Engineering of Saccharomyces cerevisiae for 24-Methylene-Cholesterol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cloning of the Full-Length Coding Region of the PhDHCR7 Gene
2.2. Strains, Media, and Culture Conditions
2.3. Strains and Plasmid Manipulation
2.4. Extraction and Quantification of Sterols
2.5. Gas Chromatography–Mass Spectroscopy (GC–MS) Analysis
2.6. 24-Methylene-Cholesterol Production by Shake-Flask Fermentation
2.7. RT-qPCR Analysis
2.8. Statistical Methods
3. Results
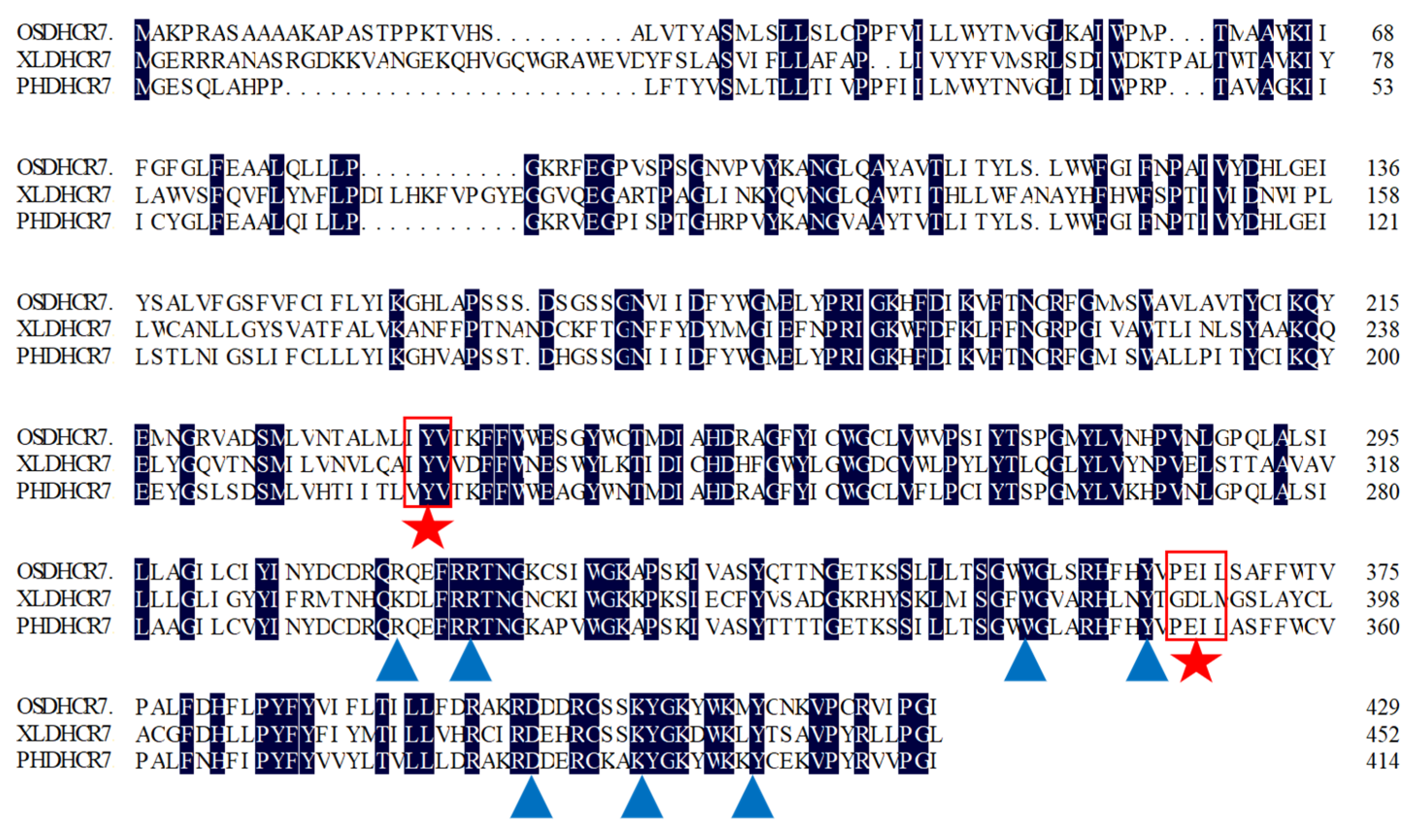
3.1. Cloning, Sequencing, and Alignment Analysis of PhDHCR7
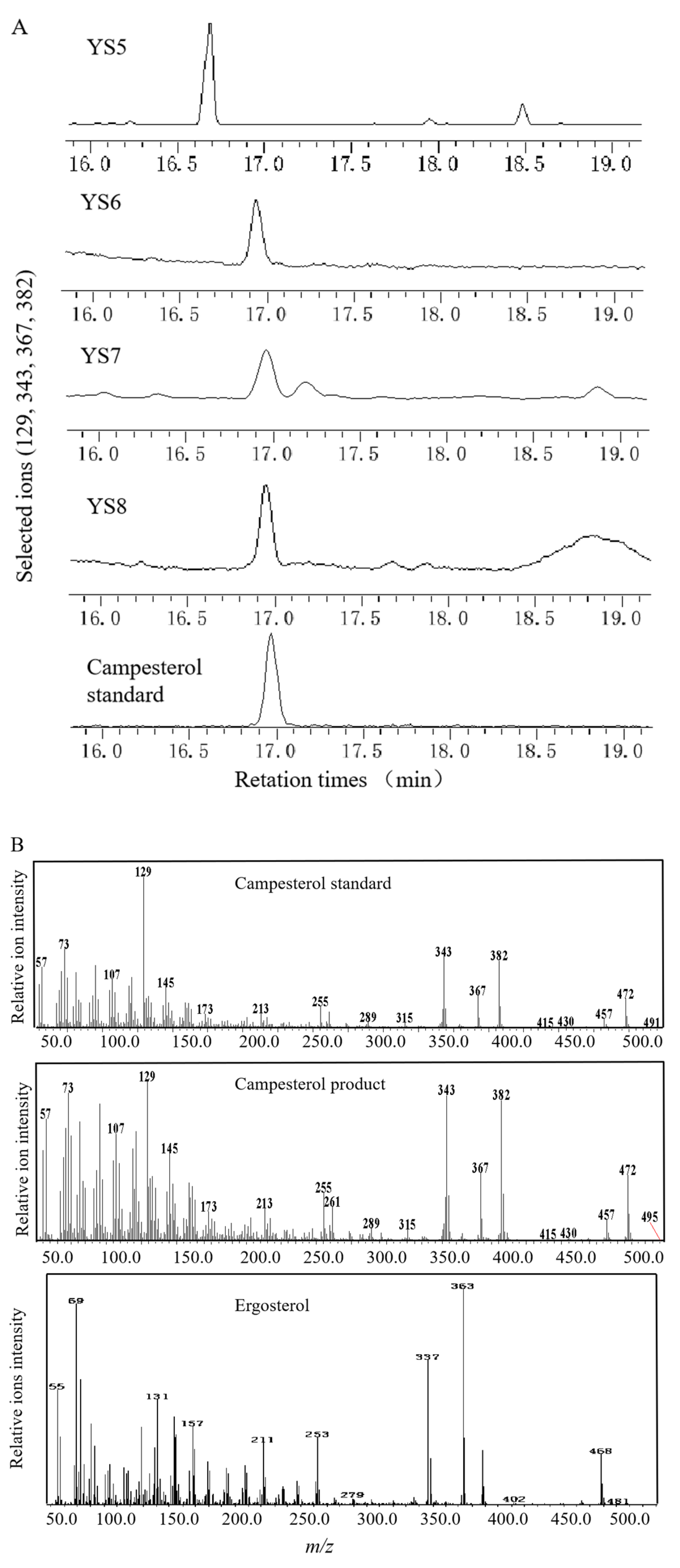
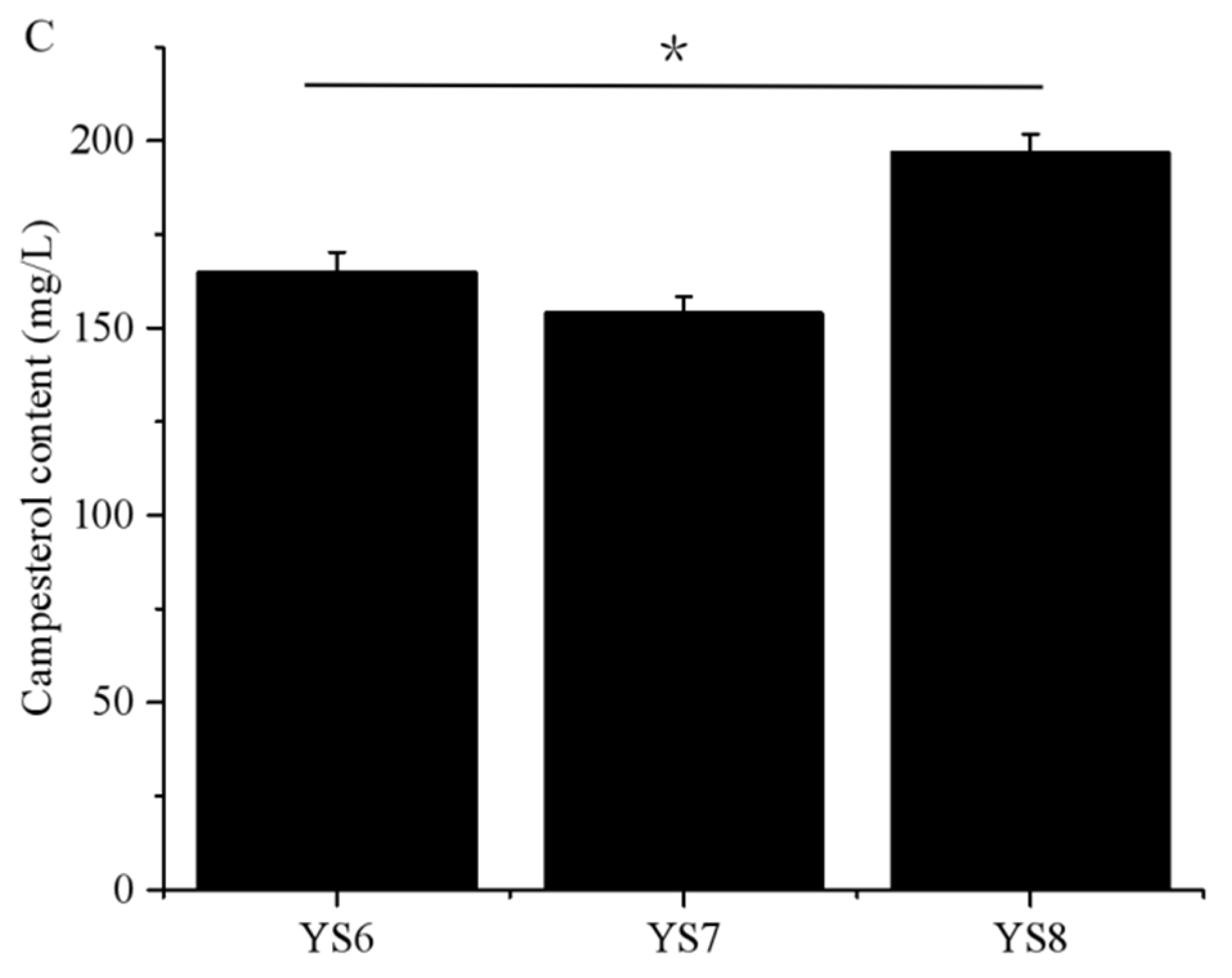
3.2. Campesterol Biosynthetic Pathway Was Constructed in S. cerevisiae via Blocking ERG5 and Introducing the Codon-Optimized DHCR7s
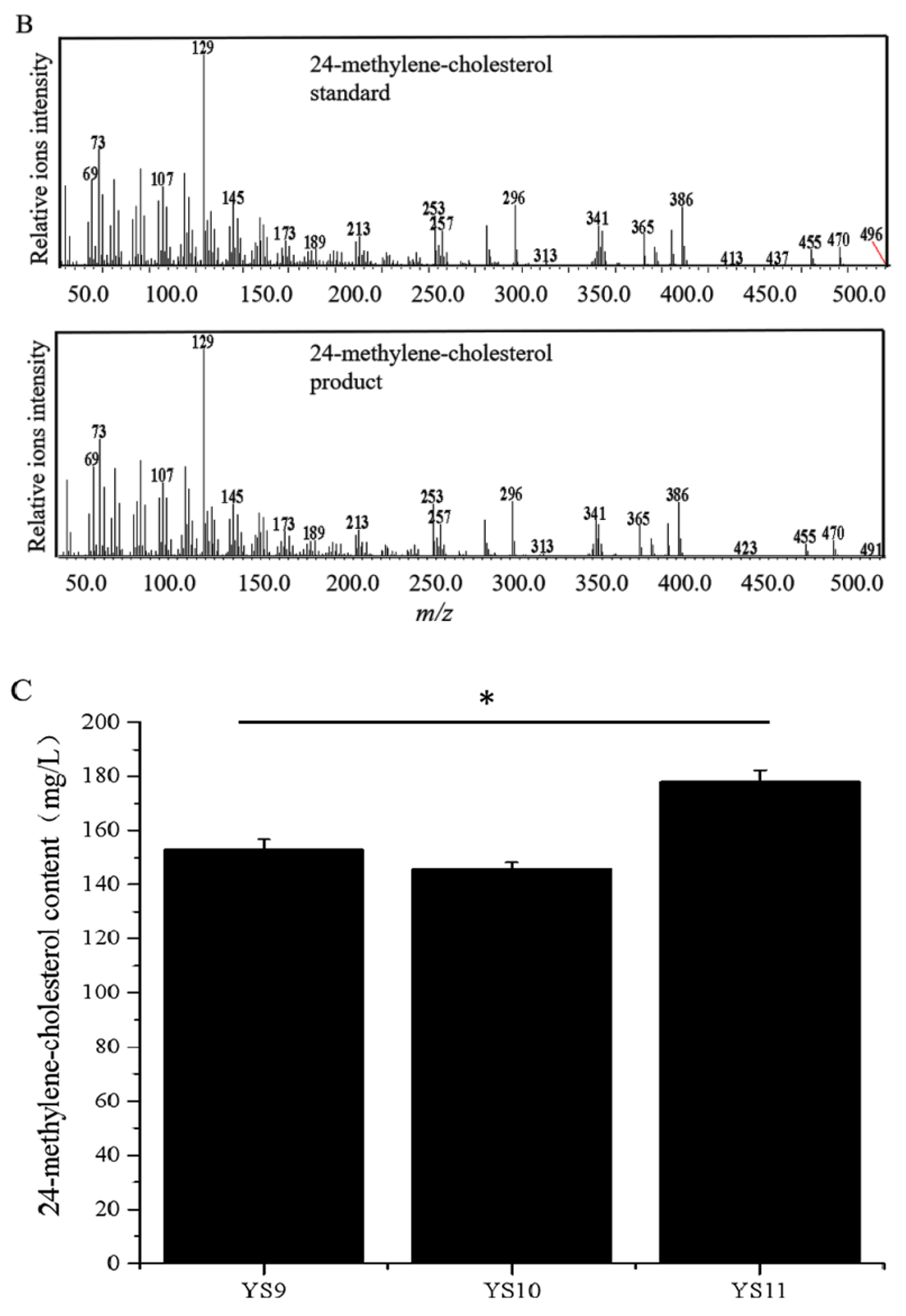
3.3. 24-Methylene-Cholesterol Was Further Produced by Disrupting ERG4
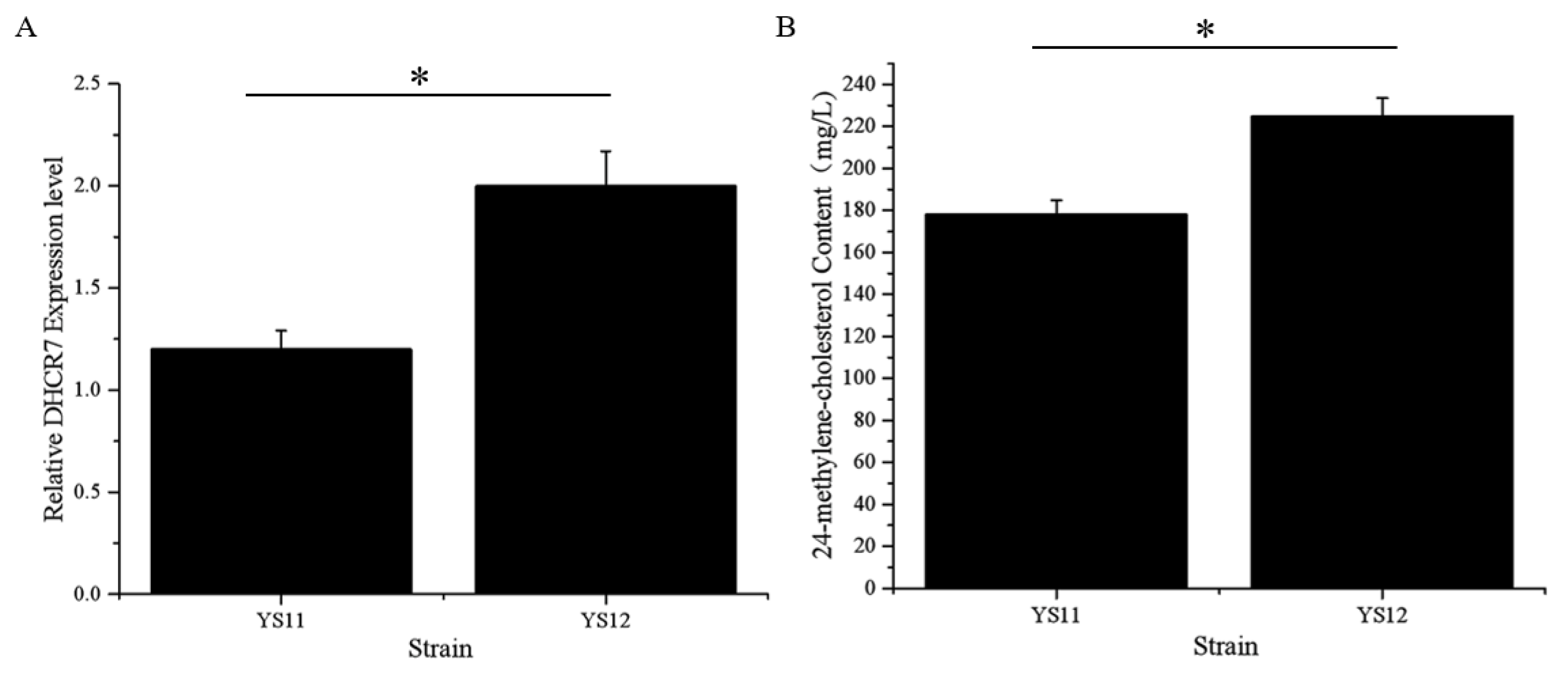
3.4. Overproduction of 24-Methylene-Cholesterol by Increasing the Number of XlDHCR7 Copies
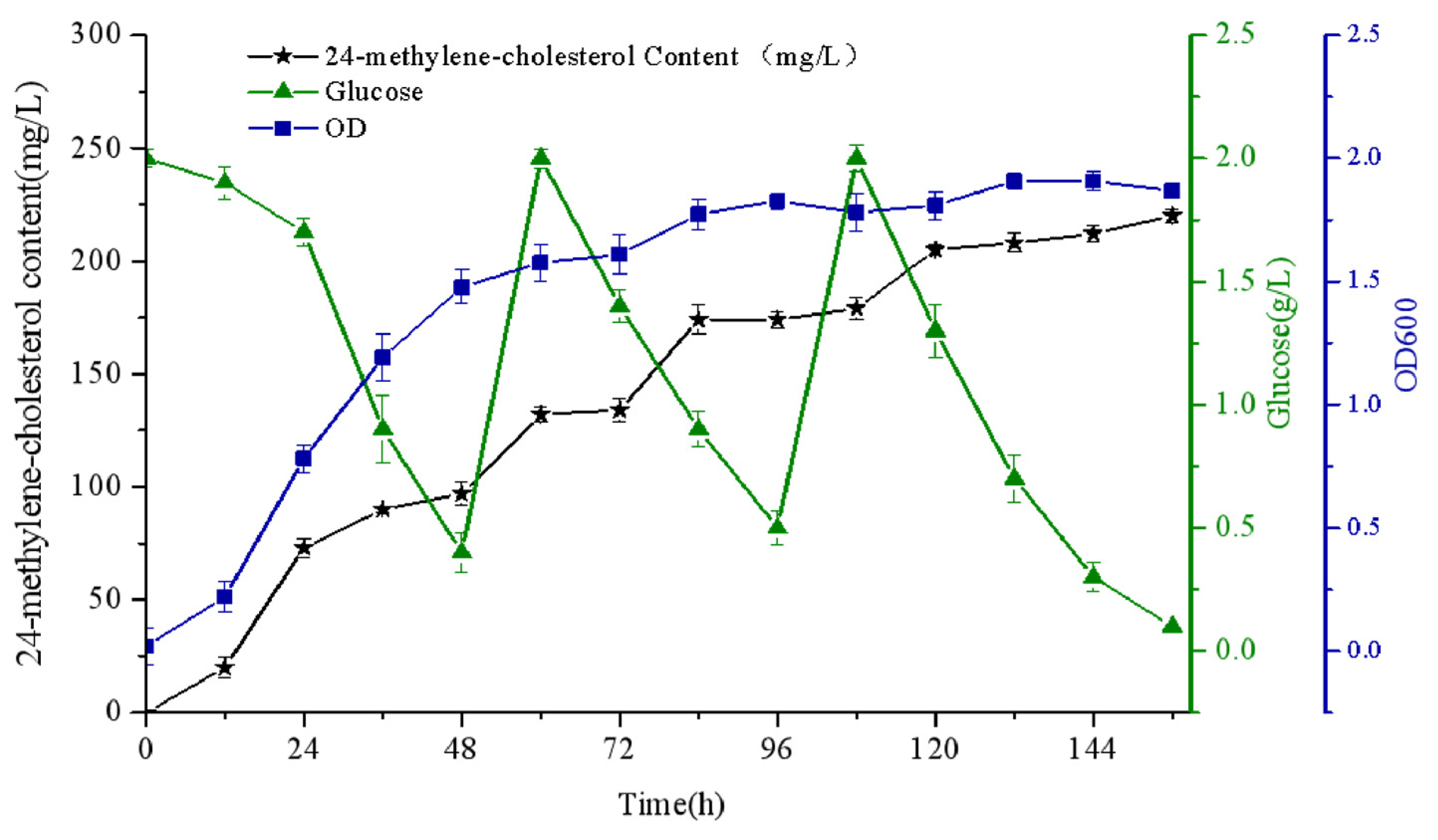
3.5. Characteristics of the Optimal Strain YS12 in Shake-Flask Fermentation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demonty, I.; Ras, R.T.; Knaap, V.D.H.C.M.; Duchateau, G.S.M.J.E.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous Dose-Response Response Relationship of the LDL-Cholesterol-Lowering Effect of Phytosterol Intake 1,2. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao-Xing, D.; Wen-Hai, X.; Ying, W.; Zhou, X.; Zhang, Y.; Liu, D.; Yuan, Y.-J. Engineering Yarrowia lipolytica for Campesterol Overproduction. PLoS ONE 2016, 11, e0146773. [Google Scholar]
- Ro, D.K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.; Reichstein, T.; Schindler, O.; Lederer, E. Isolation of 24-Methylene-cholesterol from Honey Bees (Apis mellifica L). Nature 1959, 184 (Suppl. S10), 732. [Google Scholar] [CrossRef]
- Barbier, M.; Hugel, M.F.; Lederer, E. Isolation of 24-methylene cholesterol from the pollen of different plants. Bull. Soc. Chim. Biol. 1960, 42, 91. [Google Scholar]
- Dubey, S.; Kallubai, M.; Subramanyam, R. Improving the inhibition of β-amyloid aggregation by withanolide and withanoside derivatives. Int. J. Biol. Macromol. 2021, 173, 56–65. [Google Scholar] [CrossRef]
- Dar, N.J.; Satti, N.K.; Dutt, P.; Hamid, A.; Ahmad, M. Attenuation of Glutamate-Induced Excitotoxicity by Withanolide-A in Neuron-Like Cells: Role for PI3K/Akt/MAPK Signaling Pathway. Mol. Neurobiol. 2017, 55, 2725–2739. [Google Scholar] [CrossRef]
- Ma, Y.M.; Han, W.; Li, J.; Hu, L.-H.; You, J.-B. Physalin B not only inhibits the ubiquitin-proteasome pathway but also induces incomplete autophagic response in human colon cancer cells. Acta Pharmacol. Sin. 2015, 36, 517–527. [Google Scholar] [CrossRef]
- Shin, J.M.; Lee, K.M.; Lee, H.J.; Yoon, J.H.; Nho, C.W. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes. BMC Complement. Altern. Med. 2019, 19, 1904. [Google Scholar] [CrossRef] [Green Version]
- In-vitro and in-vivo antitumour activity of physalins B and D from Physalis angulate. J. Pharm. Pharmacol. 2006, 58, 235–241.
- Lenton, J.R.; Goad, L.J.; Goodwin, T.W. Sitosterol biosynthesis in Hordeum vulgare. Phytochemistry 1975, 14, 1523–1528. [Google Scholar] [CrossRef]
- Wriessnegger, T.; Leitner, E.; Belegratis, M.; Ingolic, E.; Daum, G. Lipid analysis of mitochondrial membranes from the yeast Pichia pastoris. Chem. Phys. Lipids 2007, 1791, 166–172. [Google Scholar] [CrossRef]
- Uomori, A.; Nakagawa, Y.; Yoshimatsu, S.; Seo, S.; Sankawa, U.; Takeda, K. Biosynthesis of campesterol and dihydrobrassicasterol in cultured cells of Amsonia elliptica. Phytochemistry 1992, 31, 1569–1572. [Google Scholar] [CrossRef]
- Tsukagoshi, Y.; Suzuki, H.; Seki, H.; Muranaka, T.; Ohyama, K.; Fujimoto, Y. Ajuga Δ24-Sterol Reductase Catalyzes the Direct Reductive Conversion of 24-Methylenecholesterol to Campesterol. J. Biol. Chem. 2016, 291, 8189–8198. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.D.; Tan, S.Y.; Dong, G.R.; Niu, Y.J.; Hu, C.Y.; Meng, Y.H. Increased campesterol synthesis by improving lipid content in engineered Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2020, 104, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Haines, M.; Storch, M.; Freemont, P.S. Combinatorial metabolic pathway assembly approaches and toolkits for modular assembly. Metab. Eng. 2020, 63, 81–101. [Google Scholar] [CrossRef]
- Tan, G.Y.; Liu, T. Rational synthetic pathway refactoring of natural products biosynthesis in actinobacteria. Metab. Eng. 2016, 39, 228. [Google Scholar] [CrossRef]
- Lian, J.; Mishra, S.; Zhao, H. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab. Eng. 2018, 50, 85–108. [Google Scholar] [CrossRef]
- Ye, L.; Lv, X.; Yu, H. Engineering microbes for isoprene production. Metab. Eng. 2016, 38, 125–138. [Google Scholar] [CrossRef]
- Fukushima, A.; Nakamura, M.; Suzuki, H.; Yamazaki, M.; Knoch, E.; Mori, T.; Hirai, G.; Sodeoka, M.; Saito, K. Comparative Characterization of the Leaf Tissue of Physalis alkekengi and Physalis peruviana Using RNA-seq and Metabolite Profiling. Front. Plant Sci. 2016, 7, 1883. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Meng, H.; Li, J.; Wang, J.; Li, Q.; Wang, Y.; Zhang, Y. Identification of Novel Knockout Targets for Improving Terpenoids Biosynthesis in Saccharomyces cerevisiae. PLoS ONE 2014, 9, e112615. [Google Scholar] [CrossRef] [PubMed]
- Karst, F.; Lacroute, F. Ergosterol biosynthesis in Saccharomyces cerevisiae. Mol. Gen. Genet. 1977, 154, 269–277. [Google Scholar] [CrossRef]
- Prabhu, A.V.; Sharpe, L.J.; Brown, A.J. The sterol-based transcriptional control of human 7-dehydrocholesterol reductase (DHCR7): Evidence of a cooperative regulatory program in cholesterol synthesis. Biochim. Biophys. Acta 2014, 1841, 1431–1439. [Google Scholar] [CrossRef]
- He, X.; Zhang, B.; Tan, H. Overexpression of a sterol C-24(28) reductase increases ergosterol production in Saccharomyces cerevisiae. Biotechnol. Lett. 2003, 25, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Zweytick, D.; Hrastnik, C.; Kohlwein, S.D.; Daum, G. Biochemical characterization and subcellular localization of the sterol C-24(28) reductase, Erg4p, from the yeast Saccharomyces cerevisiae. FEBS Lett. 2000, 470, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, S.; Satoh, K.; Nagata, T.; Kawagashira, N.; Doi, K.; Kishimoto, N.; Yazaki, J.; Ishikawa, M.; Yamada, H.; Ooka, H.; et al. Collection, Mapping, and Annotation of Over 28,000 cDNA Clones from japonica Rice. Science 2003, 301, 376–379. [Google Scholar] [CrossRef]
- Damu, A.G.; Kuo, P.C.; Su, C.R.; Kuo, T.-H.; Chen, T.-H.; Bastow, K.F.; Lee, K.-H.; Wu, T.S. Isolation, Structures, and Structure Cytotoxic Activity Relationships of withanolides and Physalins from Physalis angulata. J. Nat. Prod. 2007, 70, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Song, A.A.; Janna, O.A.; Abdullah, M.P.; Shafee, N.; Othman, R.; Noor, N.M.; Rahim, R.A. Engineering the lactococcal mevalonate pathway for increased sesquiterpene production. FEMS Microbiol. Lett. 2014, 355, 177–184. [Google Scholar] [CrossRef] [Green Version]


| Strain Genotypes and Corresponding Products in This Study | |||
|---|---|---|---|
| Strains | Source | Genotype | Major sterol |
| YS5 | [21] | MATα(leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) | Ergosterol |
| YS6 | This study | ERG5::URA3-pTEF2-DHCR7(Oryza sativa)-tCYC1 | Campesterol |
| YS7 | This study | ERG5::URA3-pTEF2-DHCR7 (Physalis angulate)-tCYC1 | Campesterol |
| YS8 | This study | ERG5::URA3-pTEF2-DHCR7(Xenopus laevis)-tCYC1 | Campesterol |
| YS9 | This study | ERG5::URA3-pTEF2-DHCR7(Oryza sativa)-tCYC1 ERG4::TRP1 | 24-Methylene-cholesterol |
| YS10 | This study | ERG5::URA3-pTEF2-DHCR7(Physalis angulate)-tCYC1 ERG4::TRP1 | 24-Methylene-cholesterol |
| YS11 | This study | ERG5::URA3-pTEF2-DHCR7(Xenopus laevis)-tCYC1 ERG4::TRP1 | 24-Methylene-cholesterol |
| YS12 | This study | ERG5::URA3-pTEF2-DHCR7(Xenopus laevis)-tCYC1 ERG4::TRP1ERG4::HIS3-pTEF2-DHCR7(Xenopus laevis)-tCYC1 | 24-Methylene-cholesterol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, C.; Zhang, Y. Engineering of Saccharomyces cerevisiae for 24-Methylene-Cholesterol Production. Biomolecules 2021, 11, 1710. https://doi.org/10.3390/biom11111710
Yang J, Li C, Zhang Y. Engineering of Saccharomyces cerevisiae for 24-Methylene-Cholesterol Production. Biomolecules. 2021; 11(11):1710. https://doi.org/10.3390/biom11111710
Chicago/Turabian StyleYang, Jiao, Changfu Li, and Yansheng Zhang. 2021. "Engineering of Saccharomyces cerevisiae for 24-Methylene-Cholesterol Production" Biomolecules 11, no. 11: 1710. https://doi.org/10.3390/biom11111710
APA StyleYang, J., Li, C., & Zhang, Y. (2021). Engineering of Saccharomyces cerevisiae for 24-Methylene-Cholesterol Production. Biomolecules, 11(11), 1710. https://doi.org/10.3390/biom11111710






