The Role of Estrogens and Vitamin D in Cardiomyocyte Protection: A Female Perspective
Abstract
1. Introduction
2. Cardiovascular Health: A Sex Hormone Matter
Estrogens in Males, Androgens in Females
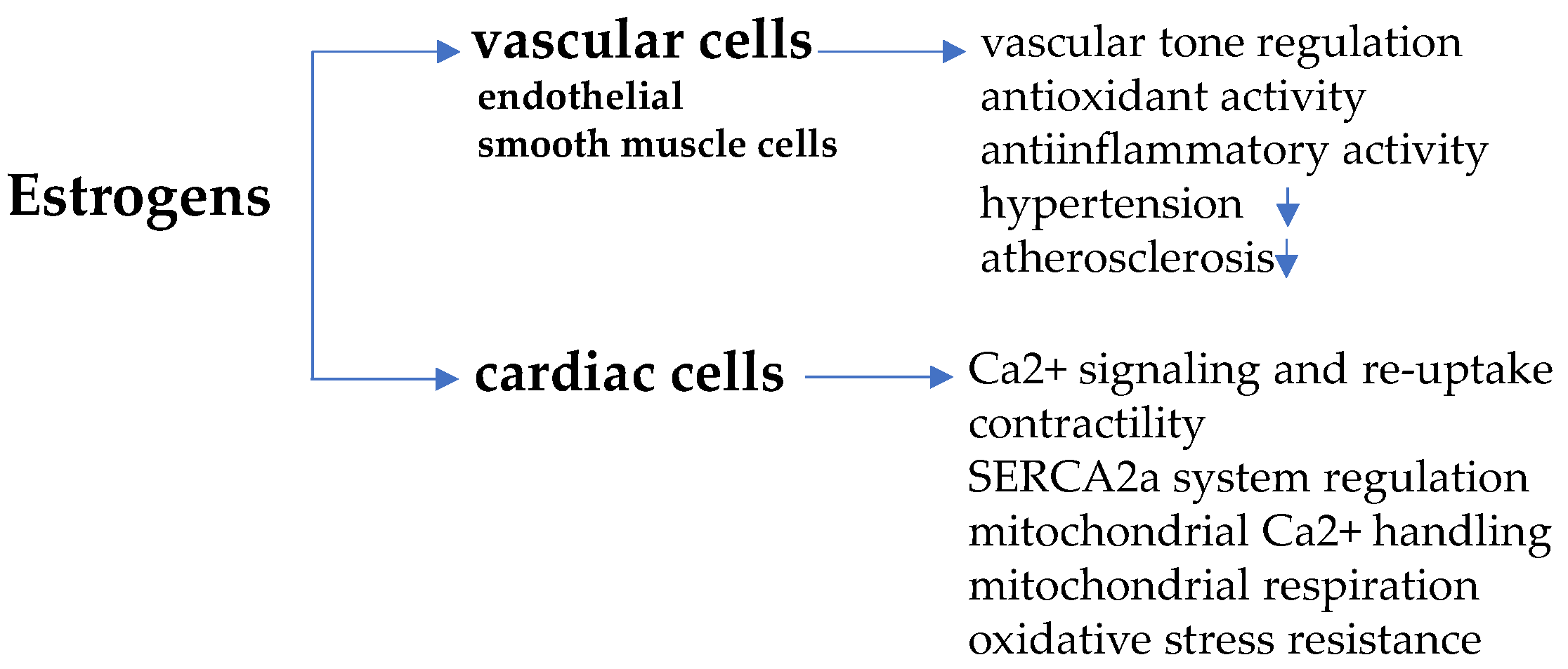
3. Estrogens and Cardiomyocyte Remodeling
3.1. Estrogen Receptors
3.2. Sex-Dimorphic Mitochondrial Function
4. Cardiovascular Health beyond Sex Hormones: Vitamin D Matters
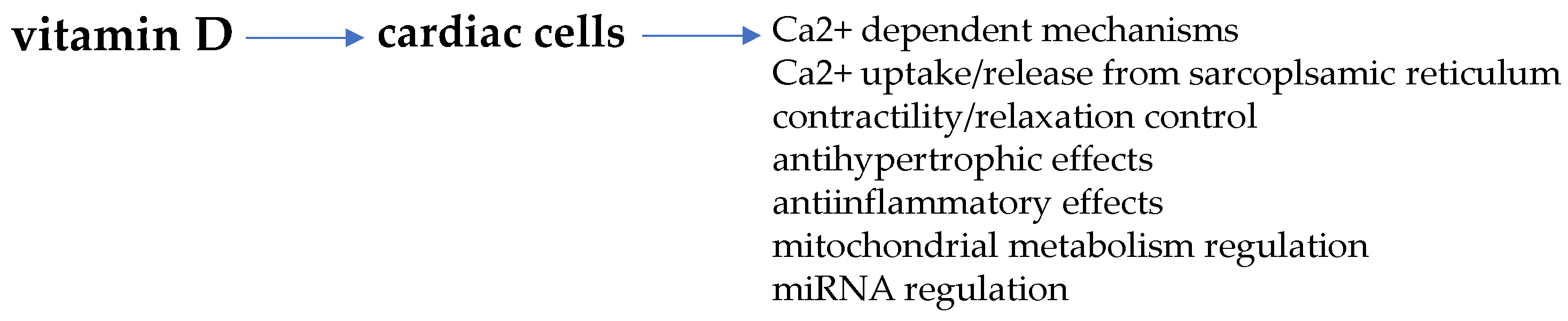
5. Vitamin D and Cardiomyocyte Remodeling
Vitamin D and Sex-Dimorphic Cardiac Metabolic Flexibility
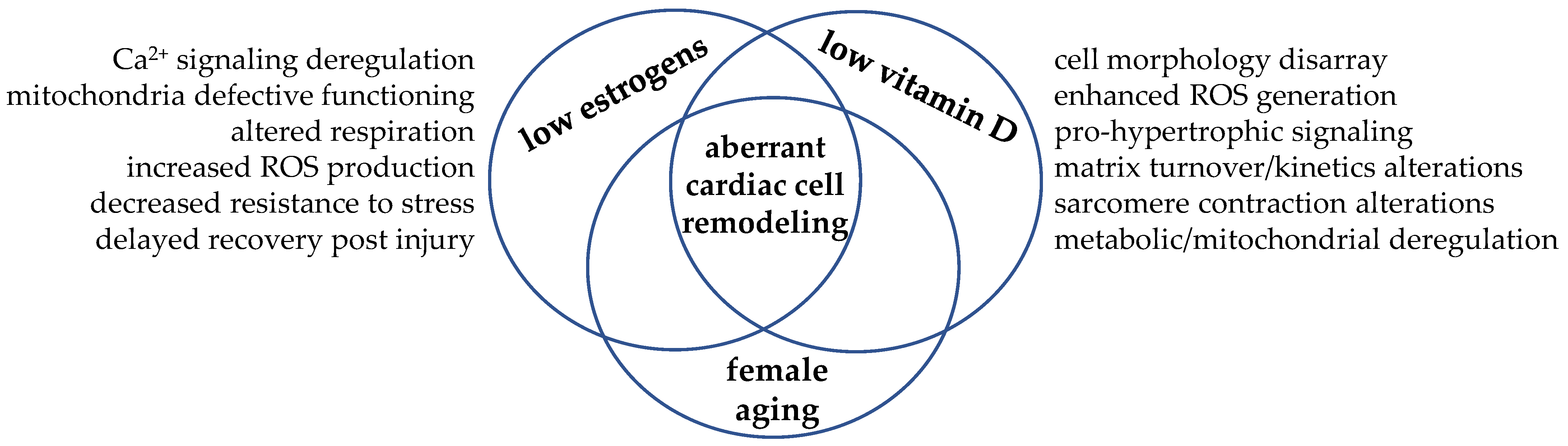
6. Vitamin D and Estrogen Cooperation: Learning from Examples
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Merz, A.A.; Cheng, S. Sex differences in cardiovascular ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Crandall, C.J.; Barrett-Connor, E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: A systematic review. Endocrinol. Metab. Clin. 2013, 42, 227–253. [Google Scholar] [CrossRef]
- Kessler, E.L.; Rivaud, M.R.; Vos, M.A.; Van Veen, T.A. Sex-specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol. Sex Differ. 2019, 10, 7. [Google Scholar] [CrossRef]
- Miller, R.J.H.; Mikami, Y.; Heydari, B.; Wilton, S.B.; James, M.T.; Howarth, A.G.; White, J.A.; Lydell, C.P. Sex-specific relationships between patterns of ventricular remodelling and clinical outcomes. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 983–990. [Google Scholar] [CrossRef]
- Oneglia, A.; Nelson, M.D.; Merz, C.N.B. Sex Differences in Cardiovascular Aging and Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Piro, M.; Bona, D.R.; Abbate, A.; Biasucci, L.M.; Crea, F. Sex-related differences in myocardial remodeling. J. Am. Coll. Cardiol. 2010, 55, 1057–1065. [Google Scholar] [CrossRef]
- Kararigas, G.; Bito, V.; Tinel, H.; Becher, E.; Baczko, I.; Knosalla, C.; Albrecht-Küpper, B.; Sipido, K.R.; Regitz-Zagrosek, V. Transcriptome Characterization of Estrogen-Treated Human Myocardium Identifies Myosin Regulatory Light Chain Interacting Protein as a Sex-Specific Element Influencing Contractile Function. J. Am. Coll. Cardiol. 2012, 59, 410–417. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef]
- Kim, I.M.; Norris, K.C.; Artaza, J.N. Vitamin D and cardiac differentiation. Vitam. Horm. 2016, 100, 299–320. [Google Scholar] [CrossRef]
- Sithara, T.; Drosatos, K. Metabolic Complications in Cardiac Aging. Front. Physiol. 2021, 12, 579. [Google Scholar] [CrossRef]
- Filardi, T.; Ghinassi, B.; Di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019, 20, 3299. [Google Scholar] [CrossRef]
- Ren, J.; Ceylan-Isik, A.F. Diabetic cardiomyopathy. Endocrine 2004, 25, 73–83. [Google Scholar] [CrossRef]
- Ren, J.; Kelley, R.O. Cardiac health in women with metabolic syndrome: Clinical aspects and pathophysiology. Obesity 2009, 17, 1114–1123. [Google Scholar] [CrossRef]
- Adekunle, A.O.; Adzika, G.K.; Mprah, R.; Ndzie Noah, M.L.; Adu-Amankwaah, J.; Rizvi, R.; Akhter, N.; Sun, H. Predominance of Heart Failure with Preserved Ejection Fraction in Postmenopausal Women: Intra-and Extra-Cardiomyocyte Maladaptive Alterations Scaffolded by Estrogen Deficiency. Front. Cell Dev. Biol. 2021, 9, 2729. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Farhat, M.Y.; Lavigne, M.C.; Ramwell, P.W. The vascular protective effects of estrogen. FASEB J. 1996, 10, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Rafikova, O.; Sullivan, J.C. Estrogen: Good, bad, or both? Hypertension 2014, 63, 449–450. [Google Scholar] [CrossRef][Green Version]
- Nită, A.R.; Knock, G.A.; Heads, R.J. Signalling Mechanisms in the Cardiovascular Protective Effects of Estrogen: With a focus on rapid/membrane signalling. Curr. Res. Physiol. 2021, 4, 103–118. [Google Scholar] [CrossRef]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.Å. Mechanisms of Estrogen Action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef] [PubMed]
- Menazza, S.; Murphy, E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ. Res. 2016, 118, 994–1007. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Budish, R.A.; Kashyap, S.; Lindsey, S.H. GPER-novel membrane estrogen receptor. Clin. Sci. 2016, 130, 1005. [Google Scholar] [CrossRef]
- Nakanishi, R.; Baskaran, L.; Gransar, H.; Budoff, M.J.; Achenbach, S.; Al-Mallah, M.; Cademartiri, F.; Callister, T.Q.; Chang, H.; Chinnaiyan, K.; et al. Relationship of Hypertension to Coronary Atherosclerosis and Cardiac Events in Patients With Coronary Computed Tomographic Angiography. Hypertension 2017, 70, 293–299. [Google Scholar] [CrossRef]
- Austin, C.E. Chronic and acute effects of oestrogens on vascular contractility. J. Hypertens. 2000, 18, 1365–1378. [Google Scholar] [CrossRef]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2019, 49, 488. [Google Scholar] [CrossRef]
- Vandenplas, G.; De Bacquer, D.; Calders, P.; Fiers, T.; Kaufman, J.M.; Ouwens, D.M.; Ruige, J.B. Endogenous oestradiol and cardiovascular disease in healthy men: A systematic review and meta-analysis of prospective studies. Heart 2012, 98, 1478–1482. [Google Scholar] [CrossRef]
- Carani, C.; Qin, K.; Simoni, M.; Faustini-Fustini, M.; Serpente, S.; Boyd, J.; Korach, K.; Simpson, E.R. Effect of Testosterone and Estradiol in a Man with Aromatase Deficiency. N. Engl. J. Med. 1997, 337, 91–95. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K. Estrogen Resistance Caused by a Mutation in the Estrogen-Receptor Gene in a Man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef]
- Morishima, A.K.I.R.A.; Grumbach, M.M.; Simpson, E.R.; Fisher, C.; Qin, K.E.N.A.N. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995, 80, 3689–3698. [Google Scholar] [CrossRef]
- Vikan, T.; Schirmer, H.; Njølstad, I.; Svartberg, J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur. J. Endocrinol. 2010, 162, 747. [Google Scholar] [CrossRef][Green Version]
- Sudhir, K.; Chou, T.M.; Messina, L.M.; Hutchison, S.J.; Korach, K.S.; Chatterjee, K.; Rubanyi, G.M. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet 1977, 349, 1146–1147. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Ärnlöv, J.; Pencina, M.J.; Amin, S.; Nam, B.-H.; Benjamin, E.J.; Murabito, J.M.; Wang, T.J.; Knapp, P.E.; D’Agostino, R.B.; Bhasin, S.; et al. Endogenous sex hormones and cardiovascular disease incidence in men. Ann. Intern. Med. 2006, 145, 176–184. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Kannel, W.B. The Framingham Study: Historical insight on the impact of cardiovascular risk factors in men versus women. J. Gend. Specif. Med. JGSM Off. J. Partnersh. Women’s Health Columbia 2002, 5, 27–37. [Google Scholar]
- Subramanian, M.; Balasubramanian, P.; Garver, H.; Northcott, C.; Zhao, H.; Haywood, J.R.; Fink, G.D.; Mohankumar, S.M.J.; Mohankumar, P.S. Chronic estradiol-17β exposure increases superoxide production in the rostral ventrolateral medulla and causes hypertension: Reversal by resveratrol. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R1560–R1568. [Google Scholar] [CrossRef]
- Gurney, E.P.; Nachtigall, M.J.; Nachtigall, L.E.; Naftolin, F. The Women’s Health Initiative trial and related studies: 10 years later: A clinician’s view. J. Steroid Biochem. Mol. Biol. 2014, 142, 4–11. [Google Scholar] [CrossRef]
- Dehaini, H.; Fardoun, M.; Abou-Saleh, H.; El-Yazbi, A.; Eid, A.A.; Eid, A.H. Estrogen in vascular smooth muscle cells: A friend or a foe? Vasc. Pharmacol. 2018, 111, 15–21. [Google Scholar] [CrossRef]
- Manson, J.E. The ‘timing hypothesis’ for estrogen therapy in menopausal symptom management. Women’s Health 2015, 11, 437–440. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Shoupe, D.; Azen, S.P.; Stanczyk, F.Z.; Hwang-Levine, J.; Budoff, M.J.; Henderson, V.W. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause 2015, 22, 391–401. [Google Scholar] [CrossRef]
- Ueda, K.; Fukuma, N.; Adachi, Y.; Numata, G.; Tokiwa, H.; Toyoda, M.; Otani, A.; Hashimoto, M.; Liu, P.Y.; Takimoto, E. Sex Differences and Regulatory Actions of Estrogen in Cardiovascular System. Front. Physiol. 2021, 12, 738218. [Google Scholar] [CrossRef]
- Korytkowski, M.T.; Krug, E.I.; Daly, M.A.; DeRiso, L.; Wilson, J.W.; Winters, S.J. Does androgen excess contribute to the cardiovascular risk profile in postmenopausal women with type 2 diabetes? Metabolism 2005, 54, 1626–1631. [Google Scholar] [CrossRef]
- Macut, D.; Antić, I.B.; Bjekić-Macut, J. Cardiovascular risk factors and events in women with androgen excess. J. Endocrinol. Investig. 2015, 38, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Kim, J.Y.; Wan, C.; Xiong, J.D.; Parry, S.A.; Azziz, R.; Lujan, M.E. Comprehensive evaluation of disparities in cardiometabolic and reproductive risk between Hispanic and White women with polycystic ovary syndrome in the United States: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Grohé, C.; Kahlert, S.; Löbbert, K.; Stimpel, M.; Karas, R.H.; Vetter, H.; Neyses, L. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1977, 416, 107–112. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Eder, S.; Nordmeyer, J.; Ehler, E.; Huber, O.; Martus, P.; Weiske, J.; Pregla, R.; Hetzer, R.; Regitz-Zagrosek, V. Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J. 2006, 20, 926–934. [Google Scholar] [CrossRef]
- Taylor, A.H.; Al-Azzawi, F. Immunolocalisation of oestrogen receptor beta in human tissues. J. Mol. Endocrinol. 2020, 24, 145–155. [Google Scholar] [CrossRef]
- Lizotte, E.; Grandy, S.A.; Tremblay, A.; Allen, B.G.; Fiset, C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell. Physiol. Biochem. 2009, 23, 075–086. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef]
- Ropero, A.B.; Eghbali, M.; Minosyan, T.Y.; Tang, G.; Toro, L.; Stefani, E. Heart estrogen receptor alpha: Distinct membrane and nuclear distribution patterns and regulation by estrogen. J. Mol. Cell. Cardiol. 2016, 41, 496–510. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Leber, J.; Zhang, X.; Jaisser, F.; Messaoudi, S.; Morano, I.; Furth, P.A.; Dworatzek, E.; Regitz-Zagrosek, V. Cardiomyocyte-specific estrogen receptor alpha increases angiogenesis, lymphangiogenesis and reduces fibrosis in the female mouse heart post-myocardial infarction. J. Cell Sci. Ther. 2014, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, S.; Whitehead, T.; Schweitzer, G.G.; Fettig, N.; Kovacs, A.; Korach, K.S.; Finck, B.N.; Shoghi, K.I. An Animal Model with a Cardiomyocyte-Specific Deletion of Estrogen Receptor Alpha: Functional, Metabolic, and Differential Network Analysis. PLoS ONE 2014, 9, e101900. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Nguyen, B.T.; Jarry, H. Estrogen modulates cardiac growth through an estrogen receptor α-dependent mechanism in healthy ovariectomized mice. Mol. Cell. Endocrinol. 2014, 382, 909–914. [Google Scholar] [CrossRef]
- Schuster, I.; Mahmoodzadeh, S.; Dworatzek, E.; Jaisser, F.; Messaoudi, S.; Morano, I.; Regitz-Zagrosek, V. Cardiomyocyte-specific overexpression of oestrogen receptor β improves survival and cardiac function after myocardial infarction in female and male mice. Clin. Sci. 2016, 130, 365–376. [Google Scholar] [CrossRef]
- Forster, C.; Kietz, S.; Hultenby, K.; Warner, M.; Gustafsson, J.-A. Characterization of the ERβ−/-mouse heart. Proc. Natl. Acad. Sci. USA 2004, 101, 14234–14239. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Weil, B.; Abarbanell, A.; Herrmann, J.; Tan, J.; Kelly, M.; Meldrum, D.R. Estrogen receptor β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R972–R978. [Google Scholar] [CrossRef]
- Deschamps, A.; Murphy, E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am. J. Physiol. Circ. Physiol. 2009, 297, H1806–H1813. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Chou, J.; Lin, M.; Ferrario, C.M.; Zapata-Sudo, G.; Groban, L. Cardiomyocyte-specific deletion of the G protein-coupled estrogen receptor (GPER) leads to left ventricular dysfunction and adverse remodeling: A sex-specific gene profiling analysis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Chou, J.; Lin, M.; Ferrario, C.M.; Zapata-Sudo, G.; Groban, L. Inflammatory and mitochondrial gene expression data in GPER-deficient cardiomyocytes from male and female mice. Data Brief 2017, 10, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Jazbutyte, V.; Kehl, F.; Neyses, L.; Pelzer, T. Estrogen receptor alpha interacts with 17β-hydroxysteroid dehydrogenase type 10 in mitochondria. Biochem. Biophys. Res. Commun. 2009, 384, 450–454. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, R.; Perez, E.J.; Wen, Y.; Stevens, S.M.; Valencia, T.; Brun-Zinkernagel, A.-M.; Prokai, L.; Will, Y.; Dykens, J.; et al. Mitochondrial localization of estrogen receptor β. Proc. Natl. Acad. Sci. USA 2004, 101, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R.; Veldkamp, M.W.d.G.W.; Kirkels, J.H.; Tan, H.L. Gender disparities in cardiac cellular electrophysiology and arrhythmia susceptibility in human failing ventricular myocytes. Int. Heart J. 2005, 46, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Papp, R.; Bett, G.C.; Lis, A.; Rasmusson, R.L.; Baczkó, I.; Varró, A.; Salama, G. Genomic upregulation of cardiac Cav1. 2α and NCX1 by estrogen in women. Biol. Sex Differ. 2017, 8, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Curl, C.L.; Delbridge, L.M.; Wendt, I.R. Sex differences in cardiac muscle responsiveness to Ca2+ and L-type Ca2+ channel modulation. Eur. J. Pharmacol. 2008, 586, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Altered cardiac myocyte Ca regulation in heart failure. Physiology 2006, 21, 380–387. [Google Scholar] [CrossRef]
- Turdi, S.; Huff, A.F.; Pang, J.; He, E.Y.; Chen, X.; Wang, S.; Chen, Y.; Zhang, Y.; Ren, J. 17-β estradiol attenuates ovariectomy-induced changes in cardiomyocyte contractile function via activation of AMP-activated protein kinase. Toxicol. Lett. 2015, 232, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tishkoff, D.X.; Nibbelink, K.A.; Holmberg, K.H.; Dandu, L.; Simpson, R.U. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008, 149, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Law, C.S.; Grigsby, C.; Olsen, K.; Hong, T.-T.; Zhang, Y.; Yeghiazarians, Y.; Gardner, D.G. Cardiomyocyte-Specific Deletion of the Vitamin D Receptor Gene Results in Cardiac Hypertrophy. Circulation 2011, 124, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gardner, D.G. Liganded vitamin D receptor displays anti-hypertrophic activity in the murine heart. J. Steroid Biochem. Mol. Biol. 2013, 136, 150–155. [Google Scholar] [CrossRef]
- Guo, X.; Lin, H.; Liu, J.; Wang, D.; Li, D.; Jiang, C.; Tang, Y.; Wang, J.; Zhang, T.; Li, Y.; et al. 1,25-Dihydroxyvitamin D attenuates diabetic cardiac autophagy and damage by vitamin D receptor-mediated suppression of FoxO1 translocation. J. Nutr. Biochem. 2020, 80, 108380. [Google Scholar] [CrossRef] [PubMed]
- Sottili, M.; Cosmi, L.; Borgogni, E.; Sarchielli, E.; Maggi, L.; Francalanci, M.; Vannelli, G.; Ronconi, E.; Adorini, L.; Annunziato, F.; et al. Immunomodulatory effects of BXL-01-0029, a less hypercalcemic vitamin D analogue, in human cardiomyocytes and T cells. Exp. Cell Res. 2009, 315, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Pan, Y.; Sun, C.; Liu, Z.; Liu, N.; Fu, Y.; Li, X.; Li, Y.; Kong, J. The Protective Effect of 1,25(OH)2D3 on Myocardial Function is Mediated via Sirtuin 3-Regulated Fatty Acid Metabolism. Front. Cell Dev. Biol. 2021, 9, 966. [Google Scholar] [CrossRef]
- Peterson, L.R.; Soto, P.F.; Herrero, P.; Schechtman, K.B.; Dence, C.; Gropler, R.J. Sex differences in myocardial oxygen and glucose metabolism. J. Nucl. Cardiol. 2007, 14, 573–581. [Google Scholar] [CrossRef]
- Liu, E.; Meigs, J.B.; Pittas, A.G.; Economos, C.D.; McKeown, N.M.; Booth, S.L.; Jacques, P.F. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am. J. Clin. Nutr. 2010, 91, 1627–1633. [Google Scholar] [CrossRef]
- van Schouten, F.J.; Hirvonen, A.; Maas, L.M.; De Mol, B.A.; Kleinjans, J.C.S.; Bell, D.; Durrer, J.D. Putative susceptibility markers of coronary artery disease: Association between VDR genotype, smoking, and aromatic DNA adduct levels in human right atrial tissue. FASEB J. 1998, 12, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef]
- Crescioli, C. Vitamin D Restores Skeletal Muscle Cell Remodeling and Myogenic Program: Potential Impact on Human Health. Int. J. Mol. Sci. 2021, 22, 1760. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Targeting Age-Dependent Functional and Metabolic Decline of Human Skeletal Muscle: The Geroprotective Role of Exercise, Myokine IL-6, and Vitamin D. Int. J. Mol. Sci. 2020, 21, 1010. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Vitamin D Merging into Immune System-Skeletal Muscle Network: Effects on Human Health. Appl. Sci. 2020, 10, 5592. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Di Somma, C.; Laudisio, D.; Salzano, C.; Pugliese, G.; De Alteriis, G.; Colao, A.; Savastano, S. Sex Differences of Vitamin D Status across BMI Classes: An Observational Prospective Cohort Study. Nutrients 2019, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.K.; Hofsø, D.; Aasheim, E.T.; Tanbo, T.; Holven, K.B.; Andersen, L.F.; Røislien, J.; Hjelmesaeth, J. Impact of gender on vitamin D deficiency in morbidly obese patients: A cross-sectional study. Eur. J. Clin. Nutr. 2012, 66, 83–90. [Google Scholar] [CrossRef]
- Sanghera, D.K.; Sapkota, B.R.; Aston, C.E.; Blackett, P.R. Vitamin D status, gender differences, and cardiometabolic health disparities. Ann. Nutr. Metab. 2017, 70, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Jungert, A.; Neuhäuser-Berthold, M. Sex-specific determinants of serum 25-hydroxyvitamin D3 concentrations in an elderly German cohort: A cross-sectional study. Nutr. Metab. 2015, 12, 2. [Google Scholar] [CrossRef]
- Vasile, M.; Corinaldesi, C.; Antinozzi, C.; Crescioli, C. Vitamin D in autoimmune rheumatic diseases: A view inside gender differences. Pharmacol. Res. 2017, 117, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C.; Minisola, S. Vitamin D: Autoimmunity and gender. Curr. Med. Chem. 2017, 24, 2671–2686. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Panagiotakos, D.B. Vitamin D status, gender and cardiovascular diseases: A systematic review of prospective epidemiological studies. Expert Rev. Cardiovasc. Ther. 2019, 17, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Argacha, J.-F.; Egrise, D.; Pochet, S.; Fontaine, D.; Lefort, A.; Libert, F.; Goldman, S.; van de Borne, P.; Berkenboom, G.; Moreno-Reyes, R. Vitamin D Deficiency-induced Hypertension Is Associated with Vascular Oxidative Stress and Altered Heart Gene Expression. J. Cardiovasc. Pharmacol. 2011, 58, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Calderon, J.R.V.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Henry, R.M.A.; Snijder, M.B.; Van Dam, R.M.; Nijpels, G.; Stehouwer, C.D.A.; Kamp, O.; Tomaschitz, A.; Pieber, T.R.; Dekker, J.M. Vitamin D deficiency and myocardial structure and function in older men and women: The Hoorn Study. J. Endocrinol. Investig. 2010, 33, 612–617. [Google Scholar] [CrossRef]
- Akin, F.; Ayça, B.; Köse, N.; Celik, O.; Yilmaz, Y.; Akin, M.N.; Arinc, H.; Ozkok, A.; Covic, A.; Kanbay, M. Serum Vitamin D and C-Reactive Protein Levels Are Independently Associated with Diastolic Dysfunction. J. Investig. Med. 2014, 62, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Berthold, H.K. Vitamin D and Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.E.; Tangpricha, V. Vitamin D therapy and cardiovascular health. Curr. Hypertens. Rep. 2011, 13, 187–191. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Guérin, A.P.; Verbeke, F.H.; Pannier, B.; Boutouyrie, P.; Marchais, S.J.; Mëtivier, F. Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J. Am. Soc. Nephrol. 2007, 18, 613–620. [Google Scholar] [CrossRef]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2009, 200, 207. [Google Scholar] [CrossRef]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef]
- Tintut, Y.; Demer, L.L. Potential Impact of the Steroid Hormone, Vitamin D, on the Vasculature Vitamin D-hormones and cardiovascular disease. Am. Heart J. 2021, 239, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; Cainzos-Achirica, M.; Heravi, A.S.; Appel, L.J. Vitamin D, calcium supplements, and implications for cardiovascular health: JACC focus seminar. J. Am. Coll. Cardiol. 2021, 77, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Verheyen, N.; Grübler, M.R.; Tomaschitz, A.; März, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef]
- Wang, T.J. Vitamin D and cardiovascular disease. Annu. Rev. Med. 2016, 67, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hershey, S.; Ahmed, S.; Nibbelink, K.; Simpson, R.U. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J. Steroid Biochem. Mol. Biol. 2007, 103, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Dekker, J.M.; März, W. Vitamin D deficiency and myocardial diseases. Mol. Nutr. Food Res. 2010, 54, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, W.; Wang, B.; Yi, Z.; Gong, P.; Xiong, Y. 1α,25-Dihydroxyvitamin D3 ameliorates diabetes-induced bone loss by attenuating FoxO1-mediated autophagy. J. Biol. Chem. 2021, 296, 100287. [Google Scholar] [CrossRef] [PubMed]
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996, 27, 1201–1206. [Google Scholar] [CrossRef]
- Schumacher, S.M.; Prasad, S.V.N. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 2018, 20, 117. [Google Scholar] [CrossRef]
- Rababa’H, A.M.; Guillory, A.N.; Mustafa, R.; Hijjawi, T. Oxidative Stress and Cardiac Remodeling: An Updated Edge. Curr. Cardiol. Rev. 2018, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Assalin, H.B.; Rafacho, B.P.M.; dos Santos, P.P.; Ardisson, L.P.; Roscani, M.G.; Chiuso-Minicucci, F.; Barbisan, L.F.; Fernandes, A.A.H.; Azevedo, P.S.; Minicucci, M.F.; et al. Impact of the Length of Vitamin D Deficiency on Cardiac Remodeling. Circ. Heart Fail. 2013, 6, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Simpson, R.U. Interaction between vitamin D receptor with caveolin-3 and regulation by 1,25-dihydroxyvitamin D3 in adult rat cardiomyocytes. J. Steroid Biochem. Mol. Biol. 2010, 121, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, L.; Marchiani, S.; Ferruzzi, P.; Muratori, M.; Crescioli, C.; Forti, G.; Maggi, M.; Baldi, E. Non-genomic effects of the androgen receptor and Vitamin D agonist are involved in suppressing invasive phenotype of prostate cancer cells. Steroids 2006, 71, 304–309. [Google Scholar] [CrossRef]
- Antinozzi, C.; Corinaldesi, C.; Giordano, C.; Pisano, A.; Cerbelli, B.; Migliaccio, S.; Di Luigi, L.; Stefanantoni, K.; Vannelli, G.B.; Minisola, S.; et al. Potential role for the VDR agonist elocalcitol in metabolic control: Evidences in human skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 2017, 167, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Karlic, H.; Varga, F. Impact of vitamin D metabolism on clinical epigenics. Clin Epigenet. 2011, 2, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Inui, M.; Martello, G.; Piccolo, S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Assalin, H.B.; Gontijo, J.A.R.; Boer, P.A. miRNAs, target genes expression and morphological analysis on the heart in gestational protein-restricted offspring. PLoS ONE 2019, 14, e0210454. [Google Scholar] [CrossRef]
- Hobert, O. Gene regulation by transcription factors and microRNAs. Science 2008, 319, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.I.; Kuryłowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Kozniewski, K.; Puzianowska-Kuznicka, M. Vitamin D receptor gene expression in adipose tissue of obese individuals is regulated by miRNA and correlates with the pro-inflammatory cytokine level. Int. J. Mol. Sci. 2019, 20, 5272. [Google Scholar] [CrossRef]
- Singh, P.K.; Long, M.D.; Battaglia, S.; Hu, Q.; Liu, S.; Sucheston-Campbell, L.E.; Campbell, M.J. VDR regulation of microRNA differs across prostate cell models suggesting extremely flexible control of transcription. Epigenetics 2015, 10, 40–49. [Google Scholar] [CrossRef]
- Singh, T.; Adams, B.D. The regulatory role of miRNAs on VDR in breast cancer. Transcription 2017, 8, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.; Wang, X.; Lanza, I.; Schaible, N.S.; Salisbury, J.; Nair, K.S.; Terzic, A.; Sieck, G.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef]
- Aguennouz, M.; Giudice, C.L.; Licata, N.; Rodolico, C.; Musumeci, O.; Fanin, M.; Migliorato, A.; Ragusa, M.; Macaione, V.; Di Giorgio, R.M.; et al. MicroRNA signatures predict dysregulated vitamin D receptor and calcium pathways status in limb girdle muscle dystrophies (LGMD) 2A/2B. Cell Biochem. Funct. 2016, 34, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S.; Adams, J.S.; Hewison, M. Vitamin D and microRNAs in bone. Crit. Rev. Eukaryot. Gene Expr. 2013, 23, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac Metabolism and its Interactions with Contraction, Growth, and Survival of Cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- dos Santos, R.L.; Da Silva, F.B.; Ribeiro, R.F.; Stefanon, I. Sex hormones in the cardiovascular system. Horm. Mol. Biol. Clin. Investig. 2014, 18, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Wittnich, C.; Tan, L.; Wallen, J.; Belanger, M. Sex differences in myocardial metabolism and cardiac function: An emerging concept. Pflügers Arch.-Eur. J. Physiol. 2013, 465, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.A.; Khalsa, S.S.S.; Vyas, A.K.; Rahimian, R. Sex-Specific Impacts of Exercise on Cardiovascular Remodeling. J. Clin. Med. 2021, 10, 3833. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, M.; Nojima, T.; Ishibashi, T.; Koda, T.; Kaneda, K.; Rosier, R.N.; Puzas, J.E. 17β-Estradiol increases the receptor number and modulates the action of 1,25-dihydroxyvitamin D3 in human osteosarcoma-derived osteoblast-like cells. Calcif. Tissue Int. 1995, 57, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Padwa, B.L.; Zhou, S.; Mullokandova, J.; LeBoff, M.S.; Glowacki, J. Synergistic effect of 1α,25-dihydroxyvitamin D3 and 17β-estradiol on osteoblast differentiation of pediatric MSCs. J. Steroid Biochem. Mol. Biol. 2018, 177, 103–108. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Zhou, Y. A synergetic role of 1,25-dihydroxyvitamin D3 in 17β-estradial induced-proliferation and differentiation of osteoblastic MC3T3-E1 cells. Eur. J. Pharmacol. 2011, 659, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ronda, A.C.; Buitrago, C.; Colicheo, A.; de Boland, A.R.; Roldán, E.; Boland, R. Activation of MAPKs by 1α,25(OH)2-Vitamin D3 and 17β-estradiol in skeletal muscle cells leads to phosphorylation of Elk-1 and CREB transcription factors. J. Steroid Biochem. Mol. Biol. 2007, 103, 462–466. [Google Scholar] [CrossRef]
- Spach, K.M.; Hayes, C.E. Vitamin D3 Confers Protection from Autoimmune Encephalomyelitis Only in Female Mice. J. Immunol. 2005, 175, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Nashold, F.E.; Spach, K.M.; Spanier, J.A.; Hayes, C.E. Estrogen Controls Vitamin D3-Mediated Resistance to Experimental Autoimmune Encephalomyelitis by Controlling Vitamin D3 Metabolism and Receptor Expression. J. Immunol. 2009, 183, 3672–3681. [Google Scholar] [CrossRef] [PubMed]
- Kragt, J.J.; Van Amerongen, B.M.; Killestein, J.; Dijkstra, C.D.; Uitdehaag, B.M.J.; Polman, C.H.; Lips, P. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult. Scler. J. 2009, 15, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Aarskog, D.; Aksnes, L.; Markestad, T.; Rødland, O. Effect of Estrogen on Vitamin D Metabolism in Tall Girls. J. Clin. Endocrinol. Metab. 1983, 57, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.; McAdoo, T.; Hatley, L.; Lester, G.; Thierry, M. Fluctuation of serum concentration of 1,25-dihydroxyvitamin D3 during the menstrual cycle. Am. J. Obstet. Gynecol. 1982, 144, 880–884. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Pilz, S.; Boehm, B.O.; Grammer, T.B.; Obermayer-Pietsch, B.; März, W. Combination of low free testosterone and low vitamin D predicts mortality in older men referred for coronary angiography. Clin. Endocrinol. 2012, 77, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Berthold, H.K.; Gouni-Berthold, I.; Gummert, J.F.; et al. Vitamin D supplementation does not prevent the testosterone decline in males with advanced heart failure: The EVITA trial. Eur. J. Nutr. 2018, 58, 673–680. [Google Scholar] [CrossRef]
- Huang, H.; Guo, J.; Chen, Q.; Chen, X.; Yang, Y.; Zhang, W.; Liu, Y.; Chen, X.; Yang, D. The synergistic effects of vitamin D and estradiol deficiency on metabolic syndrome in Chinese postmenopausal women. Menopause 2019, 26, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Gangula, P.R.; Dong, Y.L.; Al-Hendy, A.; Richard-Davis, G.; Montgomery-Rice, V.; Haddad, G.; Millis, R.; Nicholas, S.B.; Moseberry, D. Protective cardiovascular and renal actions of vitamin D and estrogen. Front. Biosci. 2013, 5, 134–148. [Google Scholar] [CrossRef][Green Version]
- Somjen, D.; Katzburg, S.; Baz, M.; Stern, N.; Posner, G.H. Modulation of the response to estradiol-17β of rat vascular tissues by a non calcemic vitamin D analog. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, J. Peroxisome Proliferator-Activated Receptors and the Heart: Lessons from the Past and Future Directions. PPAR Res. 2015, 2015, 271983. [Google Scholar] [CrossRef] [PubMed]
- Massion, P.B.; Feron, O.; Dessy, C.; Balligand, J.L. Nitric oxide and cardiac function: Ten years after, and continuing. Circ. Res. 2003, 93, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin. Hypertens. 2018, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: A meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.D.; Jia, J.J.; Dong, P.S.; Zhao, D.; Li, D.L.; Zhang, H.F. Effect of vitamin D on ventricular remodelling in heart failure: A meta-analysis of randomised controlled trials. BMJ Open 2018, 8, e020545. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Fuleihan, G.E.; Bouillon, R.; Clarke, B.; Chakhtoura, M.; Cooper, C.; McClung, M.R.; Singh, R. Serum 25-hydroxyvitamin D levels: Variability, knowledge gaps and the concept of a desirable range. J. Bone Miner. Res. 2015, 30, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]

| Receptor Type | Mediated Function | Refs. |
|---|---|---|
| ERα | growth control post-injury recovery (more efficient in females) | [50,51] |
| ERβ | cell/tissue structure, gap junction post-ischemic recovery (only in females) anti-fibrotic activity (more efficient in males) | [54,55,56] |
| ERα/ERβ (in mitochondria) | mitochondria sex-dimorphism Ca2+ homeostasis, ROS formation, cell apoptosis, EC coupling (more efficient in females) cardiotoxicity protection, injury resistance (more efficient in females) | [60,61,62,63,64,65,66] |
| GPER | structure and function protection (more efficient in males) | [58,59] |
| VDR | anti-hypertophic/anti-fibrotic activity contractility/relaxation control E2-dependent fatty acid uptake/β-oxidation reduction (females) anti-inflammatory/anti-autophagic activity | [70,71,72,73,74,75,76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crescioli, C. The Role of Estrogens and Vitamin D in Cardiomyocyte Protection: A Female Perspective. Biomolecules 2021, 11, 1815. https://doi.org/10.3390/biom11121815
Crescioli C. The Role of Estrogens and Vitamin D in Cardiomyocyte Protection: A Female Perspective. Biomolecules. 2021; 11(12):1815. https://doi.org/10.3390/biom11121815
Chicago/Turabian StyleCrescioli, Clara. 2021. "The Role of Estrogens and Vitamin D in Cardiomyocyte Protection: A Female Perspective" Biomolecules 11, no. 12: 1815. https://doi.org/10.3390/biom11121815
APA StyleCrescioli, C. (2021). The Role of Estrogens and Vitamin D in Cardiomyocyte Protection: A Female Perspective. Biomolecules, 11(12), 1815. https://doi.org/10.3390/biom11121815







