Soluble Complement Component 1q Receptor 1 (sCD93) Is Associated with Graft Function in Kidney Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
2.2. Laboratory Tests
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Studied Kidney Transplant Recipients
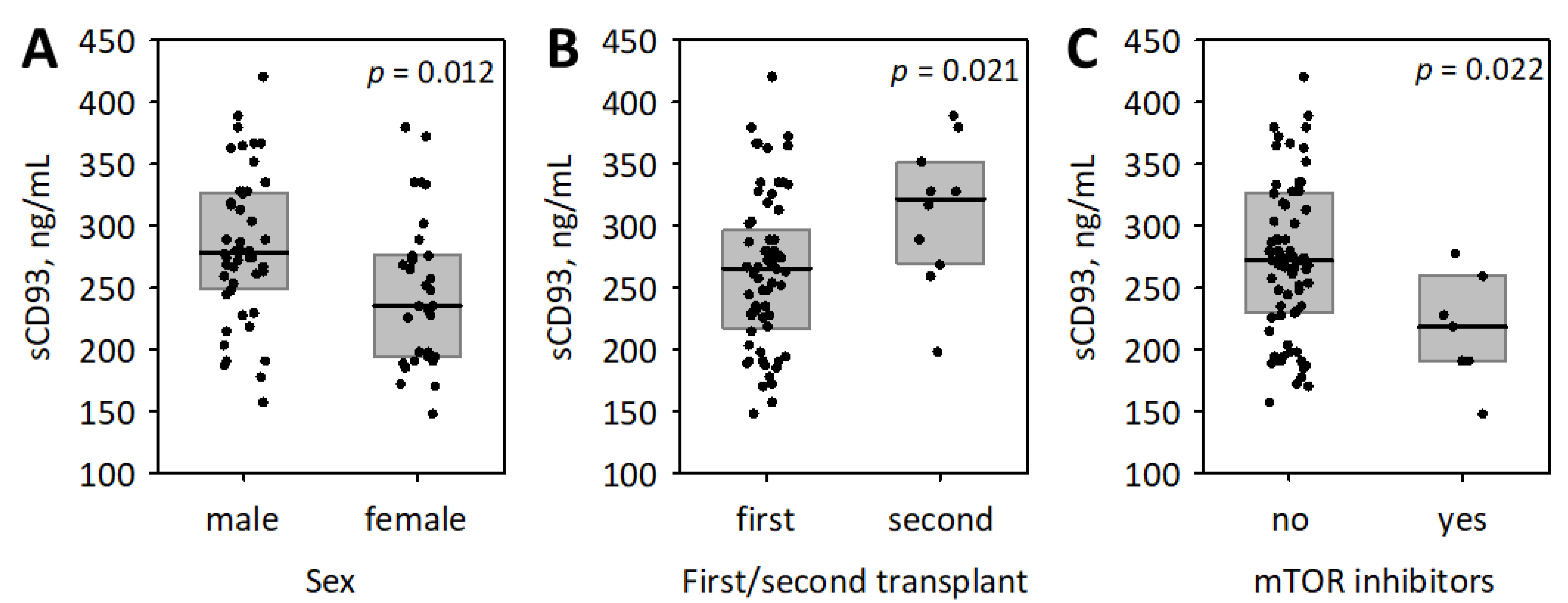
3.2. The Associations between sCD93, Other Studied Inflammatory Markers and the Baseline Characteristics of Patients
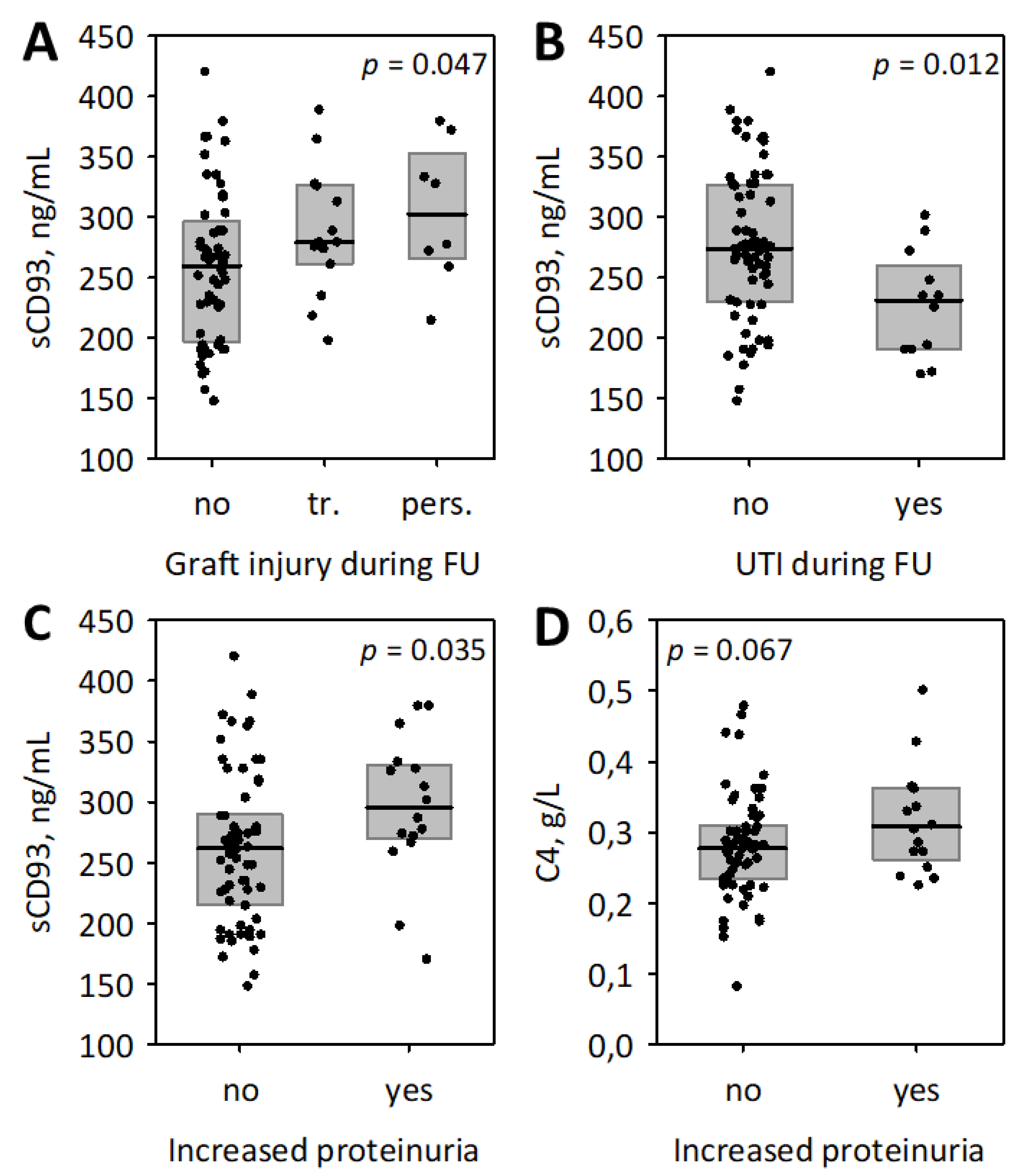
3.3. The Associations between Studied Inflammatory Markers and Follow-Up Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenlee-Wacker, M.C.; Briseño, C.; Galvan, M.; Moriel, G.; Velázquez, P.; Bohlson, S.S. Membrane-Associated CD93 Regulates Leukocyte Migration and C1q-Hemolytic Activity during Murine Peritonitis. J. Immunol. 2011, 187, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, R.R.; Henschen-Edman, A.H.; Burgess, W.H.; Tenner, A.J. cDNA cloning and primary structure analysis of C1qRp, the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity 1997, 6, 119–129. [Google Scholar] [CrossRef]
- Nepomuceno, R.R.; Tenner, A.J. C1qRp, the C1q receptor that enhances phagocytosis, is detected specifically in human cells of myeloid lineage, endothelial cells, and platelets. J. Immunol. 1998, 160, 1929–1935. [Google Scholar]
- Bohlson, S.S.; Silva, R.; Fonseca, M.I.; Tenner, A.J. CD93 Is Rapidly Shed from the Surface of Human Myeloid Cells and the Soluble Form Is Detected in Human Plasma. J. Immunol. 2005, 175, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, M.C.; Sullivan, S.A.; Bohlson, S.S. Detection and characterization of soluble CD93 released during inflammation. Inflamm. Res. 2009, 58, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, H.S.; Choi, M.Y.; Kim, H.Z.; Moon, S.J.; Ha, J.Y.; Choi, A.; Park, Y.W.; Park, J.S.; Shin, E.-C.; et al. Significance of Soluble CD93 in Type 2 Diabetes as a Biomarker for Diabetic Nephropathy: Integrated Results from Human and Rodent Studies. J. Clin. Med. 2020, 9, 1394. [Google Scholar] [CrossRef]
- McGreal, E.P.; Ikewaki, N.; Akatsu, H.; Morgan, B.P.; Gasque, P. Human C1qRp Is Identical with CD93 and the mNI-11 Antigen But Does Not Bind C1q. J. Immunol. 2002, 168, 5222–5232. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, P.J.; Fossati-jimack, L.; Taylor, P.R.; Bygrave, A.E.; Thompson, R.D.; Nourshargh, S.; Walport, M.J.; Botto, M.; Norsworthy, P.J.; Fossati-jimack, L.; et al. Murine CD93 (C1qRp) Contributes to the Removal of Apoptotic Cells In Vivo but Is Not Required for C1q-Mediated Enhancement of Phagocytosis. J. Immunol. 2004, 172, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.W.D.; Lau, D.H.C.; Liu, E.Y.; Ellins, J.; Vrieze, A.M.; Pawlak, E.N.; Dikeakos, J.D.; Heit, B. Soluble CD93 is an apoptotic cell opsonin recognized by αxβ2. Eur. J. Immunol. 2019, 49, 600–610. [Google Scholar] [CrossRef]
- Khan, K.A.; McMurray, J.L.; Mohammed, F.; Bicknell, R. C-type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019, 286, 3299–3332. [Google Scholar] [CrossRef]
- Nativel, B.; Ramin-Mangata, S.; Mevizou, R.; Figuester, A.; Andries, J.; Iwema, T.; Ikewaki, N.; Gasque, P.; Viranaïcken, W. CD93 is a cell surface lectin receptor involved in the control of the inflammatory response stimulated by exogenous DNA. Immunology 2019, 158, 85–93. [Google Scholar] [CrossRef]
- Youn, J.C.; Yu, H.T.; Jeon, J.W.; Lee, H.S.; Jang, Y.; Park, Y.W.; Park, Y.B.; Shin, E.C.; Ha, J.W. Soluble CD93 levels in patients with acute myocardial infarction and its implication on clinical outcome. PLoS ONE 2014, 9, e96538. [Google Scholar] [CrossRef]
- Duvetorp, A.; Olsen, R.; Skarstedt, M.; Söderman, J.; Seifert, O. Psoriasis and Pro-angiogenetic Factor CD93: Gene Expression and Association with Gene Polymorphism Suggests a Role in Disease Pathogenesis. Acta Derm.-Venereol. 2017, 97, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Asano, Y.; Noda, S.; Akamata, K.; Aozasa, N.; Taniguchi, T.; Takahashi, T.; Ichimura, Y.; Toyama, T.; Sumida, H.; et al. Augmented production of soluble CD93 in patients with systemic sclerosis and clinical association with severity of skin sclerosis. Br. J. Dermatol. 2012, 167, 542–547. [Google Scholar] [CrossRef]
- Moosig, F.; Fähndrich, E.; Knorr-Spahr, A.; Böttcher, S.; Ritgen, M.; Zeuner, R.; Kneba, M.; Schröder, J.O. C1qRP (CD93) expression on peripheral blood monocytes in patients with systemic lupus erythematosus. Rheumatol. Int. 2006, 26, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, J.; Takemori, A.; Suemori, K.; Matsumoto, T.; Akita, Y.; Sada, K.; Yuzawa, Y.; Amano, K.; Takasaki, Y.; Harigai, M.; et al. Targeted proteomics reveals promising biomarkers of disease activity and organ involvement in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Res. Ther. 2017, 19, 218. [Google Scholar] [CrossRef]
- Sigari, N.; Jalili, A.; Mahdawi, L.; Ghaderi, E.; Shilan, M. Soluble CD93 as a novel biomarker in asthma exacerbation. Allergy Asthma Immunol. Res. 2016, 8, 461–465. [Google Scholar] [CrossRef]
- Park, H.J.; Han, H.; Lee, S.C.; Son, Y.W.; Sim, D.W.; Park, K.H.; Park, Y.H.; Jeong, K.Y.; Park, J.W.; Lee, J.H. Soluble CD93 in serum as a marker of allergic inflammation. Yonsei Med. J. 2017, 58, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Oh, E.Y.; Han, H.J.; Park, K.H.; Jeong, K.Y.; Park, J.W.; Lee, J.H. Soluble CD93 in allergic asthma. Sci. Rep. 2020, 10, 323. [Google Scholar] [CrossRef]
- Ikewaki, N.; Sonoda, T.; Ogawa, O.; Migita, H.; Tange, Y. Serum levels of soluble CD93 in patients with chronic renal failure. J. Kyushu Univ. Health Welf. 2016, 17, 81–88. [Google Scholar]
- Van Loon, E.; Senev, A.; Lerut, E.; Coemans, M.; Callemeyn, J.; Van Keer, J.M.; Daniëls, L.; Kuypers, D.; Sprangers, B.; Emonds, M.-P.; et al. Assessing the Complex Causes of Kidney Allograft Loss. Transplantation 2020, 104, 2557–2566. [Google Scholar] [CrossRef]
- Chapman, J.R. The KDIGO clinical practice guidelines for the care of kidney transplant recipients. Transplantation 2010, 89, 644–645. [Google Scholar] [CrossRef]
- Cosio, F.G.; El Ters, M.; Cornell, L.D.; Schinstock, C.A.; Stegall, M.D. Changing kidney allograft histology early posttransplant: Prognostic implications of 1-year protocol biopsies. Am. J. Transpl. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- O’Callaghan, J.M.; Knight, S.R. Noninvasive biomarkers in monitoring kidney allograft health. Curr. Opin. Organ Transpl. 2019, 24, 411–415. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Kielar, M.; Dumnicka, P.; Gala-Błądzińska, A.; Będkowska-Prokop, A.; Ignacak, E.; Maziarz, B.; Ceranowicz, P.; Kuśnierz-Cabala, B. Urinary NGAL Measured after the First Year Post Kidney Transplantation Predicts Changes in Glomerular Filtration over One-Year Follow-Up. J. Clin. Med. 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; de Francisco, A.L.M.; de Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Mälarstig, A.; Silveira, A.; Wågsäter, D.; Öhrvik, J.; Bäcklund, A.; Samnegård, A.; Khademi, M.; Hellenius, M.L.; Leander, K.; Olsson, T.; et al. Plasma CD93 concentration is a potential novel biomarker for coronary artery disease. J. Intern. Med. 2011, 270, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.I.; Carpenter, P.M.; Park, M.; Palmarini, G.; Nelson, E.L.; Tenner, A.J. C1qRP, a myeloid cell receptor in blood, is predominantly expressed on endothelial cells in human tissue Abstract: C1qRP is a type I cell surface glycoprotein that has been shown to enhance ingestion of suboptimally opsonized targets by phagocytes. J. Leukoc. Biol. 2001, 70, 793–800. [Google Scholar]
- Donadio, C. Serum and urinary markers of early impairment of GFR in chronic kidney disease patients: Diagnostic accuracy of urinary β-trace protein. Am. J. Physiol. Ren. Physiol. 2010, 299, F1407–F1423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, E.; Wang, S.; Zhang, L.; Zhang, Y.; Malicdan, M.C.; Mao, Y.; Christoffersen, C.; Tabak, L.A.; Schjoldager, K.T.; Ten Hagen, K.G. Galnt11 regulates kidney function by glycosylating the endocytosis receptor megalin to modulate ligand binding. Proc. Natl. Acad. Sci. USA 2019, 116, 25196–25202. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ricart, M.; Torramade-Moix, S.; Pascual, G.; Palomo, M.; Moreno-Castaño, A.B.; Martinez-Sanchez, J.; Vera, M.; Cases, A.; Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins 2020, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, R.S.; Santos, A.F.; Barreto, F.C.; Stinghen, A.E.M. How do Uremic Toxins Affect the Endothelium? Toxins 2020, 12, 412. [Google Scholar] [CrossRef]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, F.; Mulè, G.; Cottone, S.; Soresi, M.; Giannitrapani, L.; Vadalà, A.; Sparacino, V.; Calabrese, S.; Picone, F.P.; Montalto, G.; et al. Circulating Levels of Adhesion Molecules in Chronic Kidney Disease Correlate with the Stage of Renal Disease and with C-Reactive Protein. Arch. Med. Res. 2007, 38, 534–538. [Google Scholar] [CrossRef]
- Lee, B.T.; Ahmed, F.A.; Hamm, L.L.; Teran, F.J.; Chen, C.-S.; Liu, Y.; Shah, K.; Rifai, N.; Batuman, V.; Simon, E.E.; et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Cottone, S.; Palermo, A.; Vaccaro, F.; Mulè, G.; Guarneri, M.; Arsena, R.; Vadalà, A.; Cerasola, G. Inflammation and endothelial activation are linked to renal function in long-term kidney transplantation. Transpl. Int. 2007, 20, 82–87. [Google Scholar] [CrossRef]
- Malyszko, J.; Malyszko, J.S.; Pawlak, K.; Mysliwiec, M. Endothelial Function and Novel Adhesion Molecule CD44 in Kidney Allograft Recipients. Transpl. Proc. 2008, 40, 3470–3473. [Google Scholar] [CrossRef]
| Characteristic | Values |
|---|---|
| Mean age ± SD, years | 53 ± 13 |
| Male sex, n (%) | 47 (60) |
| Median time from transplantation (Q1; Q3), years | 8.0 (5.0; 15.0) |
| Primary cause of kidney disease | |
| Glomerular diseases, n (%) | 29 (37) |
| Tubulointerstitial diseases, n (%) | 10 (13) |
| Vascular diseases, n (%) | 3 (4) |
| Cystic/congenital diseases, n (%) | 10 (13) |
| Unknown, n (%) | 26 (33) |
| First transplant, n (%) | 68 (87) |
| Second transplant, n (%) | 10 (13) |
| Deceased donor, n (%) | 77 (99) |
| Induction therapy, n (%) | 8 (10) |
| No data, n (%) | 23 (29) |
| Median cold ischemia time (Q1; Q3), min | 1200 (840; 1500) |
| No data, n (%) | 19 (24) |
| Median warm ischemia time (Q1; Q3), min | 31 (26; 40) |
| No data, n (%) | 19 (24) |
| Median number of donor-recipient HLA mismatches (Q1; Q3) | 3 (3; 4) |
| No data, n (%) | 52 (67) |
| Median peak pretransplant PRA (Q1; Q3), % | 0 (0; 3) |
| Maximum peak pretransplant PRA, % | 50 |
| No data, n (%) | 52 (67) |
| Median last pretransplant PRA (Q1; Q3), % | 0 (0; 0) |
| Maximum last pretransplant PRA, % | 50 |
| No data, n (%) | 52 (67) |
| Delayed graft function, n (%) | 21 (27) |
| No data, n (%) | 18 (23) |
| Immunosuppressive therapy | |
| glucocorticoids, n (%) | 75 (96) |
| MMF or MPA, n (%) | 73 (94) |
| tacrolimus, n (%) | 48 (62) |
| cyclosporine, n (%) | 24 (31) |
| mTOR inhibitor, n (%) | 7 (9) |
| Diabetes, n (%) | 13 (17) |
| Hypoglycemic agents | |
| oral, n (%) | 10 (13) |
| insulin, n (%) | 5 (6) |
| Median daily diuresis (Q1; Q3), L | 2500 (2000; 3000) |
| Mean BMI ± SD, kg/m2 | 26.9 ± 4.9 |
| Mean systolic pressure ± SD, mmHg | 133.9 ± 15.0 |
| Mean diastolic pressure ± SD, mmHg | 83.8 ± 10.7 |
| Laboratory Test | Results | Reference Range |
|---|---|---|
| Urine albumin, mg/L | 28.5 (7.0; 200.0) | <20 |
| Urine albumin/creatinine ratio, mg/g | 39.3 (10.2; 222.1) | <30 |
| Serum creatinine, µmol/L | 128 (92; 168) | F: 44–80; M: 62–106 |
| eGFR, mL/min/1.73 m2 | 47 (36; 71) | >60 |
| Hemoglobin, g/dL | 13.2 ± 1.7 | F: 12.0–16.0; M: 14.0–18.0 |
| White blood cell count, ×103/µL | 7.36 (5.85; 8.42) | 4.5–10.0 |
| Triglycerides, mmol/L | 1.67 (1.22; 2.14) | <2.26 |
| Total cholesterol, mmol/L | 5.01 (4.41; 5.61) | 3.50–5.20 |
| Glucose, mmol/L | 5.53 (5.15; 6.02) | 3.30–5.60 |
| Serum albumin, g/L | 44 (42; 46) | 35–52 |
| C-reactive protein, mg/L | 1.51 (1.00; 3.31) | <3.0 |
| Procalcitonin, ng/mL | 0.061 (0.044; 0.095) | <0.5 |
| Interleukin 6, pg/mL | 4.71 (2.51; 7.63) | <7.0 |
| C3, g/L | 1.22 (1.10; 1.36) | 0.9–1.8 |
| C4, g/L | 0.280 (0.236; 0.323) | 0.1–0.4 |
| sCD93, ng/mL | 269 (227; 316) | 90–223 * |
| Independent Variable | Simple Regression | Multiple Regression | ||
|---|---|---|---|---|
| β ± SE | p | β ± SE | p | |
| log (interleukin 6) | −0.11 ± 0.11 | 0.3 | not included | |
| log (C-reactive protein) | –0.14 ± 0.11 | 0.2 | not included | |
| log (procalcitonin) | 0.17 ± 0.11 | 0.13 | not included | |
| log (C3) | −0.08 ± 0.12 | 0.5 | not included | |
| log (C4) | 0.07 ± 0.12 | 0.5 | not included | |
| log (serum creatinine) | 0.61 ± 0.09 | <0.001 | not included | |
| eGFR | −0.51 ± 0.10 | <0.001 | −0.47 ± 0.10 | <0.001 |
| log (urine albumin) | 0.34 ± 0.11 | 0.002 | not included | |
| log (urine ACR) | 0.37 ± 0.11 | 0.001 | 0.22 ± 0.11 | 0.040 |
| Age | −0.18 ± 0.11 | 0.11 | −0.11 ± 0.10 | 0.3 |
| log (time from transplantation) | 0.13 ± 0.11 | 0.3 | 0.05 ± 0.09 | 0.6 |
| Male sex | 0.29 ± 0.11 | 0.009 | 0.20 ± 0.10 | 0.043 |
| Diabetes | −0.04 ± 0.11 | 0.8 | −0.04 ± 0.09 | 0.6 |
| Second transplant | 0.25 ± 0.11 | 0.026 | 0.22 ± 0.09 | 0.019 |
| Treatment with mTOR inhibitors | −0.27 ± 0.11 | 0.015 | −0.31 ± 0.09 | 0.001 |
| Systolic blood pressure | 0.26 ± 0.12 | 0.028 | −0.02 ± 0.10 | 0.8 |
| Whole model | not applicable | R2 = 0.55 | p < 0.001 | |
| Independent Variable | Simple Regression | Multiple Model 1 | Multiple Model 2 | |||
|---|---|---|---|---|---|---|
| β ± SE | p | β ± SE | p | β ± SE | p | |
| Baseline eGFR | 0.92 ± 0.05 | <0.001 | 0.84 ± 0.06 | <0.001 | not included | |
| log (urine ACR) | −0.37 ± 0.11 | <0.001 | −0.11 ± 0.05 | 0.038 | −0.20 ± 0.10 | 0.057 |
| Total cholesterol | −0.29 ± 0.11 | 0.010 | 0.05 ± 0.05 | 0.3 | −0.16 ± 0.10 | 0.10 |
| log (triglycerides) | −0.28 ± 0.11 | 0.015 | −0.03 ± 0.05 | 0.6 | not included | |
| log (interleukin 6) | −0.26 ± 0.11 | 0.022 | −0.12 ± 0.06 | 0.053 | −0.28 ± 0.12 | 0.019 |
| log (procalcitonin) | −0.29 ± 0.11 | 0.010 | 0.05 ± 0.06 | 0.4 | −0.002 ± 0.12 | 1.0 |
| log (sCD93) | −0.50 ± 0.10 | <0.001 | −0.04 ± 0.06 | 0.5 | −0.40 ± 0.11 | <0.001 |
| Age | −0.13 ± 0.11 | 0.3 | −0.03 ± 0.05 | 0.5 | −0.17 ± 0.09 | 0.08 |
| log (time from Tx) | −0.23 ± 0.11 | 0.047 | −0.04 ± 0.05 | 0.4 | −0.12 ± 0.09 | 0.19 |
| Male sex | −0.13 ± 0.11 | 0.3 | 0.02 ± 0.05 | 0.7 | −0.11 ± 0.10 | 0.2 |
| Diabetes | −0.16 ± 0.11 | 0.15 | −0.08 ± 0.05 | 0.09 | −0.13 ± 0.09 | 0.2 |
| Whole model | not applicable | R2 = 0.93 | p < 0.001 | R2 = 0.50 | p < 0.001 | |
| Independent Variable | Simple Regression | Multiple Model 1 | Multiple Model 2 | |||
|---|---|---|---|---|---|---|
| β ± SE | p | β ± SE | p | β ± SE | p | |
| Baseline eGFR | 0.91 ± 0.05 | <0.001 | 0.81 ± 0.07 | <0.001 | not included | |
| log (urine ACR) | −0.36 ± 0.11 | 0.002 | −0.08 ± 0.06 | 0.2 | −0.20 ± 0.10 | 0.050 |
| Total cholesterol | −0.32 ± 0.11 | 0.005 | −0.01 ± 0.06 | 0.9 | −0.20 ± 0.09 | 0.035 |
| log (triglycerides) | −0.26 ± 0.11 | 0.020 | −0.004 ± 0.05 | 0.9 | not included | |
| log (interleukin 6) | −0.24 ± 0.11 | 0.033 | −0.09 ± 0.07 | 0.2 | −0.25 ± 0.12 | 0.040 |
| log (procalcitonin) | −0.30 ± 0.11 | 0.007 | −0.001 ± 0.07 | 1.0 | −0.08 ± 0.12 | 0.7 |
| log (sCD93) | −0.52 ± 0.10 | <0.001 | −0.06 ± 0.07 | 0.4 | −0.40 ± 0.11 | <0.001 |
| Age | −0.13 ± 0.11 | 0.3 | −0.004 ± 0.06 | 0.9 | −0.14 ± 0.09 | 0.14 |
| log (time from Tx) | −0.24 ± 0.11 | 0.037 | −0.06 ± 0.05 | 0.2 | −0.14 ± 0.09 | 0.14 |
| Male sex | −0.18 ± 0.11 | 0.13 | −0.02 ± 0.06 | 0.7 | −0.15 ± 0.10 | 0.13 |
| Diabetes | −0.12 ± 0.11 | 0.3 | −0.05 ± 0.05 | 0.4 | −0.09 ± 0.09 | 0.3 |
| Whole model | not applicable | R2 = 0.85 | p < 0.001 | R2 = 0.51 | p < 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kielar, M.; Dumnicka, P.; Ignacak, E.; Będkowska-Prokop, A.; Gala-Błądzińska, A.; Maziarz, B.; Ceranowicz, P.; Kuśnierz-Cabala, B. Soluble Complement Component 1q Receptor 1 (sCD93) Is Associated with Graft Function in Kidney Transplant Recipients. Biomolecules 2021, 11, 1623. https://doi.org/10.3390/biom11111623
Kielar M, Dumnicka P, Ignacak E, Będkowska-Prokop A, Gala-Błądzińska A, Maziarz B, Ceranowicz P, Kuśnierz-Cabala B. Soluble Complement Component 1q Receptor 1 (sCD93) Is Associated with Graft Function in Kidney Transplant Recipients. Biomolecules. 2021; 11(11):1623. https://doi.org/10.3390/biom11111623
Chicago/Turabian StyleKielar, Małgorzata, Paulina Dumnicka, Ewa Ignacak, Alina Będkowska-Prokop, Agnieszka Gala-Błądzińska, Barbara Maziarz, Piotr Ceranowicz, and Beata Kuśnierz-Cabala. 2021. "Soluble Complement Component 1q Receptor 1 (sCD93) Is Associated with Graft Function in Kidney Transplant Recipients" Biomolecules 11, no. 11: 1623. https://doi.org/10.3390/biom11111623
APA StyleKielar, M., Dumnicka, P., Ignacak, E., Będkowska-Prokop, A., Gala-Błądzińska, A., Maziarz, B., Ceranowicz, P., & Kuśnierz-Cabala, B. (2021). Soluble Complement Component 1q Receptor 1 (sCD93) Is Associated with Graft Function in Kidney Transplant Recipients. Biomolecules, 11(11), 1623. https://doi.org/10.3390/biom11111623








