Comparison of Conventional and Radiomic Features between 18F-FBPA PET/CT and PET/MR
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
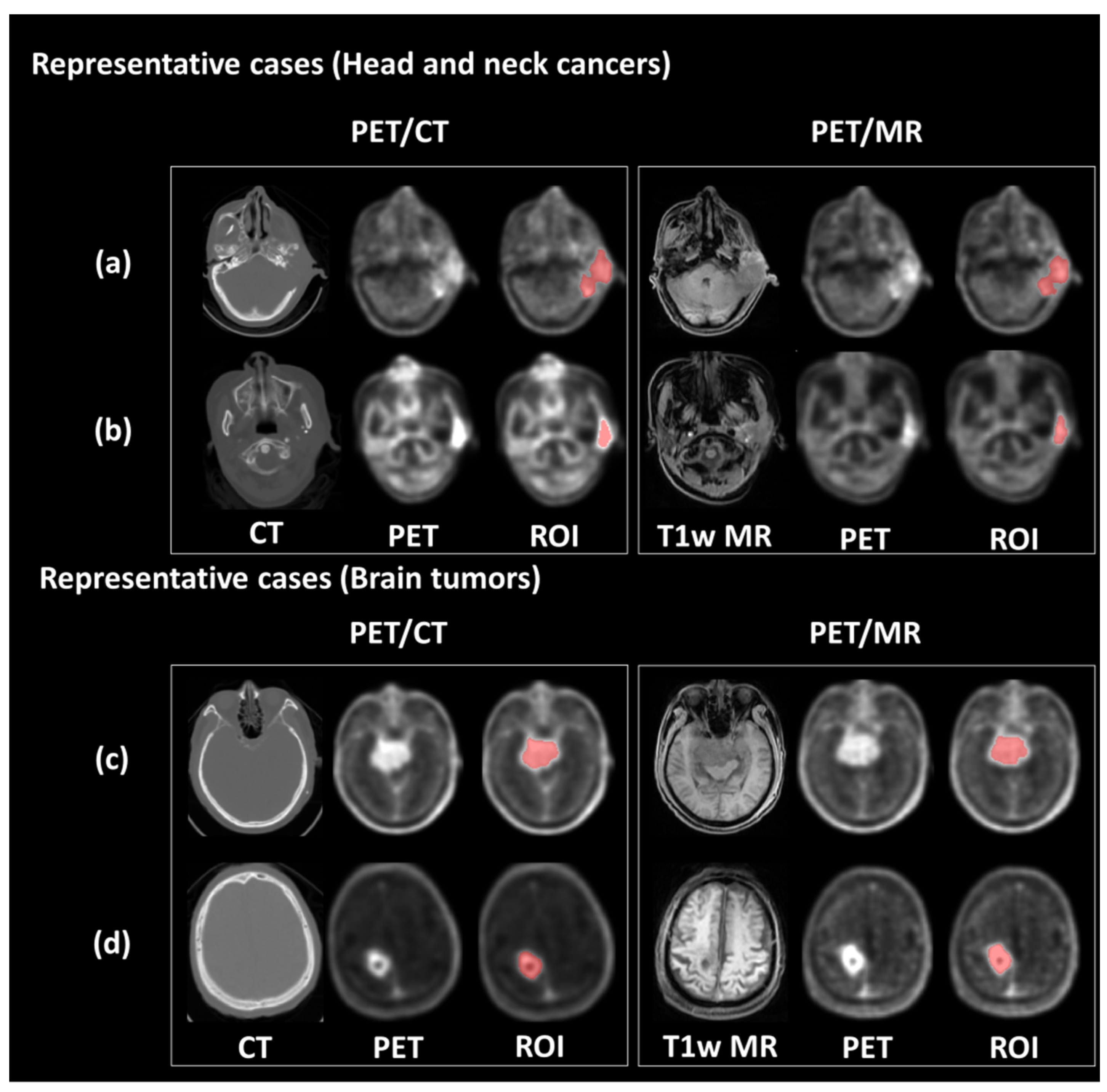
2.2. Preprocessing of PET Data
2.3. Radiomic Feature Extraction
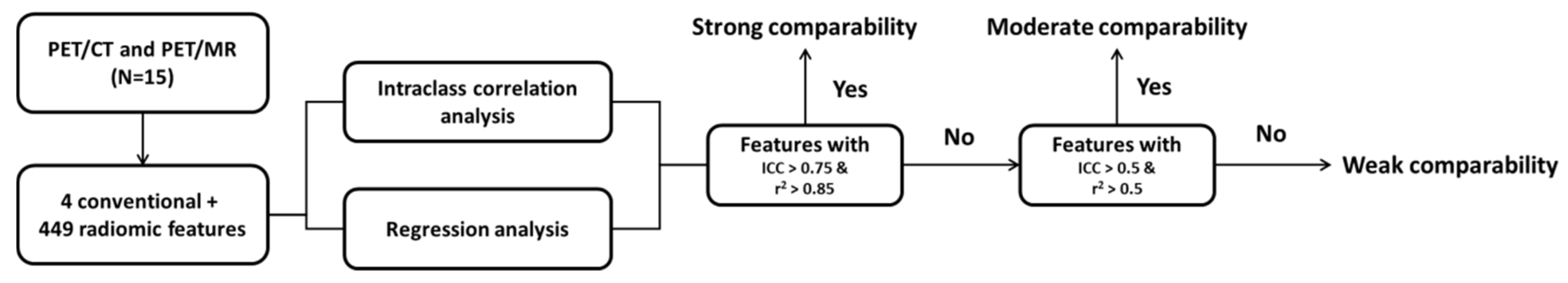
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
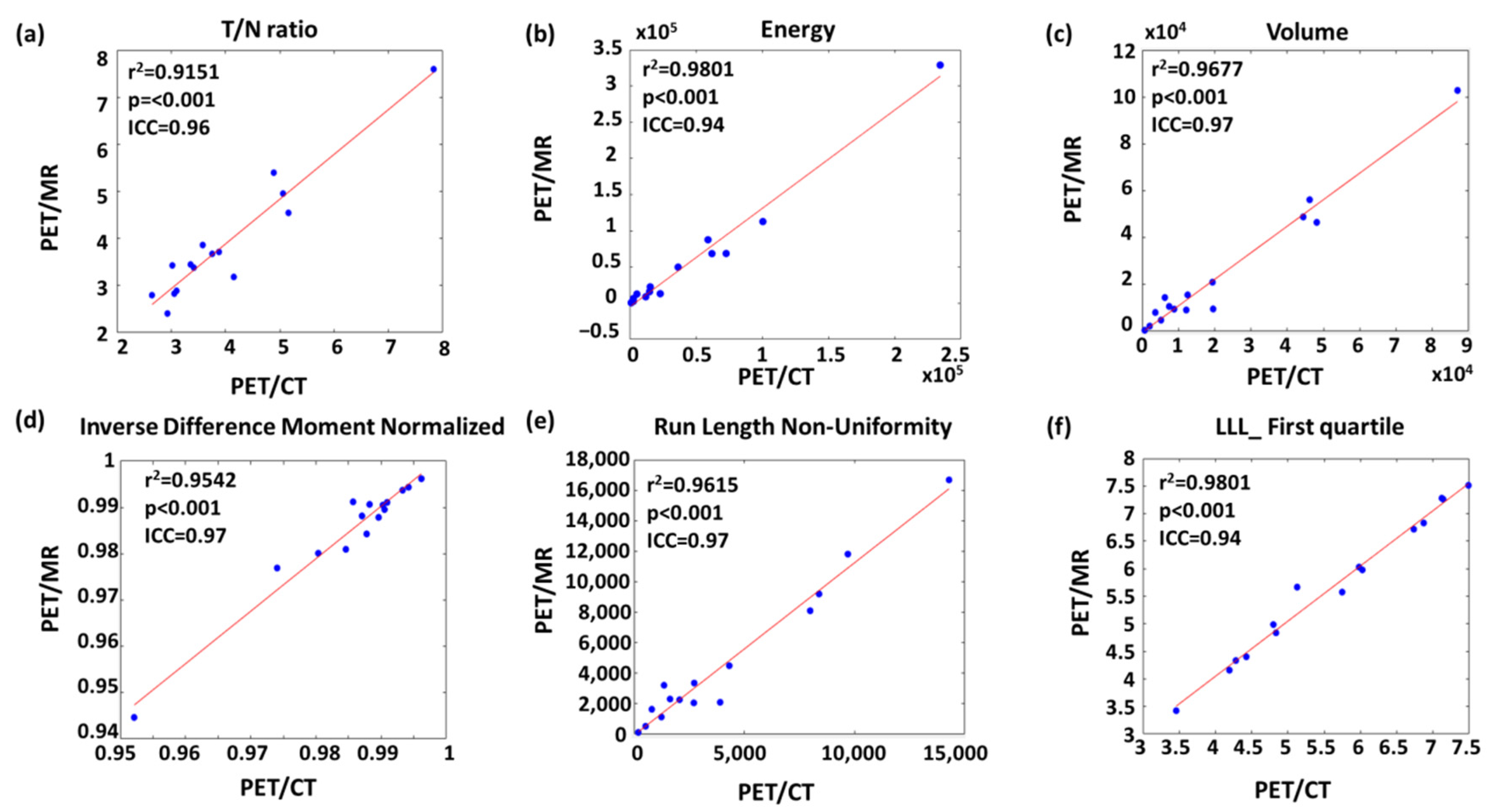
3.2. Linear Correlation between PET/CT and PET/MR Features
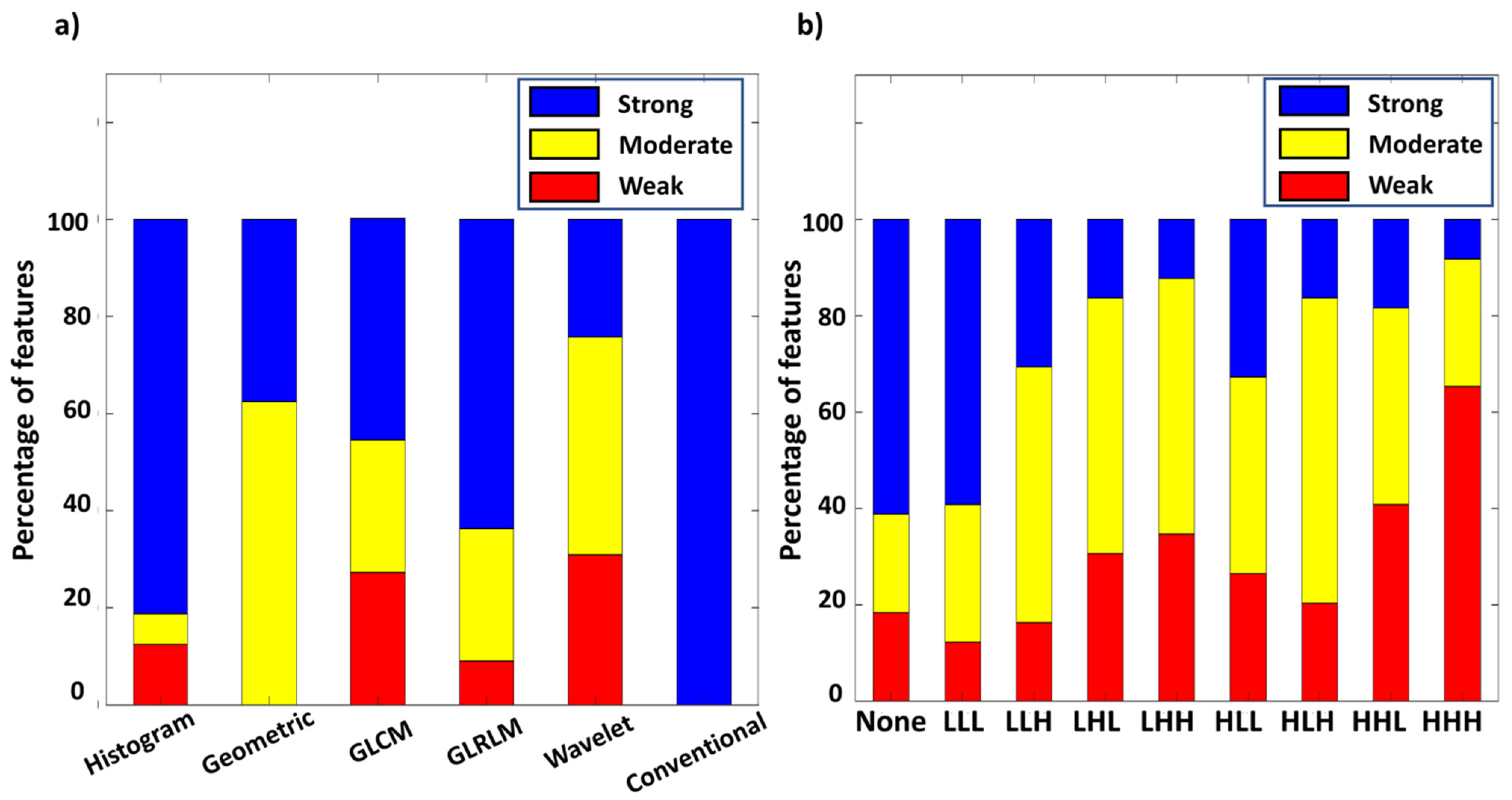
3.3. Intraclass Correlation between PET/CT and PET/MR Features
3.4. Features with High Comparability for Interchange between PET/CT and PET/MR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef]
- Kawabata, S.; Miyatake, S.-I.; Nonoguchi, N.; Hiramatsu, R.; Iida, K.; Miyata, S.; Yokoyama, K.; Doi, A.; Kuroda, Y.; Kuroiwa, T. Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patients. Appl. Radiat. Isot. 2009, 67, S15–S18. [Google Scholar] [CrossRef]
- Nedunchezhian, K.; Aswath, N.; Thiruppathy, M.; Thirugnanamurthy, S. Boron neutron capture therapy–A literature review. J. Clin. Diagn. Res. 2016, 10, ZE01. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Ikeda, H.; Nagamori, S.; Wiriyasermkul, P.; Tanaka, Y.; Naka, S.; Kanai, Y.; Hagiwara, K.; Aoki, M.; Shimosegawa, E. 18 F-FBPA as a tumor-specific probe of L-type amino acid transporter 1 (LAT1): A comparison study with 18 F-FDG and 11 C-Methionine PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 321–331. [Google Scholar] [CrossRef]
- Barth, R.F.; Soloway, A.H.; Fairchild, R.G. Boron neutron capture therapy for cancer. Sci. Am. 1990, 263, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Vicente, M.H.; Harling, O.K.; Kiger, W.; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 1–21. [Google Scholar] [CrossRef]
- Mehta, S.C.; Lu, D.R. Targeted drug delivery for boron neutron capture therapy. Pharm. Res. 1996, 13, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Aihara, T.; Morita, N.; Kamitani, N.; Kumada, H.; Ono, K.; Hiratsuka, J.; Harada, T. Boron neutron capture therapy for advanced salivary gland carcinoma in head and neck. Int. J. Clin. Oncol. 2014, 19, 437–444. [Google Scholar] [CrossRef]
- Takahashi, Y.; Imahori, Y.; Mineura, K. Prognostic and therapeutic indicator of fluoroboronophenylalanine positron emission tomography in patients with gliomas. Clin. Cancer Res. 2003, 9, 5888–5895. [Google Scholar] [PubMed]
- Imahori, Y.; Ueda, S.; Ohmori, Y.; Kusuki, T.; Ono, K.; Fujii, R.; Ido, T. Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J. Nucl. Med. 1998, 39, 325–333. [Google Scholar]
- Aihara, T.; Hiratsuka, J.; Morita, N.; Uno, M.; Sakurai, Y.; Maruhashi, A.; Ono, K.; Harada, T. First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck J. Sci. Spec. Head Neck 2006, 28, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Watabe, T.; Nagamori, S.; Naka, S.; Ikeda, H.; Kongpracha, P.; Horitsugi, G.; Kanai, Y.; Shimosegawa, E.; Kanai, Y. Distribution of LAT1-targeting PET tracer was independent of the tumor blood flow in rat xenograft models of C6 glioma and MIA PaCa-2. Ann. Nucl. Med. 2019, 33, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Beshr, R.; Isohashi, K.; Watabe, T.; Naka, S.; Horitsugi, G.; Romanov, V.; Kato, H.; Miyatake, S.-I.; Shimosegawa, E.; Hatazawa, J. Preliminary feasibility study on differential diagnosis between radiation-induced cerebral necrosis and recurrent brain tumor by means of [18 F] fluoro-borono-phenylalanine PET/CT. Ann. Nucl. Med. 2018, 32, 702–708. [Google Scholar] [CrossRef]
- Ehman, E.C.; Johnson, G.B.; Villanueva-Meyer, J.E.; Cha, S.; Leynes, A.P.; Larson, P.E.Z.; Hope, T.A. PET/MRI: Where might it replace PET/CT? J. Magn. Reson. Imaging 2017, 46, 1247–1262. [Google Scholar] [CrossRef]
- Lyons, K.; Seghers, V.; Sorensen, J.I.; Zhang, W.; Paldino, M.J.; Krishnamurthy, R.; Rohren, E.M. Comparison of standardized uptake values in normal structures between PET/CT and PET/MRI in a tertiary pediatric hospital: A prospective study. Am. J. Roentgenol. 2015, 205, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Grootjans, W.; Tixier, F.; van der Vos, C.S.; Vriens, D.; Le Rest, C.C.; Bussink, J.; Oyen, W.J.; de Geus-Oei, L.-F.; Visvikis, D.; Visser, E.P. The impact of optimal respiratory gating and image noise on evaluation of intratumor heterogeneity on 18F-FDG PET imaging of lung cancer. J. Nucl. Med. 2016, 57, 1692–1698. [Google Scholar] [CrossRef]
- Leijenaar, R.T.; Carvalho, S.; Velazquez, E.R.; Van Elmpt, W.J.; Parmar, C.; Hoekstra, O.S.; Hoekstra, C.J.; Boellaard, R.; Dekker, A.L.; Gillies, R.J. Stability of FDG-PET Radiomics features: An integrated analysis of test-retest and inter-observer variability. Acta Oncol. 2013, 52, 1391–1397. [Google Scholar] [CrossRef]
- Su, Y.; Rubin, B.B.; McConathy, J.; Laforest, R.; Qi, J.; Sharma, A.; Priatna, A.; Benzinger, T.L. Impact of MR-based attenuation correction on neurologic PET studies. J. Nucl. Med. 2016, 57, 913–917. [Google Scholar] [CrossRef]
- Fahey, F.H.; Kinahan, P.E.; Doot, R.K.; Kocak, M.; Thurston, H.; Poussaint, T.Y. Variability in PET quantitation within a multicenter consortium. Med. Phys. 2010, 37, 3660–3666. [Google Scholar] [CrossRef]
- Vuong, D.; Tanadini-Lang, S.; Huellner, M.W.; Veit-Haibach, P.; Unkelbach, J.; Andratschke, N.; Kraft, J.; Guckenberger, M.; Bogowicz, M. Interchangeability of radiomic features between [18F]-FDG PET/CT and [18F]-FDG PET/MR. Med. Phys. 2019, 46, 1677–1685. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Tsuyoshi, H.; Kanno, M.; Yamada, S.; Kobayashi, M.; Narita, N.; Kimura, H.; Fujieda, S.; Yoshida, Y.; Okazawa, H. Selected PET radiomic features remain the same. Oncotarget 2018, 9, 20734. [Google Scholar] [CrossRef][Green Version]
- Stroobants, S.; Goeminne, J.; Seegers, M.; Dimitrijevic, S.; Dupont, P.; Nuyts, J.; Martens, M.; Van den Borne, B.; Cole, P.; Sciot, R. 18FDG-positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec®). Eur. J. Cancer 2003, 39, 2012–2020. [Google Scholar] [CrossRef]
- Schwarz, J.D.; Bader, M.; Jenicke, L.; Hemminger, G.; Jänicke, F.; Avril, N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J. Nucl. Med. 2005, 46, 1144–1150. [Google Scholar]
- Avril, N.; Sassen, S.; Schmalfeldt, B.; Naehrig, J.; Rutke, S.; Weber, W.A.; Werner, M.; Graeff, H.; Schwaiger, M.; Kuhn, W. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J. Clin. Oncol 2005, 23, 7445–7453. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-F.; Hsu, F.-T.; Hsieh, K.L.-C.; Kao, Y.-C.J.; Cheng, S.-J.; Hsu, J.B.-K.; Tsai, P.-H.; Chen, R.-J.; Huang, C.-C.; Yen, Y. Machine learning–based radiomics for molecular subtyping of gliomas. Clin. Cancer Res. 2018, 24, 4429–4436. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Wu, C.-C.; Lee, C.-C.; Huang, H.-E.; Lee, W.-K.; Chung, W.-Y.; Wu, H.-M.; Guo, W.-Y.; Wu, Y.-T.; Lu, C.-F. Prediction of pseudoprogression and long-term outcome of vestibular schwannoma after Gamma Knife radiosurgery based on preradiosurgical MR radiomics. Radiother. Oncol. 2020, 155, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Du, S.; Sun, H.; Gao, S.; Xin, J.; Lu, Z. Metabolic parameters with different thresholds for evaluating tumor recurrence and their correlations with hematological parameters in locally advanced squamous cell cervical carcinoma: An observational 18F-FDG PET/CT study. Quant. Imaging Med. Surg. 2019, 9, 440. [Google Scholar] [CrossRef]
- Dhruv, B.; Mittal, N.; Modi, M. Study of Haralick’s and GLCM texture analysis on 3D medical images. Int. J. Neurosci. 2019, 129, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Karacavus, S.; Yılmaz, B.; Tasdemir, A.; Kayaaltı, Ö.; Kaya, E.; İçer, S.; Ayyıldız, O. Can laws be a potential PET image texture analysis approach for evaluation of tumor heterogeneity and histopathological characteristics in NSCLC? J. Digit. Imaging 2018, 31, 210–223. [Google Scholar] [CrossRef] [PubMed]
- García-Olalla, Ó.; Fernández-Robles, L.; Alegre, E.; Castejón-Limas, M.; Fidalgo, E. Boosting Texture-Based Classification by Describing Statistical Information of Gray-Levels Differences. Sensors 2019, 19, 1048. [Google Scholar] [CrossRef]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Eliasziw, M.; Donner, A. Sample size and optimal designs for reliability studies. Stat. Med. 1998, 17, 101–110. [Google Scholar] [CrossRef]
- Parmar, C.; Velazquez, E.R.; Leijenaar, R.; Jermoumi, M.; Carvalho, S.; Mak, R.H.; Mitra, S.; Shankar, B.U.; Kikinis, R.; Haibe-Kains, B. Robust radiomics feature quantification using semiautomatic volumetric segmentation. PLoS ONE 2014, 9, e102107. [Google Scholar] [CrossRef]
- Klyuzhin, I.S.; Fu, J.F.; Shenkov, N.; Rahmim, A.; Sossi, V. Use of generative disease models for analysis and selection of radiomic features in PET. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 3, 178–191. [Google Scholar] [CrossRef]
- Herrera, M.S.; González, S.J.; Minsky, D.M.; Kreiner, A.J. Evaluation of performance of an accelerator-based BNCT facility for the treatment of different tumor targets. Phys. Med. 2013, 29, 436–446. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, D.N. A history of boron neutron capture therapy of brain tumours: Postulation of a brain radiation dose tolerance limit. Brain 1991, 114, 1609–1629. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, H.-P.W.; Pichler, B.J.; Schmand, M.; Burbar, Z.; Michel, C.; Ladebeck, R.; Jattke, K.; Townsend, D.; Nahmias, C.; Jacob, P.K. Simultaneous MR/PET imaging of the human brain: Feasibility study. Radiology 2008, 248, 1028–1035. [Google Scholar] [CrossRef]
- Boss, A.; Bisdas, S.; Kolb, A.; Hofmann, M.; Ernemann, U.; Claussen, C.D.; Pfannenberg, C.; Pichler, B.J.; Reimold, M.; Stegger, L. Hybrid PET/MRI of intracranial masses: Initial experiences and comparison to PET/CT. J. Nucl. Med. 2010, 51, 1198–1205. [Google Scholar] [CrossRef]
- Weber, M.-A.; Henze, M.; Tüttenberg, J.; Stieltjes, B.; Meissner, M.; Zimmer, F.; Burkholder, I.; Kroll, A.; Combs, S.E.; Vogt-Schaden, M. Biopsy targeting gliomas: Do functional imaging techniques identify similar target areas? Investig. Radiol. 2010, 45, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Aihara, T.; Morita, N. BNCT for advanced or recurrent head and neck cancer. In Neutron Capture Therapy; Springer: Berlin/Heidelberg, Germany, 2012; pp. 417–424. [Google Scholar]
- Lan, T.-L.; Chou, F.-I.; Lin, K.-H.; Pan, P.-S.; Lee, J.-C.; Huang, W.-S.; Liu, Y.-M.; Chao, Y.; Chen, Y.-W. Using salvage Boron Neutron Capture Therapy (BNCT) for recurrent malignant brain tumors in Taiwan. Appl. Radiat. Isot. 2020, 160, 109105. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.; Deist, T.M.; Peerlings, J.; De Jong, E.E.; Van Timmeren, J.; Sanduleanu, S.; Larue, R.T.; Even, A.J.; Jochems, A. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Keereman, V.; Mollet, P.; Berker, Y.; Schulz, V.; Vandenberghe, S. Challenges and current methods for attenuation correction in PET/MR. Magn. Reson. Mater. Phys. Biol. Med. 2013, 26, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Garcia, D.; Sawiak, S.J.; Knesaurek, K.; Narula, J.; Fuster, V.; Machac, J.; Fayad, Z.A. Comparison of MR-based attenuation correction and CT-based attenuation correction of whole-body PET/MR imaging. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1574–1584. [Google Scholar] [CrossRef]
- Rousset, O.; Rahmim, A.; Alavi, A.; Zaidi, H. Partial volume correction strategies in PET. PET Clin. 2007, 2, 235–249. [Google Scholar] [CrossRef]
- Kurihara, H.; Hiroi, K.; Honda, N.; Kono, Y.; Arai, Y. Time Course of Tumor SUV in FBPA PET of Brain Tumor. 2014. Available online: https://epos.myesr.org/poster/esr/ecr2014/C-2374 (accessed on 6 January 2021).
- Galavis, P.E.; Hollensen, C.; Jallow, N.; Paliwal, B.; Jeraj, R. Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol. 2010, 49, 1012–1016. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron neutron capture therapy: A review of clinical applications. Front. Oncol. 2021, 11, 351. [Google Scholar] [CrossRef]

| Characteristic | Value | Percentage or Range |
|---|---|---|
| Patients (N = 15) | ||
| Average age | 55.4 | 13–88 |
| Gender (Male:Female) | 8:7 | |
| Tumor types | ||
| Head and neck cancer | ||
| Ear sarcoma | 1 | 6.7% |
| Mandible osteosarcoma | 1 | 6.7% |
| Tongue cancer | 1 | 6.7% |
| Brain tumor | ||
| Glioblastoma | 4 | 26.4% |
| Glioma | 1 | 6.7% |
| Diffuse intrinsic pontine glioma | 1 | 6.7% |
| Brain metastasis from lung | 1 | 6.7% |
| Oligoastrocytoma | 1 | 6.7% |
| Oligodendroglioma | 1 | 6.7% |
| Astrocytoma | 2 | 13.3% |
| Meningioma | 1 | 6.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-Y.; Jen, J.-H.; Chen, Y.-W.; Li, C.-Y.; Wang, L.-W.; Liu, R.-S.; Huang, W.-S.; Lu, C.-F. Comparison of Conventional and Radiomic Features between 18F-FBPA PET/CT and PET/MR. Biomolecules 2021, 11, 1659. https://doi.org/10.3390/biom11111659
Liao C-Y, Jen J-H, Chen Y-W, Li C-Y, Wang L-W, Liu R-S, Huang W-S, Lu C-F. Comparison of Conventional and Radiomic Features between 18F-FBPA PET/CT and PET/MR. Biomolecules. 2021; 11(11):1659. https://doi.org/10.3390/biom11111659
Chicago/Turabian StyleLiao, Chien-Yi, Jun-Hsuang Jen, Yi-Wei Chen, Chien-Ying Li, Ling-Wei Wang, Ren-Shyan Liu, Wen-Sheng Huang, and Chia-Feng Lu. 2021. "Comparison of Conventional and Radiomic Features between 18F-FBPA PET/CT and PET/MR" Biomolecules 11, no. 11: 1659. https://doi.org/10.3390/biom11111659
APA StyleLiao, C.-Y., Jen, J.-H., Chen, Y.-W., Li, C.-Y., Wang, L.-W., Liu, R.-S., Huang, W.-S., & Lu, C.-F. (2021). Comparison of Conventional and Radiomic Features between 18F-FBPA PET/CT and PET/MR. Biomolecules, 11(11), 1659. https://doi.org/10.3390/biom11111659






