CRISPR/dCas9 Transcriptional Activation of Endogenous Apolipoprotein AI and Paraoxonase 1 in Enterocytes Alleviates Endothelial Cell Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures
2.3. Overexpression of ApoA-I and PON1 in Caco-2 Cells
2.4. Incubation of EC with Conditioned Media from Caco-2 Transfected Cells
2.5. Isolation of Total RNA and Quantification of Gene Expression
2.6. Quantification of Protein Expression in the Cellular Lysate
2.7. Measurement of Secreted Proteins Levels in the Cell Culture Medium
2.8. Statistical Analysis of the Data
3. Results
3.1. Transcriptional Activation of Endogenous ApoAI by CRISPR/dCas9 Technology Increases ApoAI Expression and Secretion and Stimulates PON-1 Production in Caco-2 Cells
3.2. Transcriptional Activation of Endogenous PON1 by CRISPR/dCas9 Technology Increases PON1 Expression and Secretion and Stimulates ApoAI Protein Production in Caco-2 Cells
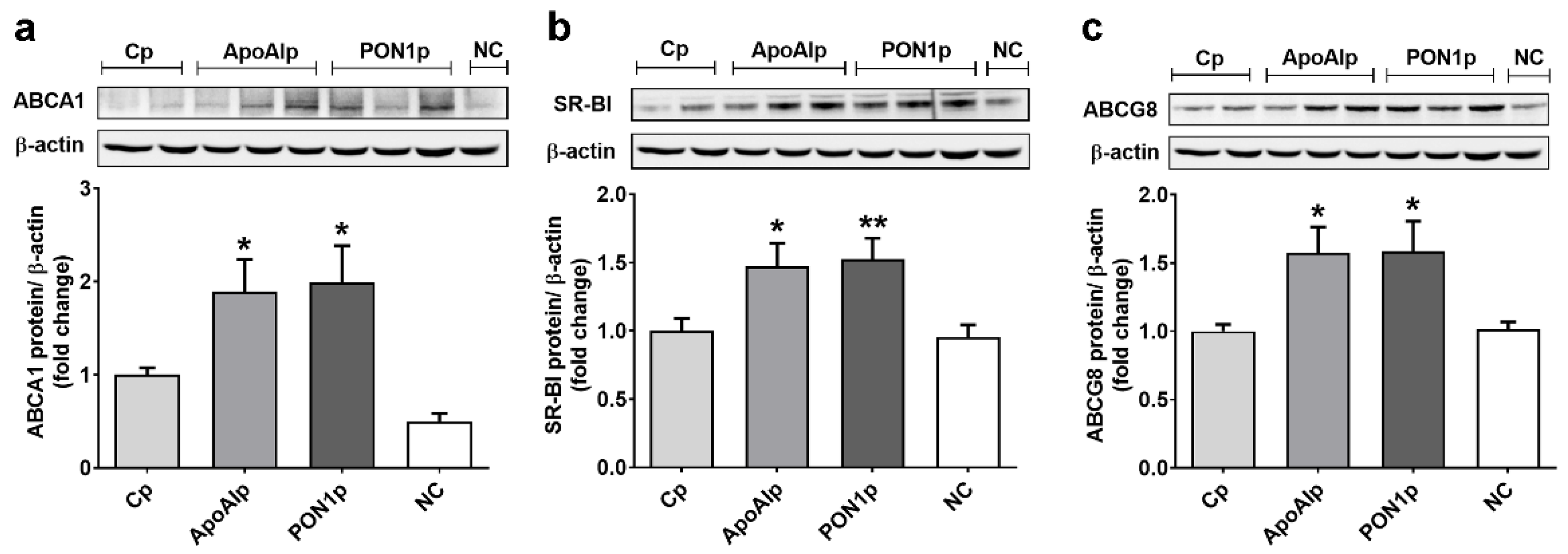
3.3. Overexpression of Endogenous ApoAI and PON1 Stimulates ABCA1, SR-BI, and ABCG8 Protein Expression in Caco-2 Cells
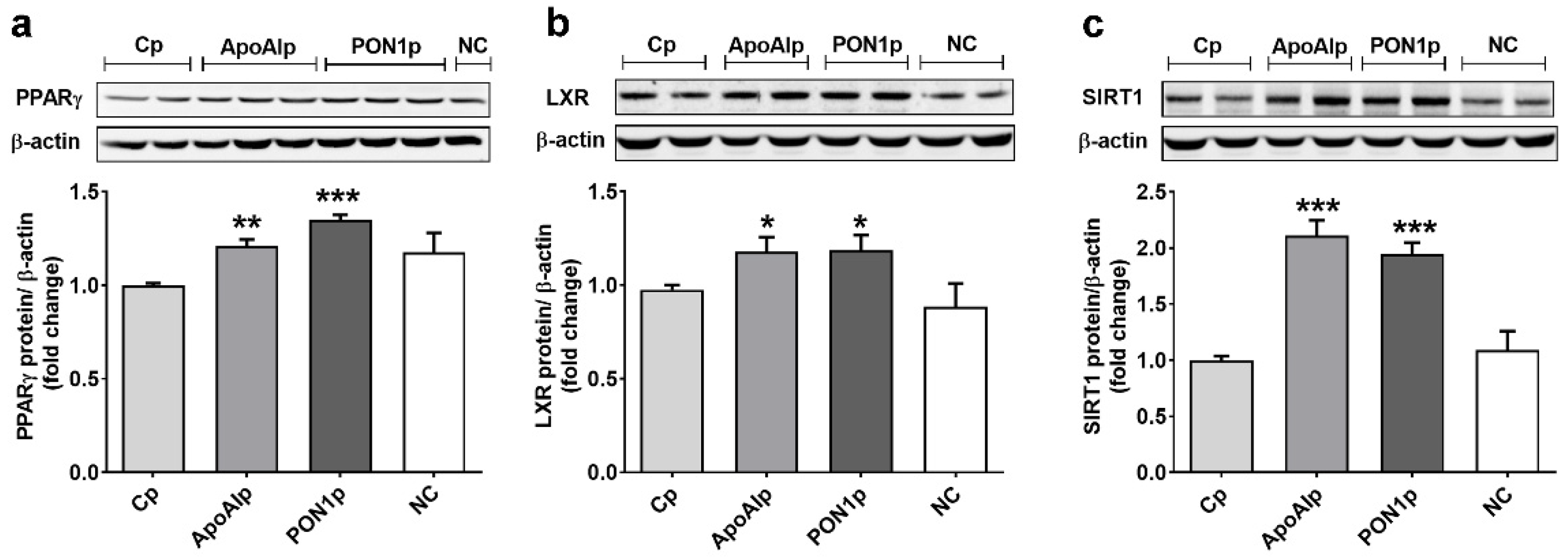
3.4. Overexpression of Endogenous ApoAI and PON-1 Upregulates the Expression of Lipid Related Transcription Factors in Caco-2 Cells
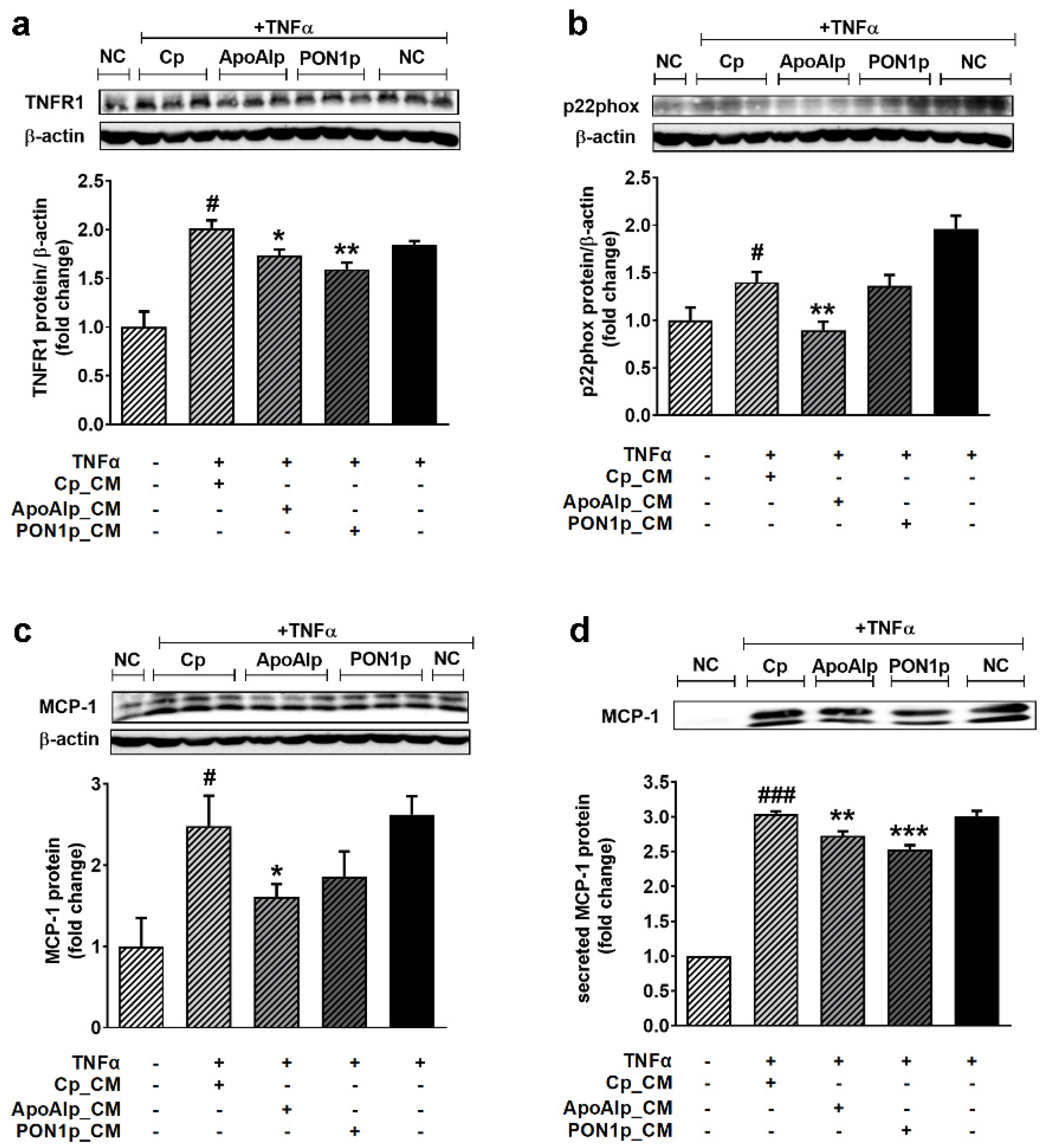
3.5. Conditioned Media from Caco-2 Cells Overexpressing Endogenous ApoAI or PON1 Have Anti-Inflammatory and Antioxidant Effects on TNFα-Activated EC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diab. Rep. 2019, 19, 44. [Google Scholar] [CrossRef]
- Sima, A.V.; Stancu, C.S.; Simionescu, M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009, 335, 191–203. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Little, P.J.; Askew, C.D.; Xu, S.; Kamato, D. Endothelial Dysfunction and Cardiovascular Disease: History and Analysis of the Clinical Utility of the Relationship. Biomedicines 2021, 9, 699. [Google Scholar] [CrossRef]
- Eren, E.; Yilmaz, N.; Aydin, O. High Density Lipoprotein and it’s Dysfunction. Open Biochem. J. 2012, 6, 78–93. [Google Scholar] [CrossRef]
- Bhatt, A.; Rohatgi, A. HDL Cholesterol Efflux Capacity: Cardiovascular Risk Factor and Potential Therapeutic Target. Curr. Atheroscler. Rep. 2016, 18, 2. [Google Scholar] [CrossRef]
- Arora, S.; Patra, S.K.; Saini, R. HDL-A molecule with a multi-faceted role in coronary artery disease. Clin. Chim. Acta 2016, 452, 66–81. [Google Scholar] [CrossRef]
- Assmann, G.; Gotto, A.M., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation 2004, 109, III-8–III-14. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef]
- Reiner, Z. Resistance and intolerance to statins. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Messas, N.; Dube, M.P.; Tardif, J.C. Pharmacogenetics of Lipid-Lowering Agents: An Update Review on Genotype-Dependent Effects of HDL-Targetingand Statin Therapies. Curr. Atheroscler. Rep. 2017, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Larach, D.B.; deGoma, E.M.; Rader, D.J. Targeting high density lipoproteins in the prevention of cardiovascular disease? Curr. Cardiol. Rep. 2012, 14, 684–691. [Google Scholar] [CrossRef]
- Luscher, T.F.; Landmesser, U.; von Eckardstein, A.; Fogelman, A.M. High-density lipoprotein: Vascular protective effects, dysfunction, and potential as therapeutic target. Circ. Res. 2014, 114, 171–182. [Google Scholar] [CrossRef]
- Carnuta, M.G.; Stancu, C.S.; Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; et al. Dysfunctional high-density lipoproteins have distinct composition, diminished anti-inflammatory potential and discriminate acute coronary syndrome from stable coronary artery disease patients. Sci. Rep. 2017, 7, 7295. [Google Scholar] [CrossRef]
- Stancu, C.S.; Toma, L.; Sima, A.V. Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res. 2012, 349, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Is ABCA1 a lipid transfer protein? J. Lipid. Res. 2018, 59, 749–763. [Google Scholar] [CrossRef]
- Dahabreh, D.F.; Medh, J.D. Activation of peroxisome proliferator activated receptor-gamma results in an atheroprotective apolipoprotein profile in HepG2 cells. Adv. Biol. Chem. 2012, 2, 218–225. [Google Scholar] [CrossRef][Green Version]
- Costet, P.; Luo, Y.; Wang, N.; Tall, A.R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000, 275, 28240–28245. [Google Scholar] [CrossRef]
- Shen, W.J.; Asthana, S.; Kraemer, F.B.; Azhar, S. Scavenger receptor B type 1: Expression, molecular regulation, and cholesterol transport function. J. Lipid. Res. 2018, 59, 1114–1131. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging (Albany N. Y.) 2016, 8, 2290–2307. [Google Scholar] [CrossRef] [PubMed]
- Riwanto, M.; Rohrer, L.; Roschitzki, B.; Besler, C.; Mocharla, P.; Mueller, M.; Perisa, D.; Heinrich, K.; Altwegg, L.; von Eckardstein, A.; et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: Role of high-density lipoprotein-proteome remodeling. Circulation 2013, 127, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Sanda, G.M.; Toma, L.; Barbalata, T.; Moraru, O.E.; Niculescu, L.S.; Sima, A.V.; Stancu, C.S. Clusterin, paraoxonase 1 and myeloperoxidase alterations induce HDL dysfunction and contribute to peripheral artery disease; aggravation by type 2 diabetes mellitus. Biofactors 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Valanti, E.K.; Dalakoura-Karagkouni, K.; Sanoudou, D. Current and Emerging Reconstituted HDL-apoA-I and HDL-apoE Approaches to Treat Atherosclerosis. J. Pers. Med. 2018, 8, 34. [Google Scholar] [CrossRef]
- Hammersley, D.; Signy, M. Ezetimibe: An update on its clinical usefulness in specific patient groups. Ther. Adv. Chronic. Dis. 2017, 8, 4–11. [Google Scholar] [CrossRef]
- Ross, S.; D′Mello, M.; Anand, S.S.; Eikelboom, J.; Consortium, C.A.D.; Stewart, A.F.; Samani, N.J.; Roberts, R.; Pare, G. Effect of Bile Acid Sequestrants on the Risk of Cardiovascular Events: A Mendelian Randomization Analysis. Circ. Cardiovasc. Genet. 2015, 8, 618–627. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Darabi, M.; Guillas-Baudouin, I.; Le Goff, W.; Chapman, M.J.; Kontush, A. Therapeutic applications of reconstituted HDL: When structure meets function. Pharmacol. Ther. 2016, 157, 28–42. [Google Scholar] [CrossRef]
- Andrews, J.; Janssan, A.; Nguyen, T.; Pisaniello, A.D.; Scherer, D.J.; Kastelein, J.J.; Merkely, B.; Nissen, S.E.; Ray, K.; Schwartz, G.G.; et al. Effect of serial infusions of reconstituted high-density lipoprotein (CER-001) on coronary atherosclerosis: Rationale and design of the CARAT study. Cardiovasc. Diagn. Ther. 2017, 7, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Karalis, I.; Jukema, J.W. HDL Mimetics Infusion and Regression of Atherosclerosis: Is It Still Considered a Valid Therapeutic Option? Curr. Cardiol. Rep. 2018, 20, 66. [Google Scholar] [CrossRef]
- Mohammadi, A.; Bazrafshani, M.R.; Oshaghi, E.A. Effect of garlic extract on some serum biochemical parameters and expression of npc1l1, abca1, abcg5 and abcg8 genes in the intestine of hypercholesterolemic mice. Indian J. Biochem. Biophys. 2013, 50, 500–504. [Google Scholar]
- Niesor, E.J. Will Lipidation of ApoA1 through Interaction with ABCA1 at the Intestinal Level Affect the Protective Functions of HDL? Biology 2015, 4, 17–38. [Google Scholar] [CrossRef]
- Stancu, C.S.; Carnuta, M.G.; Sanda, G.M.; Toma, L.; Deleanu, M.; Niculescu, L.S.; Sasson, S.; Simionescu, M.; Sima, A.V. Hyperlipidemia-induced hepatic and small intestine ER stress and decreased paraoxonase 1 expression and activity is associated with HDL dysfunction in Syrian hamsters. Mol. Nutr. Food Res. 2015, 59, 2293–2302. [Google Scholar] [CrossRef]
- Barbalata, T.; Deleanu, M.; Carnuta, M.G.; Niculescu, L.S.; Raileanu, M.; Sima, A.V.; Stancu, C.S. Hyperlipidemia Determines Dysfunctional HDL Production and Impedes Cholesterol Efflux in the Small Intestine: Alleviation by Ginger Extract. Mol. Nutr. Food Res. 2019, 63, e1900029. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Liu, H.; Cheng, K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J. Control. Release 2017, 266, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, X.; Yuan, Y.; Zhu, H.; Zhou, W.; Huang, H.; Feng, M. Inhibitory effect of apolipoprotein A-I on matrix metalloproteinase-2 expression in vivo and in vitro. Acta Biochim. Biophys. Sin. 2013, 45, 194–202. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.J.; Liu, L.C.; Guo, C.; Shen, W.W.; Cao, J.; Du, F.; Wu, D.F.; Yu, H. Hepatic paraoxonase 1 ameliorates dysfunctional high-density lipoprotein and atherosclerosis in scavenger receptor class B type I deficient mice. Ann. Transl. Med. 2021, 9, 1063. [Google Scholar] [CrossRef]
- Vrins, C.L. From blood to gut: Direct secretion of cholesterol via transintestinal cholesterol efflux. World J. Gastroenterol. 2010, 16, 5953–5957. [Google Scholar] [CrossRef]
- Yu, X.H.; Qian, K.; Jiang, N.; Zheng, X.L.; Cayabyab, F.S.; Tang, C.K. ABCG5/ABCG8 in cholesterol excretion and atherosclerosis. Clin. Chim. Acta. 2014, 428, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Fledderus, J.; Vanchin, B.; Rots, M.G.; Krenning, G. The Endothelium as a Target for Anti-Atherogenic Therapy: A Focus on the Epigenetic Enzymes EZH2 and SIRT1. J. Pers. Med. 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Luscher, T.F. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014, 35, 1782–1791. [Google Scholar] [CrossRef]
- Robert, J.; Osto, E.; von Eckardstein, A. The Endothelium Is Both a Target and a Barrier of HDL′s Protective Functions. Cells 2021, 10, 1041. [Google Scholar] [CrossRef] [PubMed]
- Cochran, B.J.; Ong, K.L.; Manandhar, B.; Rye, K.A. APOA1: A Protein with Multiple Therapeutic Functions. Curr. Atheroscler. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Morton, J.; Bao, S.; Vanags, L.Z.; Tsatralis, T.; Ridiandries, A.; Siu, C.W.; Ng, K.M.; Tan, J.T.M.; Celermajer, D.S.; Ng, M.K.C.; et al. Strikingly Different Atheroprotective Effects of Apolipoprotein A-I in Early- Versus Late-Stage Atherosclerosis. JACC Basic Transl. Sci. 2018, 3, 187–199. [Google Scholar] [CrossRef]
- Wacker, B.K.; Dronadula, N.; Zhang, J.; Dichek, D.A. Local Vascular Gene Therapy With Apolipoprotein A-I to Promote Regression of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 316–327. [Google Scholar] [CrossRef]
- Barrett, T.J.; Distel, E.; Murphy, A.J.; Hu, J.; Garshick, M.S.; Ogando, Y.; Liu, J.; Vaisar, T.; Heinecke, J.W.; Berger, J.S.; et al. Apolipoprotein AI) Promotes Atherosclerosis Regression in Diabetic Mice by Suppressing Myelopoiesis and Plaque Inflammation. Circulation 2019, 140, 1170–1184. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Ballantyne, C.M.; Jukema, J.W.; Kastelein, J.J.P.; Koenig, W.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Borgman, M.; et al. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 806–814. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Andrews, J.; Kastelein, J.J.P.; Merkely, B.; Nissen, S.E.; Ray, K.K.; Schwartz, G.G.; Worthley, S.G.; Keyserling, C.; Dasseux, J.L.; et al. Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 815–822. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Maaninka, K.; Lappalainen, J.; Nurmi, K.; Metso, J.; Oorni, K.; Navab, M.; Fogelman, A.M.; Jauhiainen, M.; Lee-Rueckert, M.; et al. Carboxyl-Terminal Cleavage of Apolipoprotein A-I by Human Mast Cell Chymase Impairs Its Anti-Inflammatory Properties. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O′Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef] [PubMed]



| Gene | GeneBank Accession Number | Sequences of Oligonucleotide Primers |
|---|---|---|
| ApoAI | NM_000039.2 | FW: 5′-CCCTGGGATCGAGTGAAGGA-3′ RV: 5′-CTGGGACACATAGTCTCTGCC-3′ |
| PON1 | NM_000446.5 | FW: 5′-CTATGACTCAGAGAATCCTCCTGCATCAG-3′ RV: 5′-CATGGGTGCAAATCGGTCTGTTAGAGC-3′ |
| β-actin | NM_001101.3 | FW: 5′-GTCTTCCCCTCCATCGT-3′ RV: 5′-CGTCGCCCACATAGGAAT-3′ |
| Antibody | Specificity | Catalogue | Dilution | Source |
|---|---|---|---|---|
| Mouse mAb | Human ApoAI | sc-69755 | 1:600 | Santa Cruz |
| Mouse mAb | Human PON1 | ab24261 | 1:500 | Abcam |
| Rabbit pAb | Human ABCA1 | NB400105 | 1:500 | Novus Biologicals |
| Rabbit pAb | Human ABCG8 | sc-30111 | 1:500 | Santa Cruz |
| Mouse mAb | Human LXR | sc-377260 | 1:500 | Santa Cruz |
| Rabbit pAb | Human PPAR-γ | sc-7196 | 1:500 | Santa Cruz |
| Mouse mAb | Human SIRT-1 | NBP1-51641 | 1:1000 | Novus Biologicals |
| Rabbit mAb | Human SR-BI | ab217318 | 1:2000 | Abcam |
| Mouse mAb | Human TNFR1 | sc-8436 | 1:400 | Santa Cruz |
| Rabbit pAb | Human MCP-1 | ab9669 | 1:1000 | Abcam |
| Rabbit pAb | Human p22phox | ab75941 | 1:1000 | Abcam |
| Mouse mAb | Human β-actin | sc-47778 | 1:4000 | Santa Cruz |
| Rabbit Anti-Mouse IgG H&L (HRP) | ab6728 | 1:10,000 | Abcam | |
| Rabbit Anti-Goat IgG H&L (HRP) | ab6741 | 1:10,000 | Abcam | |
| Goat Anti-Rabbit IgG H&L (HRP) | ab6721 | 1:10,000 | Abcam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, L.; Barbălată, T.; Sanda, G.M.; Niculescu, L.S.; Sima, A.V.; Stancu, C.S. CRISPR/dCas9 Transcriptional Activation of Endogenous Apolipoprotein AI and Paraoxonase 1 in Enterocytes Alleviates Endothelial Cell Dysfunction. Biomolecules 2021, 11, 1769. https://doi.org/10.3390/biom11121769
Toma L, Barbălată T, Sanda GM, Niculescu LS, Sima AV, Stancu CS. CRISPR/dCas9 Transcriptional Activation of Endogenous Apolipoprotein AI and Paraoxonase 1 in Enterocytes Alleviates Endothelial Cell Dysfunction. Biomolecules. 2021; 11(12):1769. https://doi.org/10.3390/biom11121769
Chicago/Turabian StyleToma, Laura, Teodora Barbălată, Gabriela M. Sanda, Loredan S. Niculescu, Anca V. Sima, and Camelia S. Stancu. 2021. "CRISPR/dCas9 Transcriptional Activation of Endogenous Apolipoprotein AI and Paraoxonase 1 in Enterocytes Alleviates Endothelial Cell Dysfunction" Biomolecules 11, no. 12: 1769. https://doi.org/10.3390/biom11121769
APA StyleToma, L., Barbălată, T., Sanda, G. M., Niculescu, L. S., Sima, A. V., & Stancu, C. S. (2021). CRISPR/dCas9 Transcriptional Activation of Endogenous Apolipoprotein AI and Paraoxonase 1 in Enterocytes Alleviates Endothelial Cell Dysfunction. Biomolecules, 11(12), 1769. https://doi.org/10.3390/biom11121769








