Development of a Low-Molecular-Weight Aβ42 Detection System Using a Enzyme-Linked Peptide Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
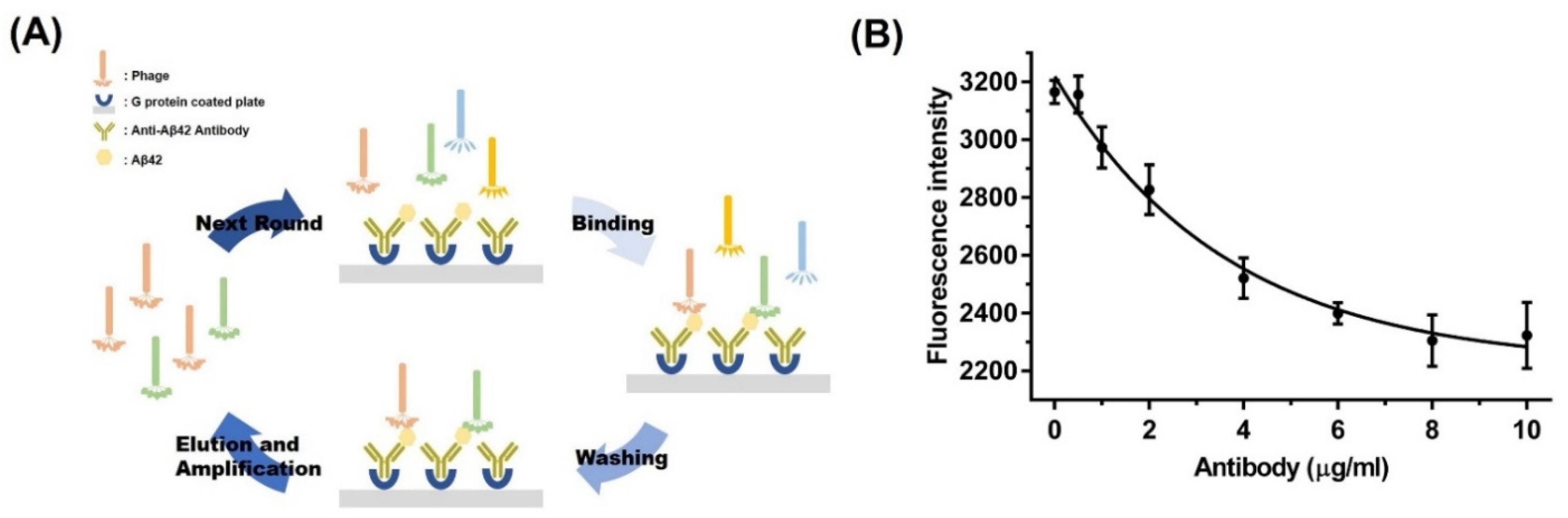
2.2. Optimization of the Binding Conditions for Phage Display
2.3. In Vitro Phage Screening against Amyloid-Beta 42
2.4. Analysis of the Screened Phages
2.5. Characterization of the Binding Properties of Synthetic Peptides
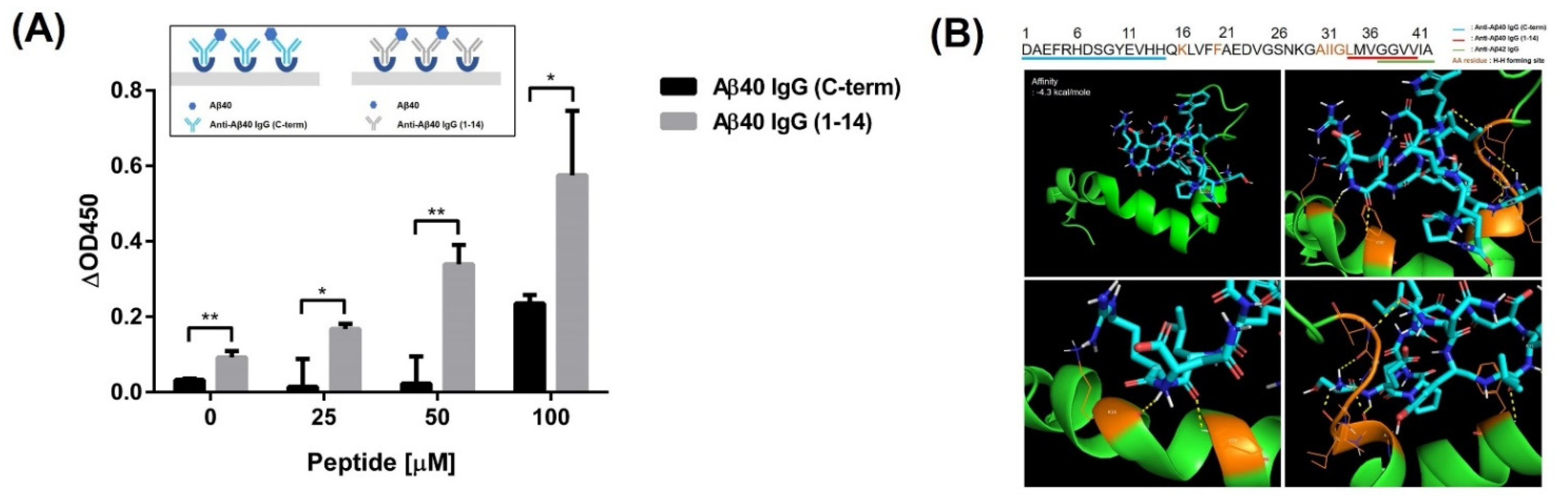
2.6. Estimation of the Binding Site of the Developed Peptides
2.7. Molecular Docking of Aβ42 and Peptide
2.8. Recovery Test of Aβ42 at Different Spiking Levels
2.9. Participants
2.10. Neuropsychological Assessment
2.11. ELISA
2.12. Verification of the Diagnostic Ability of the Peptide-Based Detection System
2.13. Statistical Analysis
3. Results
3.1. Optimization of the Binding Conditions of the Diagnostic System
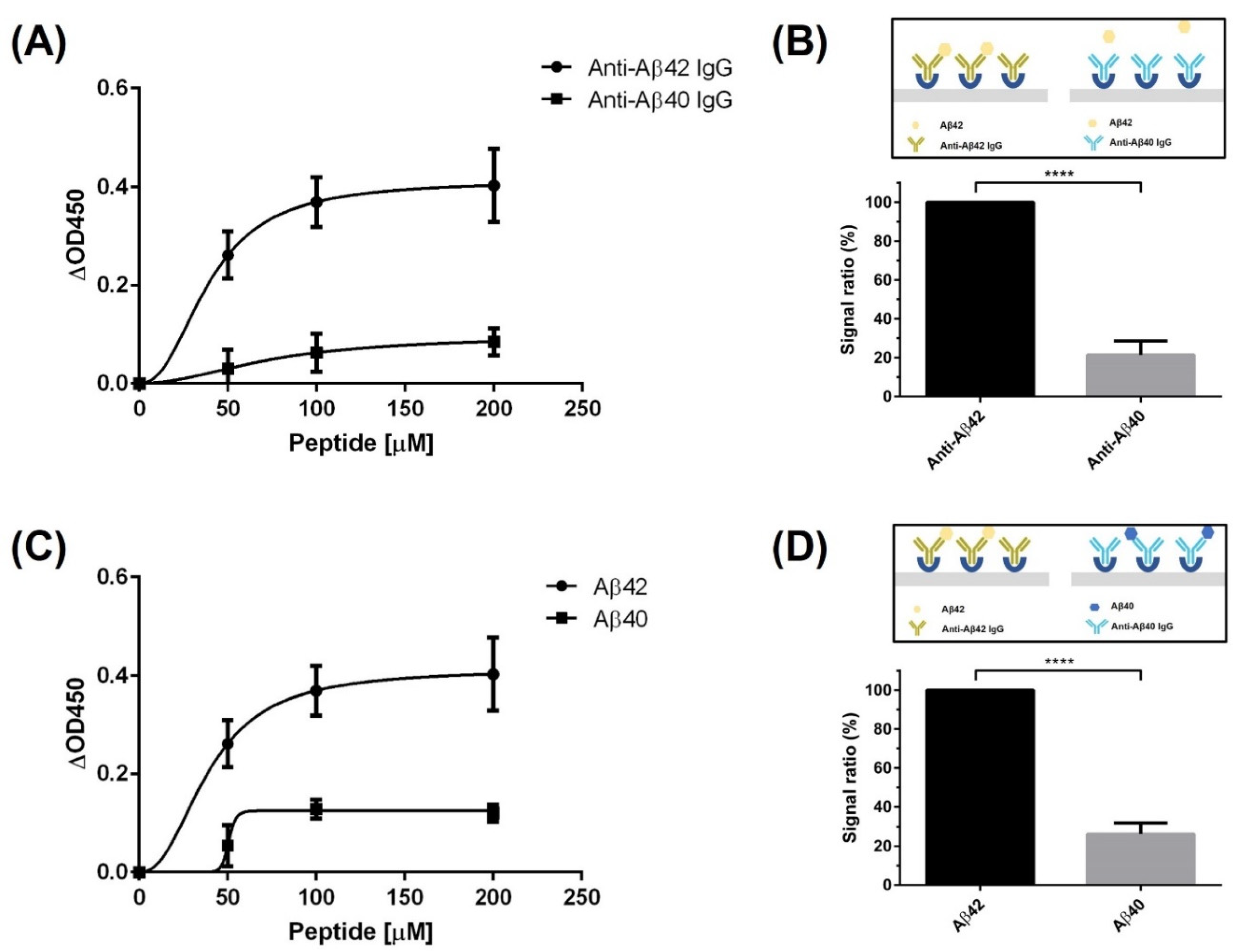
3.2. Characterization of the Peptide Using the Diagnostic System
3.3. Estimation of the Epitope Recognized by the Screened Peptide
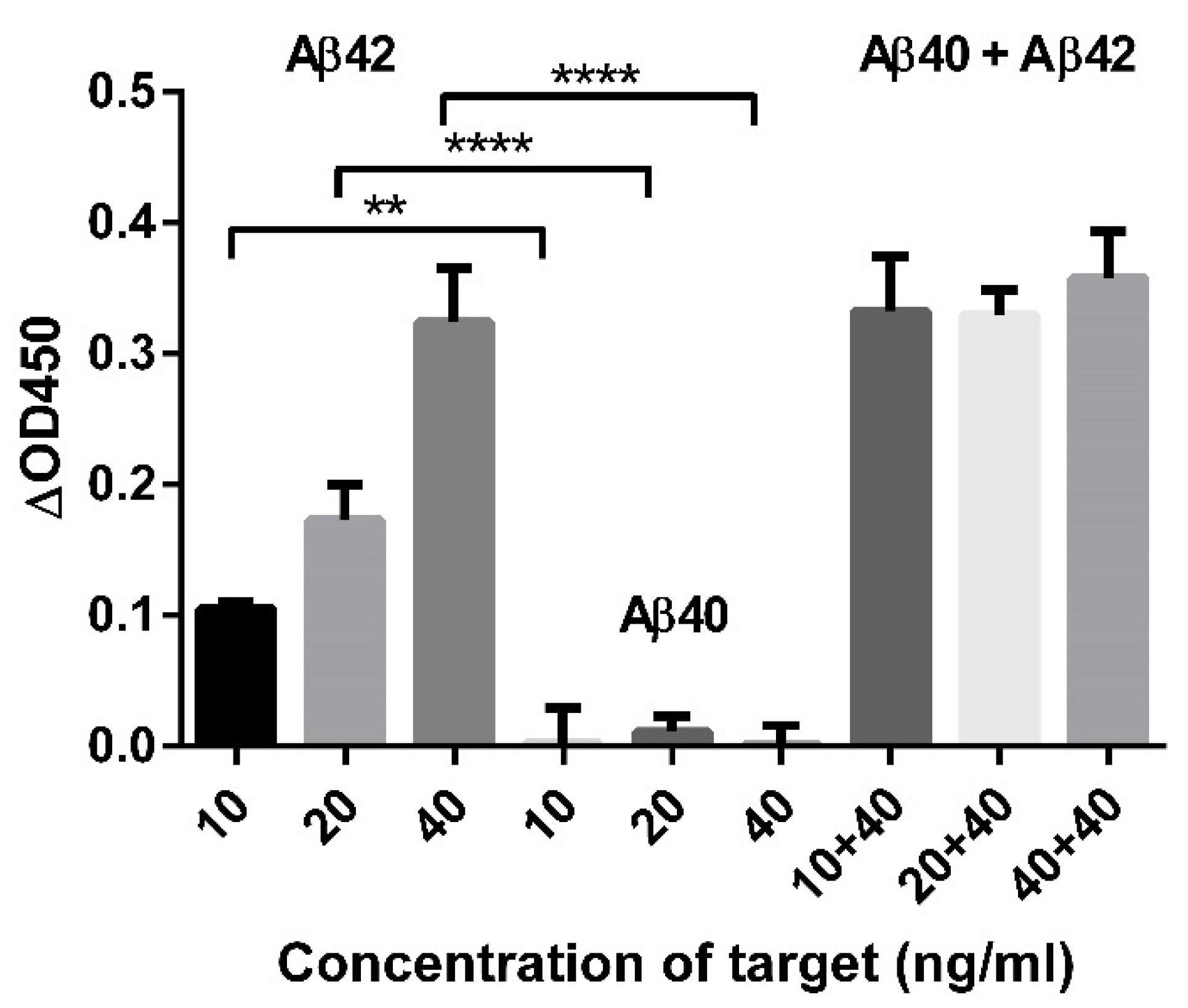
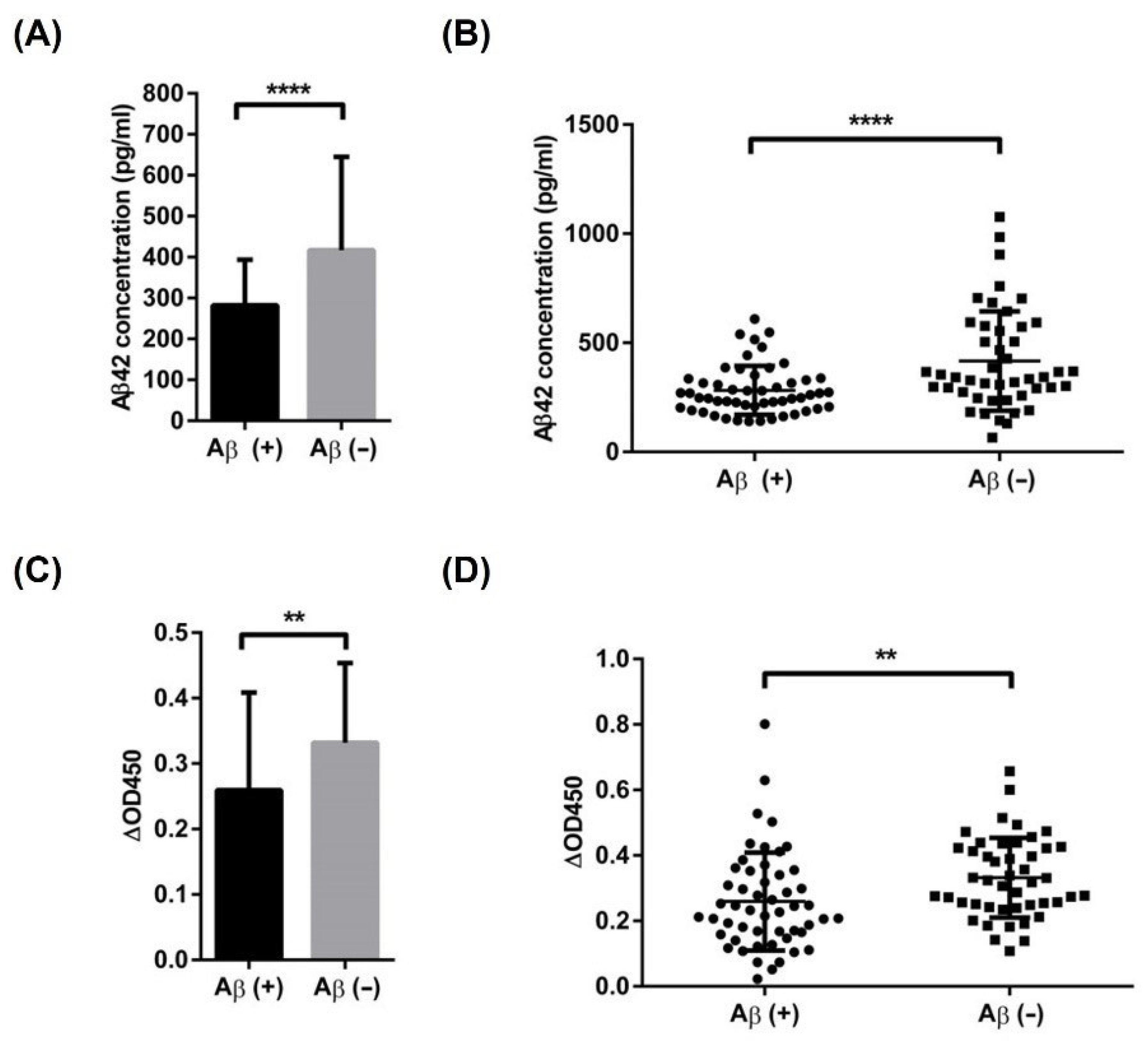
3.4. Recovery Test of Aβ42
3.5. Characteristics of Participants
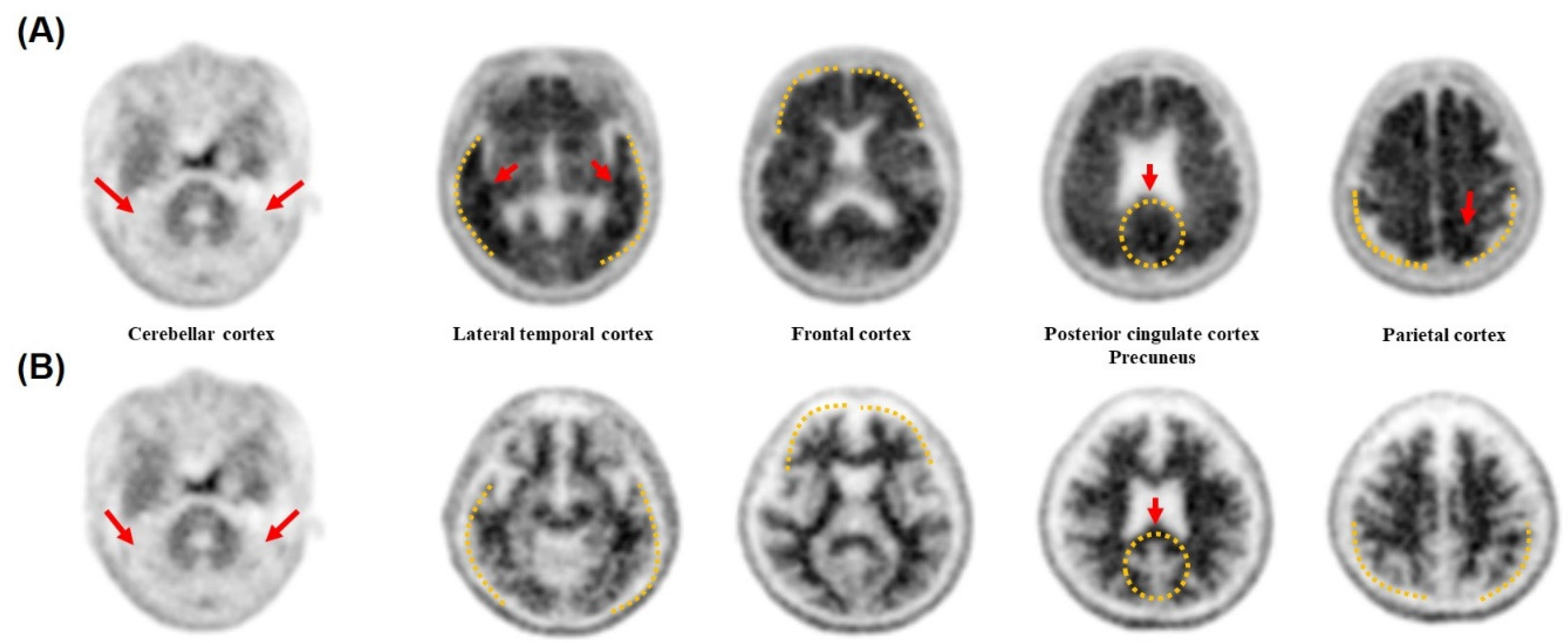
3.6. Accuracy Validation of the Diagnostic Methods by Cross Checking with the PET Images
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, S.; Chen, G.; Xu, J.; Liu, Y.; Li, G.; Chen, T.; Li, Y.; James, T.D. Current strategies for the development of fluorescence-based molecular probes for visualizing the enzymes and proteins associated with Alzheimer’s disease. Coord. Chem. Rev. 2021, 427, 213553. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, H.; Park, J.H.; Park, K.W.; Lee, K.; Kim, S.; Chae, K.; Park, M.H.; Koh, S.H.; Na, H.R. Effects of multicomponent exercise on cognitive function in elderly Korean individuals. J. Clin. Neurol. 2020, 16, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Q.; Murphy, S.L.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2018; NCHS Data Brief No. 355; National Center for Health Statistics: Hyattsville, MD, USA, 2020. [Google Scholar]
- Jang, J.W.; Park, J.H.; Kim, S.; Lee, S.H.; Lee, S.H.; Kim, Y.J. Prevalence and incidence of dementia in South Korea: A nationwide analysis of the National Health Insurance Service Senior Cohort. J. Clin. Neurol. 2021, 17, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Jeong, J.H.; Moon, S.Y.; Park, Y.K.; Hong, C.H.; Na, H.R.; Song, H.S.; Lee, S.M.; Choi, M.; Park, K.W.; et al. South Korean study to prevent cognitive impairment and protect brain health through lifestyle intervention in at-risk elderly people: Protocol of a multicenter, randomized controlled feasibility trial. J. Clin. Neurol. 2020, 16, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Alexander, G.C.; Emerson, S.; Kesselheim, A.S. Evaluation of aducanumab for Alzheimer disease: Scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA 2021, 325, 1717–1718. [Google Scholar] [CrossRef]
- Sappagh, S.E.; Saleh, H.; Sahal, R.; Abuhmed, T.; RiazulIslam, S.M.; Ali, F.; Amer, E. Alzheimer’s disease progression detection model based on an early fusion of cost-effective multimodal data. Future Gener. Comput. Syst. 2021, 115, 680–699. [Google Scholar] [CrossRef]
- Rasmussen, J.; Langerman, H. Alzheimer’s disease—Why we need early diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zetterberg, H.; Bendlin, B.B. Biomarkers for Alzheimer’s disease—Preparing for a new era of disease-modifying therapies. Mol. Psychiatry 2021, 26, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Cazarin, C.A.; Dalmagro, A.P.; Gonçalves, A.E.; Boeing, T.; Silva, L.M.D.; Corrêa, E.; Klein-Júnior, L.C.; Pinto, B.C.; Lorenzett, T.S.; Sobrinho, T.U.D.C.; et al. Usnic acid enantiomers restore cognitive deficits and neurochemical alterations induced by Aβ1–42 in mice. Behav. Brain Res. 2021, 397, 112945. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.S.; Li, L.; Sun, X.J.; Zhao, Y.W.; Zhang, J.; Geng, Z.; Fu, J.L.; Ren, Q.G. Glycogen synthase kinase-3 regulates production of amyloid-β peptides and tau phosphorylation in diabetic rat brain. Sci. World J. 2014, 2014, 878123. [Google Scholar] [CrossRef] [Green Version]
- Potter, R.; Patterson, B.W.; Elbert, D.L.; Ovod, V.; Kasten, T.; Sigurdson, W.; Mawuenyega, K.; Blazey, T.; Goate, A.; Chott, R.; et al. Increased in vivo amyloid-β 42 production, exchange, and loss in presenilin mutation carriers. Sci. Transl. Med. 2013, 5, 189ra77. [Google Scholar] [CrossRef] [Green Version]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef]
- Varvel, N.H.; Bhaskar, K.; Patil, A.R.; Pimplikar, S.W.; Herrup, K.; Lamb, B.T. Abeta oligomers induce neuronal cell cycle events in Alzheimer’s disease. J. Neurosci. 2008, 28, 10786–10793. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Mintun, M.A.; Mach, R.H.; Lee, S.Y.; Dence, C.S.; Shah, A.R.; LaRossa, G.N.; Spinner, M.L.; Klunk, W.E.; Mathis, C.A.; et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann. Neurol. 2006, 59, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, T.; Riemenschneider, M.; Förstl, H.; Henriksen, G.; Klunk, W.E.; Mathis, C.A.; Shiga, T.; Wester, H.J.; Kurz, A.; Drzezga, A. Beta amyloid in Alzheimer’s disease: Increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol. Psychiatry 2009, 65, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strozyk, D.; Blennow, K.; White, L.R.; Launer, L.J. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 2003, 60, 652–656. [Google Scholar] [CrossRef]
- Tapiola, T.; Alafuzoff, I.; Herukka, S.K.; Parkkinen, L.; Hartikainen, P.; Soininen, H.; Pirttilä, T. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 2009, 66, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Tolboom, N.; Flier, W.M.V.D.; Yaqub, M.; Boellaard, R.; Verwey, N.A.; Blankenstein, M.A.; Windhorst, A.D.; Scheltens, P.; Lammertsma, A.A.; Berckel, B.N.M.V. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J. Nucl. Med. 2009, 50, 1464–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.Y.; Kim, M.J.; Kim, D.W.; Kim, S.Y.; Park, S.; Park, C.B. Clinically accurate diagnosis of Alzheimer’s disease via multiplexed sensing of core biomarkers in human plasma. Nat. Commun. 2020, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Klafki, H.W.; Rieper, P.; Matzen, A.; Zampar, S.; Wirths, O.; Vogelgsang, J.; Osterloh, D.; Rohdenburg, L.; Oberstein, T.J.; Jahn, O.; et al. Development and technical validation of an immunoassay for the detection of app669–711 (Aβ−3–40) in biological samples. Int. J. Mol. Sci. 2020, 21, 6564. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.H.; Hung, J.T.; Lu, Y.J.; Cheng, C.M. Paper-based detection device for Alzheimer’s disease—Detecting β-amyloid peptides (1–42) in human plasma. Diagnostics 2020, 10, 272. [Google Scholar] [CrossRef]
- Aprile, F.A.; Sormanni, P.; Podpolny, M.; Chhangur, S.; Needham, L.M.; Ruggeri, F.S.; Perni, M.; Limbocker, R.; Heller, G.T.; Sneideris, T.; et al. Rational design of a conformation-specific antibody for the quantification of Aβ oligomers. Proc. Natl. Acad. Sci. USA 2020, 117, 13509–13518. [Google Scholar] [CrossRef]
- Yang, G.; Velgos, S.N.; Boddapati, S.P.; Sierks, M.R. Probing antibody-antigen interactions. Microbiol. Spectr. 2014, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.J.; Yi, S.H.; Han, J.Y.; Park, S.Y.; Jang, J.W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S.; et al. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, L.; Sobott, F.; Deyn, P.P.D.; Dam, D.V. Signal loss due to oligomerization in ELISA analysis of amyloid-beta can be recovered by a novel sample pre-treatment method. MethodX 2015, 2, 112–123. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, E.H.; Lee, S.C.; Kim, A.R.; Park, H.H.; Son, J.W.; Koh, S.H.; Yoon, M.Y. Development of peptide aptamers as alternatives for antibody in the detection of amyloid-beta 42 aggregates. Anal. Biochem. 2020, 609, 113921. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Jang, S.C.; Chung, J.; Choi, W.K.; Hong, C.; Ahn, G.R.; Kim, S.H.; Lee, B.Y.; Chung, W.J. Colorimetric allergenic fungal spore detection using peptide-modified gold nanoparticles. Sens. Actuators B Chem. 2021, 327, 128894. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, J.Y.; Wang, Y.T.; Zhang, H.C.; Chen, M.L.; Yang, T.; Wang, J.H. M13 phage-based nanoprobe for SERS detection and inactivation of Staphylococcus aureus. Talanta 2021, 221, 121668. [Google Scholar] [CrossRef]
- Feng, S.; Shi, R.; Xu, P.; Bhamore, J.R.; Bal, J.; Baek, S.H.; Park, C.Y.; Park, J.P.; Park, T.J. Colorimetric detection of creatinine using its specific binding peptides and gold nanoparticles. New J. Chem. 2020, 44, 15828–15835. [Google Scholar] [CrossRef]
- Colombo, M.; Mizzotti, C.; Masiero, S.; Kater, M.M.; Pesaresi, P. Peptide aptamers: The versatile role of specific protein function inhibitors in plant biotechnology. J. Integr. Plant Biol. 2015, 57, 892–901. [Google Scholar] [CrossRef]
- Sachdeva, S.; Joo, H.; Tsai, J.; Jasti, B.; Li, X. A rational approach for creating peptides mimicking antibody binding. Sci. Rep. 2019, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Reverdatto, S.; Burz, D.S.; Shekhtman, A. Peptide aptamers: Development and applications. Curr. Top. Med. Chem. 2015, 15, 1082–1101. [Google Scholar] [CrossRef] [Green Version]
- De, S.; Whiten, D.R.; Ruggeri, F.S.; Hughes, C.; Rodrigues, M.; Sideris, D.I.; Taylor, C.G.; Aprile, F.A.; Muyldermans, S.; Knowles, T.P.J.; et al. Soluble aggregates present in cerebrospinal fluid change in size and mechanism of toxicity during Alzheimer’s disease progression. Acta Neuropathol. Commun. 2019, 7, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glucksman, M.J.; Bhattacharjee, S.; Makowski, L. Three-dimensional structure of a cloning vector. X-ray diffraction studies of filamentous bacteriophage M13 at 7 A resolution. J. Mol. Biol. 1992, 226, 455–470. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, S.H.; Hoffmeister, R.A.; Yoon, M.Y.; Kim, S.K. Novel peptide-based inhibitors for microtubule polymerization in Phytophthora capsica. Int. J. Mol. Sci. 2019, 20, 2641. [Google Scholar] [CrossRef] [Green Version]
- Pande, J.; Szewczyk, M.M.; Grover, A.K. Phage display: Concept, innovations, applications and future. Biotechnol. Adv. 2010, 28, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Stine, W.B.; Jungbauer, L.; Yu, C.; Ladu, M.J. Preparing synthetic Aβ in different aggregation states. Methods Mol. Biol. 2011, 670, 13–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuta, T.; Asai, T.; Kiyokawa, Y.; Nakada, T.; Bessyo-Hirashima, K.; Fukaya, N.; Hyodo, K.; Takase, K.; Kikuchi, H.; Oku, N. Targeted delivery of anticancer drugs to tumor vessels by use of liposomes modified with a peptide identified by phage biopanning with human endothelial progenitor cells. Int. J. Pharm. 2017, 524, 364–372. [Google Scholar] [CrossRef]
- Cho, J.H.; Ha, N.R.; Koh, S.H.; Yoon, M.Y. Design of a PKCδ-specific small peptide as a theragnostic agent for glioblastoma. Anal. Biochem. 2016, 496, 63–70. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multi-threading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaselli, S.; Esposito, V.; Vangone, P.; Nuland, N.A.V.; Bonvin, A.M.; Guerrini, R.; Tancredi, T.; Temussi, P.A.; Picone, D. The alpha-to-beta conformational transition of Alzheimer’s Abeta-(1-42) peptide in aqueous media is reversible: A step by step conformational analysis suggests the location of beta conformation seeding. Chembiochem 2006, 7, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Sabri, O.; Seibyl, J.; Rowe, C.; Barthelt, H. Beta-amyloid imaging with florbetaben. Clin. Transl. Imaging 2015, 3, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, K.E.; Jeong, Y.J.; Park, K.W.; Kang, D.Y. Cerebral amyloid quantification in cognitively normal Korean adults using F-18 Florbetaben PET. Nucl. Med. Mol. Imaging 2019, 53, 334–339. [Google Scholar] [CrossRef]
- Bullich, S.; Seibyl, J.; Catafau, A.M.; Jovalekic, A.; Koglin, N.; Barthel, H.; Sabri, O.; Santi, S.D. Optimized classification of 18F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment. Neuroimage Clin. 2017, 15, 325–332. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Welch, N.G. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [Green Version]
- Gil, J.P.; Cruz, A.; Casals, C. Solubility of hydrophobic surfactant proteins in organic solvent/water mixtures. Structural studies on SP-B and SP-C in aqueous organic solvents and lipids. Biochim. Biophys. Acta 1993, 1168, 261–270. [Google Scholar]
- Nie, X.; Chen, Z.; Tian, Y.; Chen, S.; Qu, L.; Fan, M. Rapid detection of trace formaldehyde in food based on surface-enhanced Raman scattering coupled with assembled purge trap. Food Chem. 2020, 340, 127930. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.S.; Gnanapragasam, P.N.P.; Galimidi, R.P.; Foglesong, C.P.; West, A.P., Jr.; Bjorkman, P.J. Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc. Natl. Acad. Sci. USA 2009, 106, 7385–7390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villemagne, V.L.; Ong, K.; Mulligan, R.S.; Holl, G.; Pejoska, S.; Jones, G.; O’Keefe, G.; Ackerman, U.; Danguy, H.T.; Chan, J.G.; et al. Amyloid imaging with 18F-florbetaben in Alzheimer disease and other dementias. J. Nucl. Med. 2011, 52, 1210–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanekiyo, T.; Xu, H.; Bu, G. ApoE and Aβ in Alzheimer’s disease: Accidental encounters or partners? Neuron 2014, 81, 740–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldeiras, I.; Santana, I.; Leitão, M.J.; Ribeiro, M.H.; Pascoal, R.; Duro, D.; Lemos, R.; Santiago, B.; Almeida, M.R.; Oliveira, C.R. Cerebrospinal fluid Aβ40 is similarly reduced in patients with frontotemporal lobar degeneration and Alzheimer’s Disease. J. Neurol. Sci. 2015, 358, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, T.Q.; Andreasen, N.; Wiberg, M.K.; Westman, E.; Wahlund, L.O. Ratio of Aβ42/P-tau181p in CSF is associated with aberrant default mode network in AD. Sci. Rep. 2013, 3, 1339. [Google Scholar] [CrossRef] [PubMed]

| Round # | Binding Condition | Binding Time (min) | Washing Conditions |
|---|---|---|---|
| 1R | TBS | 120 | 0.1% TBST |
| 2R | TBS | 60 | 0.1% TBST |
| 3R | TBS | 60 | 0.1% TBST |
| Negative | TBS + 0.5% BSA | 60 | |
| 4R | TBS + 0.5% BSA | 30 | 0.1% TBST, 500 mM NaCl |
| Phage # | Sequence | Frequency (30/60) |
|---|---|---|
| 9 | S E P Q N I W Q Y L R N | 20 |
| 21 | I W M T R T N L N D V N | 10 |
| Concentration of Target (Aβ40 + Aβ42, ng/mL) | Average Recovery (%) | Standard Deviation (%) | %RSD |
|---|---|---|---|
| 10 + 40 | 97.02 | 12.46 | 12.84 |
| 20 + 40 | 96.04 | 5.81 | 6.053 |
| 40 + 40 | 104.5 | 10.51 | 10.15 |
| Amyloid-Negative | Amyloid-Positive | ||
|---|---|---|---|
| Population | Sex, n (female) | 46 (21) | 52 (32) |
| Age (SD), y * | 71.67 (8.57) | 66.73 (10.36) | |
| Education level (SD), y | 11.67 (4.68) | 11.41 (4.77) | |
| Disease duration (SD), m | 28.52 (28.89) | 35.62 (27.10) | |
| ApoE ε4 carrier, n (%) ** | 9 (23.1) | 30 (76.9) | |
| Underlying disease | DM, n (%) * | 22 (46.9) | 10 (19.2) |
| HTN, n (%) | 21 (45.7) | 25 (54.3) | |
| Hyperlipidemia, n (%) | 18 (40.9) | 26 (59.1) | |
| Global cognition | MMSE (SD) | 23.91 (4.70) | 22.00 (4.90) |
| CDR (SD) | 0.69 (0.52) | 0.72 (0.45) | |
| Laboratory test | Neutrophil, % (SD) | 56.42 (9.09) | 56.64 (8.91) |
| Monocyte, % (SD) | 8.15 (1.64) | 11.05 (19.01) | |
| ESR (SD), mm/h * | 15.29 (11.65) | 10.91 (7.00) | |
| CRP (SD) | 0.23 (0.46) | 0.21 (0.48) | |
| LDL (SD), mg/dL * | 104.68 (39.24) | 127.03 (38.21) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Lee, E.-H.; Kim, H.-J.; Kim, A.-R.; Kim, Y.-E.; Lee, J.-H.; Yoon, M.-Y.; Koh, S.-H. Development of a Low-Molecular-Weight Aβ42 Detection System Using a Enzyme-Linked Peptide Assay. Biomolecules 2021, 11, 1818. https://doi.org/10.3390/biom11121818
Kim S-H, Lee E-H, Kim H-J, Kim A-R, Kim Y-E, Lee J-H, Yoon M-Y, Koh S-H. Development of a Low-Molecular-Weight Aβ42 Detection System Using a Enzyme-Linked Peptide Assay. Biomolecules. 2021; 11(12):1818. https://doi.org/10.3390/biom11121818
Chicago/Turabian StyleKim, Sang-Heon, Eun-Hye Lee, Hyung-Ji Kim, A-Ru Kim, Ye-Eun Kim, Jae-Hong Lee, Moon-Young Yoon, and Seong-Ho Koh. 2021. "Development of a Low-Molecular-Weight Aβ42 Detection System Using a Enzyme-Linked Peptide Assay" Biomolecules 11, no. 12: 1818. https://doi.org/10.3390/biom11121818
APA StyleKim, S.-H., Lee, E.-H., Kim, H.-J., Kim, A.-R., Kim, Y.-E., Lee, J.-H., Yoon, M.-Y., & Koh, S.-H. (2021). Development of a Low-Molecular-Weight Aβ42 Detection System Using a Enzyme-Linked Peptide Assay. Biomolecules, 11(12), 1818. https://doi.org/10.3390/biom11121818







