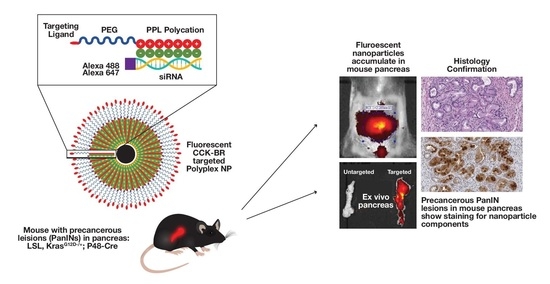
Cholecystokinin-B Receptor-Targeted Nanoparticle for Imaging and Detection of Precancerous Lesions in the Pancreas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the CCK-B Receptor-Targeted Polyplex Nanoparticle
2.2. Characterization of the Polyplex Micelle Nanoparticle In Vitro
2.3. Analysis of the CCK-B Receptor as a Target for the Polyplex Nanoparticle
2.4. Breeding and Genotyping of Mice
2.5. Administration and Imaging of KC Mice and Organs with Polyplex Nanoparticles
2.6. Immunohistochemistry for Detection of Alexa Fluor 488 Labeled Polyplex Nanoparticles in Tissues
2.7. Immunohistochemistry (IHC) for CCK-BR in KRAS Mouse Pancreas and Human Pancreas
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of the CCK-B Receptor-Targeted Polyplex Nanoparticle
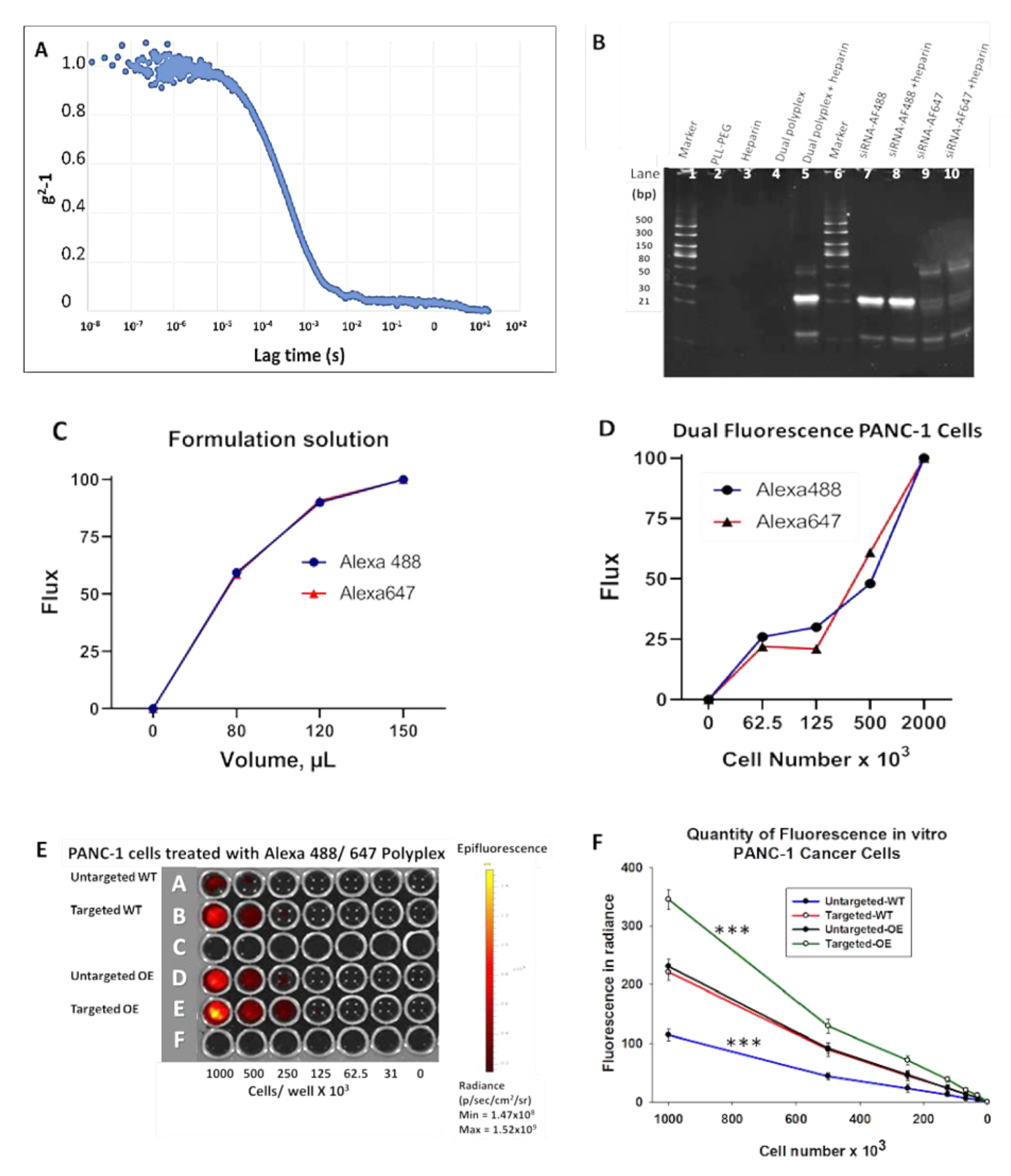
3.2. Characterization of the Polyplex Micelle Nanoparticle In Vitro
3.2.1. Heparin Displacement Assay
3.2.2. Confirmation of Dual Fluorophore Labeling In Vitro
3.2.3. CCK-B Receptor-Targeted Nanoparticles Have Enhanced Uptake in Cells
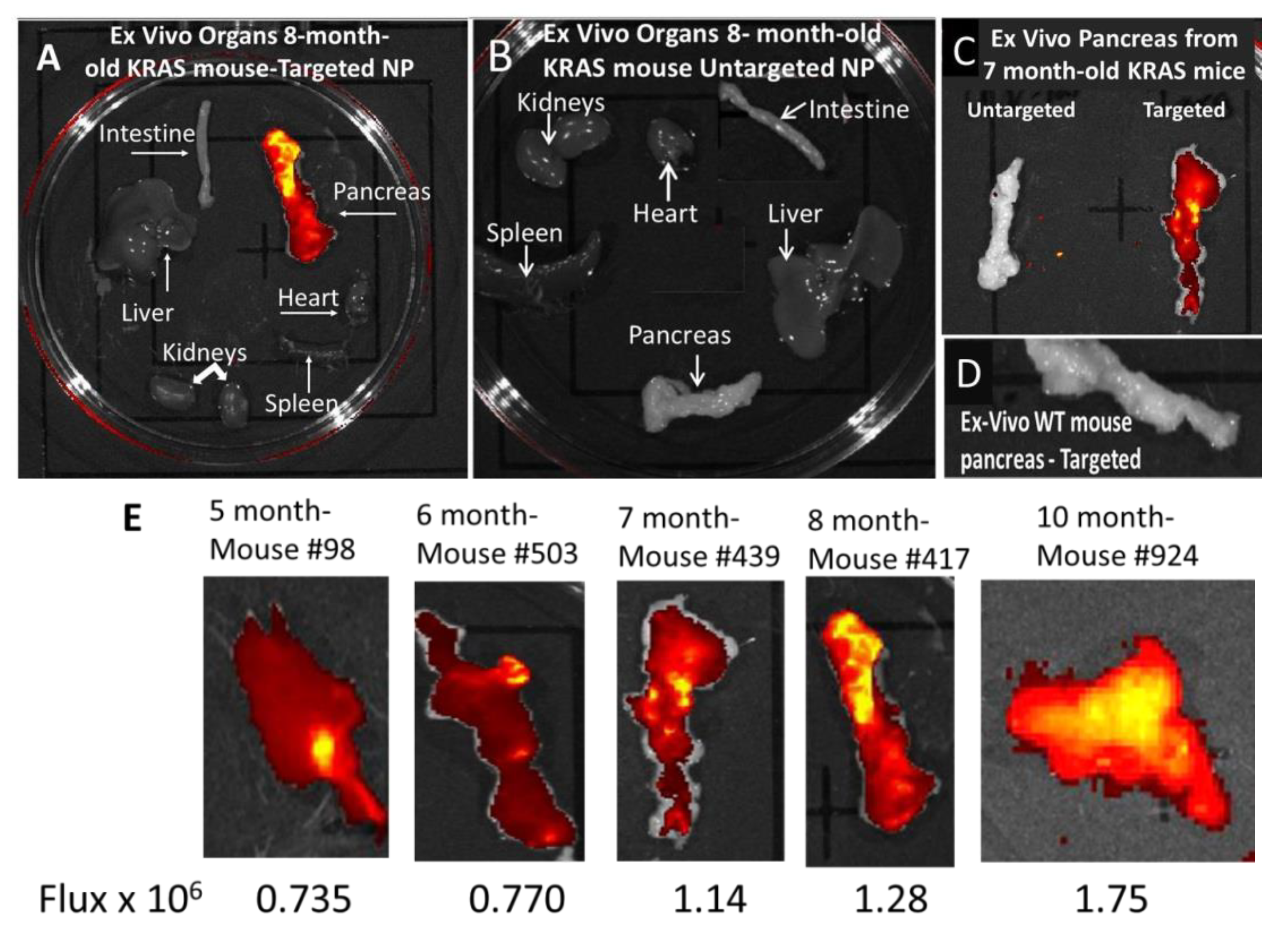
3.3. In Vivo Imaging of KC Mice with Targeted Fluorescent-Tagged Nanoparticles
3.4. Ex Vivo imaging of Organs from Mice Injected with Targeted or Untargeted Nanoparticles
3.5. Confirmation of Nanoparticle Uptake in PanINs by Immunohistochemistry
3.6. Immunoreactivity with Anti-PEG and Anti-CCK-BR Antibodies in 5-Month-Old KRAS Mouse Pancreas
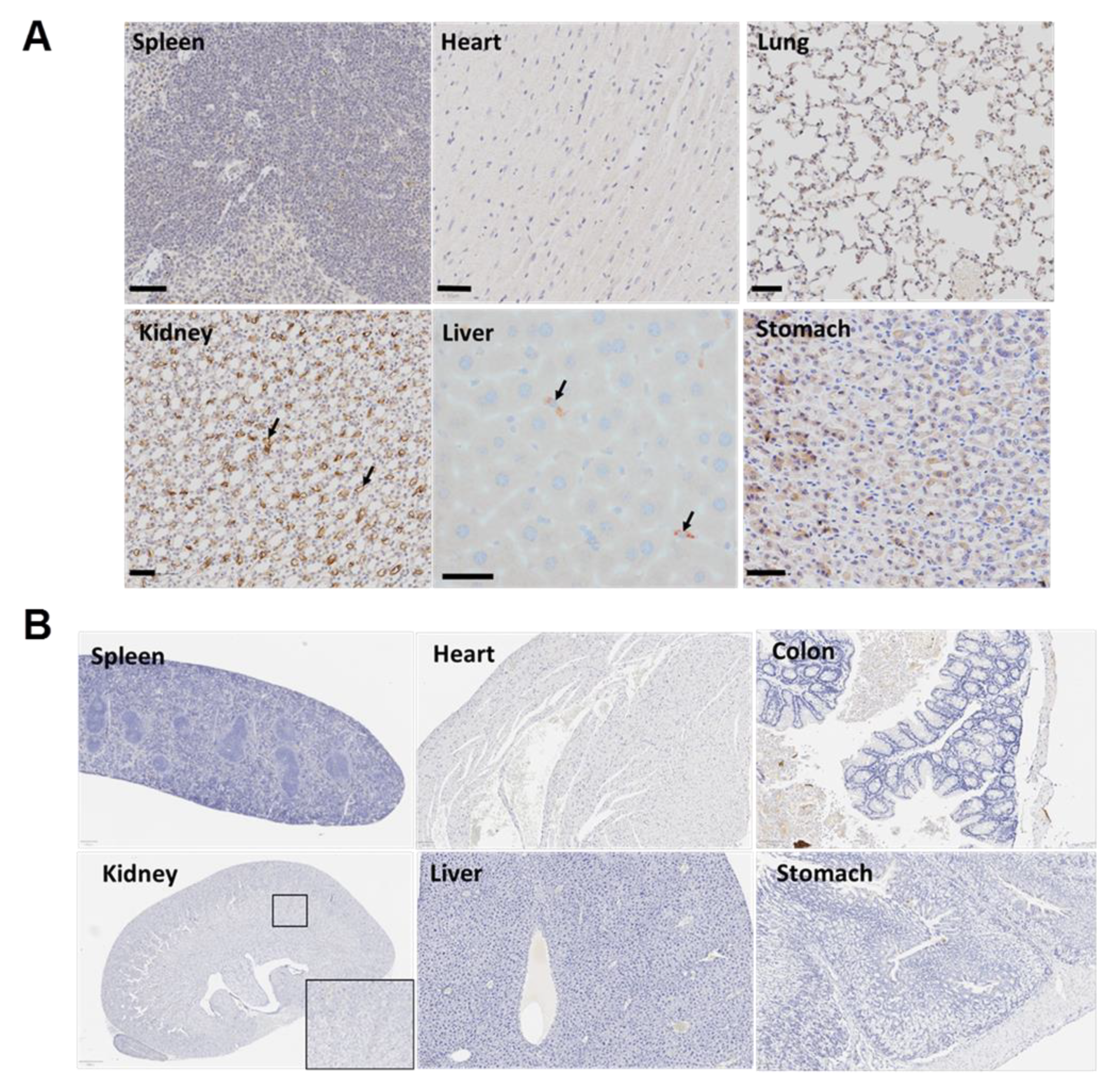
3.7. The Targeted Polyplex Nanoparticles Has Limited Off-Target Toxicity to Other Organs
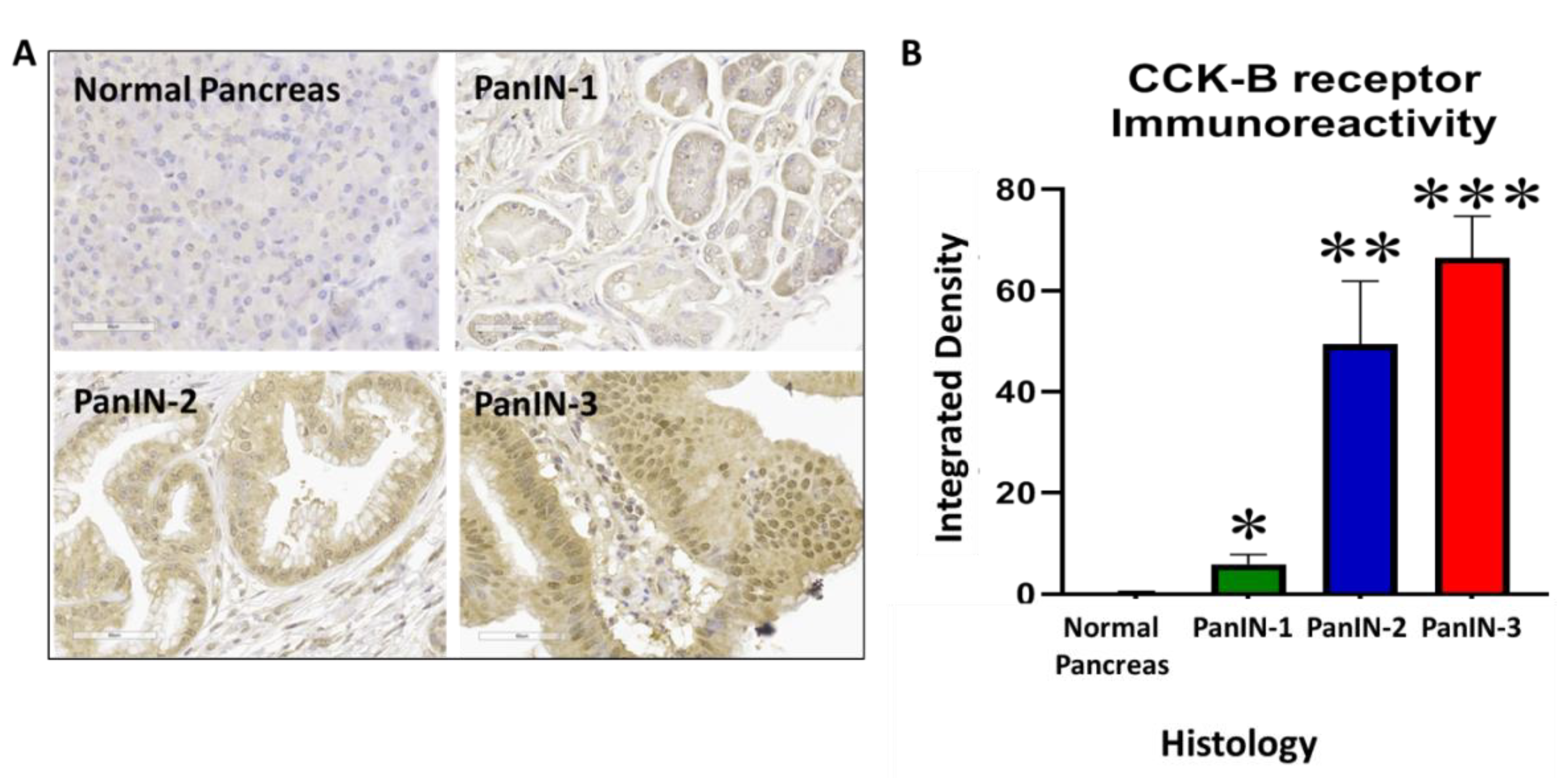
3.8. Human PanIN Lesions Express CCK-B Receptors
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Grodzinski, P.; Cai, W.; Liu, C.H. Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics. ACS Nano 2018, 12, 2106–2121. [Google Scholar] [CrossRef]
- Bogart, L.K.; Pourroy, G.; Murphy, C.J.; Puntes, V.; Pellegrino, T.; Rosenblum, D.; Peer, D.; Lévy, R. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano 2014, 8, 3107–3122. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Wong, J.C.; Lu, D.S. Staging of pancreatic adenocarcinoma by imaging studies. Clin. Gastroenterol. Hepatol. 2008, 6, 1301–1308. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (≤20 mm) pancreatic adenocarcinomas: Analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canto, M.I.; Goggins, M.; Hruban, R.H.; Petersen, G.M.; Giardiello, F.M.; Yeo, C.; Fishman, E.K.; Brune, K.; Axilbund, J.; Griffin, C.; et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clin. Gastroenterol. Hepatol. 2006, 4, 766–781. [Google Scholar] [CrossRef]
- Cao, H.; Le, D.; Yang, L.X. Current status in chemotherapy for advanced pancreatic adenocarcinoma. Anticancer Res. 2013, 33, 1785–1791. [Google Scholar]
- Tuveson, D.A.; Shaw, A.T.; Willis, N.A.; Silver, D.P.; Jackson, E.L.; Chang, S.; Mercer, K.L.; Grochow, R.; Hock, H.; Crowley, D.; et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 2004, 5, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.P.; Solomon, T.E. Cholecystokinin and pancreatic cancer: The chicken or the egg? Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G91–G101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.P.; Cooper, T.K.; McGovern, C.O.; Gilius, E.L.; Zhong, Q.; Liao, J.; Molinolo, A.A.; Gutkind, J.S.; Matters, G.L. Cholecystokinin receptor antagonist halts progression of pancreatic cancer precursor lesions and fibrosis in mice. Pancreas 2014, 43, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.P.; Rickabaugh, C.A.; McLaughlin, P.J.; Zagon, I.S. Cholecystokinin receptors and PANC-1 human pancreatic cancer cells. Am. J. Physiol. 1993, 265, G149–G155. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Liu, G.; Soundararajan, V.; McLaughlin, P.J.; Zagon, I.S. Identification and characterization of CCK-B/gastrin receptors in human pancreatic cancer cell lines. Am. J. Physiol. 1994, 266, R277–R283. [Google Scholar] [CrossRef] [PubMed]
- Saillan-Barreau, C.; Dufresne, M.; Clerc, P.; Sanchez, D.; Corominola, H.; Moriscot, C.; Guy-Crotte, O.; Escrieut, C.; Vaysse, N.; Gomis, R.; et al. Evidence for a functional role of the cholecystokinin-B/gastrin receptor in the human fetal and adult pancreas. Diabetes 1999, 48, 2015–2021. [Google Scholar] [CrossRef]
- Tamiolakis, D.; Venizelos, I.; Simopoulos, C.; Kotini, A.; Jivannakis, T.; Papadopoulos, N. Does neoplastic gastrin expression remodel the embryonal pattern of the protein? A study in human pancreas. Hepatogastroenterology 2004, 51, 249–252. [Google Scholar]
- Nadella, S.; Burks, J.; Huber, M.; Wang, J.; Cao, H.; Kallakury, B.; Tucker, R.D.; Boca, S.M.; Jermusyck, A.; Collins, I.; et al. Endogenous Gastrin Collaborates with Mutant KRAS in Pancreatic Carcinogenesis. Pancreas 2019, 48, 894–903. [Google Scholar] [CrossRef]
- Smith, J.P.; Shih, A.; Wu, Y.; McLaughlin, P.J.; Zagon, I.S. Gastrin regulates growth of human pancreatic cancer in a tonic and autocrine fashion. Am. J. Physiol. 1996, 270, R1078–R1084. [Google Scholar] [CrossRef]
- Matters, G.L.; Harms, J.F.; McGovern, C.O.; Jayakumar, C.; Crepin, K.; Smith, Z.P.; Nelson, M.C.; Stock, H.; Fenn, C.W.; Kaiser, J.; et al. Growth of human pancreatic cancer is inhibited by down-regulation of gastrin gene expression. Pancreas 2009, 38, e151–e161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.P.; Verderame, M.F.; Ballard, E.N.; Zagon, I.S. Functional significance of gastrin gene expression in human cancer cells. Regul. Pept. 2004, 117, 167–173. [Google Scholar] [CrossRef]
- Sosabowski, J.; Lee, M.; Dekker, B.; Simmons, B.; Singh, S.; Bereford, H.; Hagan, S.A.; McKenzie, A.J.; Mather, S.J.; Watson, S.A.; et al. Formulation development and manufacturing of a gastrin/CCK-2 targeting peptide as an interdiate drug product for clinical imaging study. Eur. J. Pharm. Sci. 2007, 31, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Burks, J.; Nadella, S.; Mahmud, A.; Mankongpaisarnrung, C.; Wang, J.; Hahm, J.I.; Tucker, R.D.; Shivapurkar, N.; Stern, S.T.; Smith, J.P.; et al. Cholecystokinin Receptor-Targeted Polyplex Nanoparticle Inhibits Growth and Metastasis of Pancreatic Cancer. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachi, G.; Bussolino, F.; Ciardelli, G.; Mattu, C. Nanomedicine for Imaging and Therapy of Pancreatic Adenocarcinoma. Front. Bioeng. Biotechnol. 2019, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, H.; Li, X.; Duan, N.; Hu, S.; Liu, Y.; Yue, Y.; Song, L.; Zhang, Y.; Li, D.; et al. Plectin-1 Targeted Dual-modality Nanoparticles for Pancreatic Cancer Imaging. EBioMedicine 2018, 30, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Qin, B.; Yuan, C.; Chen, Q.; Xing, D. Pancreatic Cancer detection via Galectin-1-targeted Thermoacoustic Imaging: Validation in an in vivo heterozygosity model. Theranostics 2020, 10, 9172–9185. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; An, Y.; Jia, G.; Wang, X.; He, C.; Ding, Y.; Tang, Q. Facile assembly of upconversion nanoparticle-based micelles for active targeted dual-mode imaging in pancreatic cancer. J. Nanobiotechnol. 2018, 16, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, A.B.; Aiertza, M.K.; Marradi, M.; Gil-Iceta, L.; Shekhter, Z.T.; Szczupak, B.; Jiménez-González, M.; Reese, T.; Scanziani, E.; Passoni, L.; et al. Functional Single-Chain Polymer Nanoparticles: Targeting and Imaging Pancreatic Tumors in Vivo. Biomacromolecules 2016, 17, 3213–3221. [Google Scholar] [CrossRef]
- Kelly, K.A.; Bardeesy, N.; Anbazhagan, R.; Gurumurthy, S.; Berger, J.; Alencar, H.; DePinho, R.A.; Mahmood, U.; Weissleder, R. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008, 5, e85. [Google Scholar] [CrossRef] [PubMed]
- Matters, G.L.; McGovern, C.; Harms, J.F.; Markovic, K.; Anson, K.; Jayakumar, C.; Martenis, M.; Awad, C.; Smith, J.P. Role of endogenous cholecystokinin on growth of human pancreatic cancer. Int. J. Oncol. 2011, 38, 593–601. [Google Scholar] [PubMed] [Green Version]
- Clawson, G.A.; Abraham, T.; Pan, W.; Tang, X.; Linton, S.S.; McGovern, C.O.; Loc, W.S.; Smith, J.P.; Butler, P.J.; Kester, M.; et al. A Cholecystokinin B Receptor-Specific DNA Aptamer for Targeting Pancreatic Ductal Adenocarcinoma. Nucleic Acid Ther. 2017, 27, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Hassan, P.A.; Rana, S.; Verma, G. Making sense of Brownian motion: Colloid characterization by dynamic light scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef]
- Lee, J.W.; Komar, C.A.; Bengsch, F.; Graham, K.; Beatty, G.L. Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+);LSL-Trp53(R172H/+);Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery. Curr. Protoc. Pharmacol. 2016, 73, 14. [Google Scholar] [CrossRef] [Green Version]
- McCarty, K.S., Jr.; Miller, L.S.; Cox, E.B.; Konrath, J.; McCarty, K.S., Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch. Pathol. Lab. Med. 1985, 109, 716–721. [Google Scholar] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.P. Identifying people at a high risk of developing pancreatic cancer. Nat. Rev. Cancer 2013, 13, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013, 62, 339–347. [Google Scholar] [CrossRef]
- Poruk, K.E.; Firpo, M.A.; Adler, D.G.; Mulvihill, S.J. Screening for pancreatic cancer: Why, how, and who? Ann. Surg. 2013, 257, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alauddin, M.M. Positron emission tomography (PET) imaging with (18)F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 55–76. [Google Scholar] [PubMed]
- Papagiannopoulou, D. Technetium-99m radiochemistry for pharmaceutical applications. J. Label. Comp. Radiopharm. 2017, 60, 502–520. [Google Scholar] [CrossRef] [PubMed]



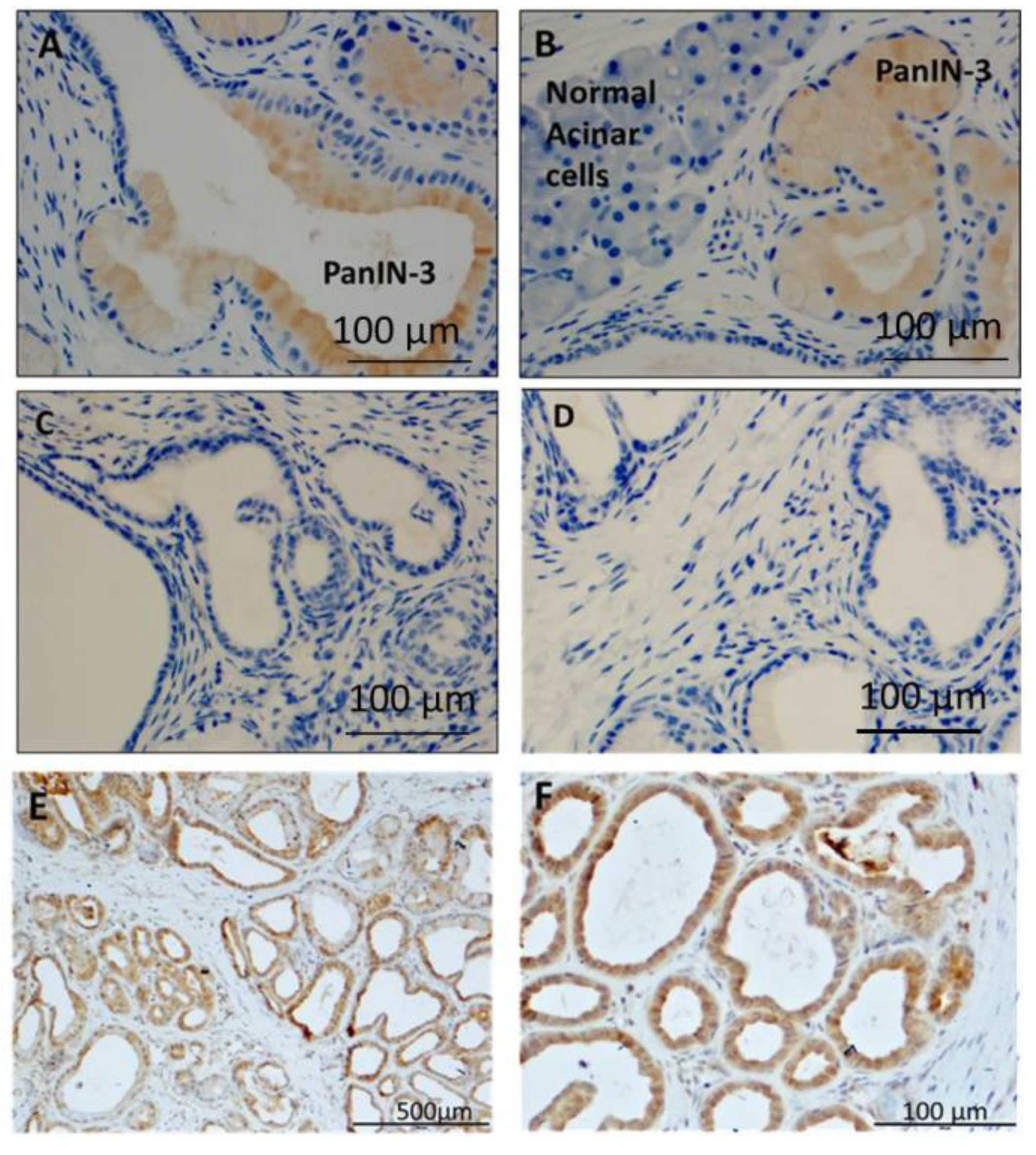
| Normal Pancreas | PanINs | Liver | Kidney | Heart | Stomach | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | % positive | H- Score | % positive | H- Score | % positive | H- Score | % positive | H- Score | % positive | H- Score | % positive | H- Score |
| ID98 | 0.01 | 0.01 | 9.33 | 21.82 | 0 | 0 | 0.02 | 0.02 | 0.06 | 0.07 | 0 | 0 |
| ID101 | 0 | 0 | 18.48 | 31.39 | 0.02 | 0.02 | 0.07 | 0.13 | 0 | 0.01 | 0 | 0 |
| ID114 | 0 | 0 | 31.72 | 58.32 | 0.01 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.P.; Cao, H.; Edmondson, E.F.; Dasa, S.S.K.; Stern, S.T. Cholecystokinin-B Receptor-Targeted Nanoparticle for Imaging and Detection of Precancerous Lesions in the Pancreas. Biomolecules 2021, 11, 1766. https://doi.org/10.3390/biom11121766
Smith JP, Cao H, Edmondson EF, Dasa SSK, Stern ST. Cholecystokinin-B Receptor-Targeted Nanoparticle for Imaging and Detection of Precancerous Lesions in the Pancreas. Biomolecules. 2021; 11(12):1766. https://doi.org/10.3390/biom11121766
Chicago/Turabian StyleSmith, Jill P., Hong Cao, Elijah F. Edmondson, Siva Sai Krishna Dasa, and Stephan T. Stern. 2021. "Cholecystokinin-B Receptor-Targeted Nanoparticle for Imaging and Detection of Precancerous Lesions in the Pancreas" Biomolecules 11, no. 12: 1766. https://doi.org/10.3390/biom11121766
APA StyleSmith, J. P., Cao, H., Edmondson, E. F., Dasa, S. S. K., & Stern, S. T. (2021). Cholecystokinin-B Receptor-Targeted Nanoparticle for Imaging and Detection of Precancerous Lesions in the Pancreas. Biomolecules, 11(12), 1766. https://doi.org/10.3390/biom11121766