Rella, F.; Casaburi, C.; Monti, M.G.; De Simone, G.; Chiariotti, L.; Palombini, L.; Bruni, C.B.; Saccà, L.; et al. Differential expression of TNF-alpha, IL-6, and IGF-1 by graded mechanical stress in normal rat myocardium. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H926–H934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodrigues, P.G.; Miranda-Silva, D.; Vitorino, R.; Linke, W.; Leite-Moreira, A.F.; Falcão-Pires, I.; Costa, S.M.; Barros, C.; Hamdani, N.; Moura, C.; et al. Early myocardial changes induced by doxorubicin in the nonfailing dilated ventricle. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H459–H475. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Umemura, M.; Narikawa, M.; Hikichi, M.; Osaw, K.; Fujita, T.; Yokoyama, U.; Ishigami, T.; Tamura, K.; Ishikawa, Y. Reactive fibrosis precedes doxorubicin-induced heart failure through sterile inflammation. ESC Hear. Fail. 2020, 7, 588–603. [Google Scholar] [CrossRef]
- Cattaruzza, M.; Hecker, M. Protein carbonylation and decarboylation: A new twist to the complex response of vascular cells to oxidative stress. Circ. Res. 2008, 102, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Cheema, A.K.; Zhang, L.; Suzuki, Y.J. Protein carbonylation as a novel mechanism in redox signaling. Circ. Res. 2008, 102, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Jeong, J.; Rao, V.A. Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity. Proc. Natl. Acad. Sci. USA 2014, 111, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Galijasevic, S. The development of myeloperoxidase inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 1–7. [Google Scholar] [CrossRef]
- Atwal, M.; Lishman, E.L.; Austin, C.A.; Cowell, I.G. Myeloperoxidase enhances etoposide and mitoxantrone-mediated DNA damage: A target for myeloprotec-tion in cancer chemotherapy. Mol. Pharmacol. 2017, 91, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Reszka, K.J.; Wagner, B.A.; Teesch, L.M.; Britigan, B.E.; Spitz, D.R.; Burns, C.P. Inactivation of anthracyclines by cellular peroxidase. Cancer Res. 2005, 65, 6346–6353. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Azami, N.; Hamzehlou, S.; Farahani, M.V.; Hushmandi, K.; Ashrafizadeh, M.; et al. Nrf2 signaling pathway in chemoprotection and doxorubicin resistance: Potential application in drug discovery. Antioxidants 2021, 10, 349. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Niu, T.; Wang, H.; Li, B.; Shao, L.; Lai, Y.; Li, H.; Janicki, J.S.; Wang, X.L.; et al. Nrf2 Deficiency Exaggerates Doxorubicin-Induced Cardiotoxicity and Cardiac Dysfunction. Oxidative Med. Cell. Longev. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Han, X.; Pan, J.; Ren, D.; Cheng, Y.; Fan, P.; Lou, H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 3140–3146. [Google Scholar] [CrossRef]
- Li, B.; Kim, D.S.; Yadav, R.K.; Kim, H.R.; Chae, H.J. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int. J. Mol. Med. 2015, 36, 53–64. [Google Scholar] [CrossRef]
- Wang, L.F.; Su, S.W.; Wang, L.; Zhang, G.Q.; Zhang, R.; Niu, Y.J.; Guo, Y.S.; Li, C.Y.; Jiang, W.B.; Liu, Y.; et al. Tert-butylhydroquinone ameliorates doxorubicin-induced cardiotoxicity by activating Nrf2 and inducing the expression of its target genes. Am. J. Transl. Res. 2015, 7, 1724–1735. [Google Scholar]
- Yu, X.; Cui, L.; Zhang, Z.; Zhao, Q.; Li, S. α-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim. Biophys. Sin. (Shanghai) 2013, 45, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yan, M.; Chen, L.; Fang, P.; Li, Z.; Wan, Z.; Cao, S.; Hou, Z.; Wei, S.; Li, W.; et al. Nrf2-dependent antioxidant response mediated the protective effect of tanshinone IIA on doxorubicin-induced cardiotoxicity. Exp. Ther. Med. 2018, 16, 3333–3344. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Lee, S.; Kwak, M.K. Effect of stable inhibition of NRF2 on doxorubicin sensitivity in human ovarian carcinoma OV90 cells. Arch. Pharm. Res. 2010, 33, 717–726. [Google Scholar] [CrossRef] [PubMed]
| Plasma | I-Control | I-DOX | A-Control | A-DOX |
|---|---|---|---|---|
| AST(U/L) | 37.67 ± 6.02 | 58.40 ± 12.10 * | 35.29 ± 14.45 | 86.25 ± 26.32 ** |
| ALT (U/L) | 9.33 ± 4.27 | 26.75 ± 3.30 * | 15.86 ± 8.65 | 28.17 ± 19.10 |
| AST/ALT ratio | 4.81 ± 2.37 | 2.76 ± 1.29 | 2.33 ± 0.80 | 9.63 ± 5.79 * |
| CK-MB (U/L) | 55.83 ± 22.27 | 69.40 ± 60.91 | 31.67 ± 19.56 | 154.5 ± 105.7 ** |
| Total-CK (U/L) | 43.33 ± 14.42 | 101.8 ± 111.2 | 22.83 ± 11.48 | 401.7 ± 369.7 ** |
| Heart | I-Control | I-DOX | A-Control | A-DOX |
| Heart weight/ brain weight ratio (%) | 0.38 ± 0.03 | 0.29 ± 0.04 ** | 0.60 ± 0.08 | 0.42 ± 0.17 ** |
| Hematoxylin-Eosin Staining | I-Control | I-DOX | A-Control | A-DOX |
|---|---|---|---|---|
| Cellular degeneration | 0.07 ± 0.25 | 0.57 ± 0.63 *** | 0.02 ± 0.13 | 2.45 ± 0.50 **** |
| Necrotic zones | 0.00 ± 0.00 | 0.30 ± 0.47 ** | 0.00 ± 0.00 | 1.40 ± 0.50 **** |
| Interstitial inflammatory cell infiltration | 0.08 ± 0.27 | 0.53 ± 0.51 *** | 0.05 ± 0.22 | 1.26 ± 0.44 **** |
| Loss of tissue organization | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.38 ± 0.50 *** |
| I-Control | I-DOX | A-Control | A-DOX | |
|---|---|---|---|---|
| tGSH (nmol/mg protein) | 3.700 ± 0.913 | 3.864 ± 1.872 | 3.853 ± 1.404 | 2.843 ± 1.556 |
| GSSG (nmol/mg protein) | 0.827 ± 0.258 | 0.693 ± 0.400 | 0.871 ± 0.244 | 0.503 ± 0.410 |
| GSH (nmol/mg protein) | 2.046 ± 0.846 | 2.478 ± 1.206 | 2.111 ± 1.213 | 1.837 ± 1.091 |
| GSH/GSSG ratio | 2.107 ± 1.306 | 3.880 ± 2.234 | 2.465 ± 1.159 | 5.143 ± 4.009 |
| ATP (nmol/mg protein) | 0.484 ± 0.086 | 0.673 ± 0.357 | 0.382 ± 0.346 | 0.322 ± 0.074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis-Mendes, A.; Padrão, A.I.; Duarte, J.A.; Gonçalves-Monteiro, S.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice. Biomolecules 2021, 11, 1725. https://doi.org/10.3390/biom11111725
Reis-Mendes A, Padrão AI, Duarte JA, Gonçalves-Monteiro S, Duarte-Araújo M, Remião F, Carvalho F, Sousa E, Bastos ML, Costa VM. Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice. Biomolecules. 2021; 11(11):1725. https://doi.org/10.3390/biom11111725
Chicago/Turabian StyleReis-Mendes, Ana, Ana Isabel Padrão, José Alberto Duarte, Salomé Gonçalves-Monteiro, Margarida Duarte-Araújo, Fernando Remião, Félix Carvalho, Emília Sousa, Maria Lourdes Bastos, and Vera Marisa Costa. 2021. "Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice" Biomolecules 11, no. 11: 1725. https://doi.org/10.3390/biom11111725
APA StyleReis-Mendes, A., Padrão, A. I., Duarte, J. A., Gonçalves-Monteiro, S., Duarte-Araújo, M., Remião, F., Carvalho, F., Sousa, E., Bastos, M. L., & Costa, V. M. (2021). Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice. Biomolecules, 11(11), 1725. https://doi.org/10.3390/biom11111725











