Diagnostic and Prognostic Utility of the Extracellular Vesicles Subpopulations Present in Pleural Effusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Study Design
2.2. Fractionation of Extracellular Vesicles
2.3. NTA Analysis
2.4. Luminex Assay with Human Premixed Multi-Analyte Kit
2.5. Multiplex Bead-Based EV Flow Cytometry Assay
2.6. Western Blot Analysis
3. Results
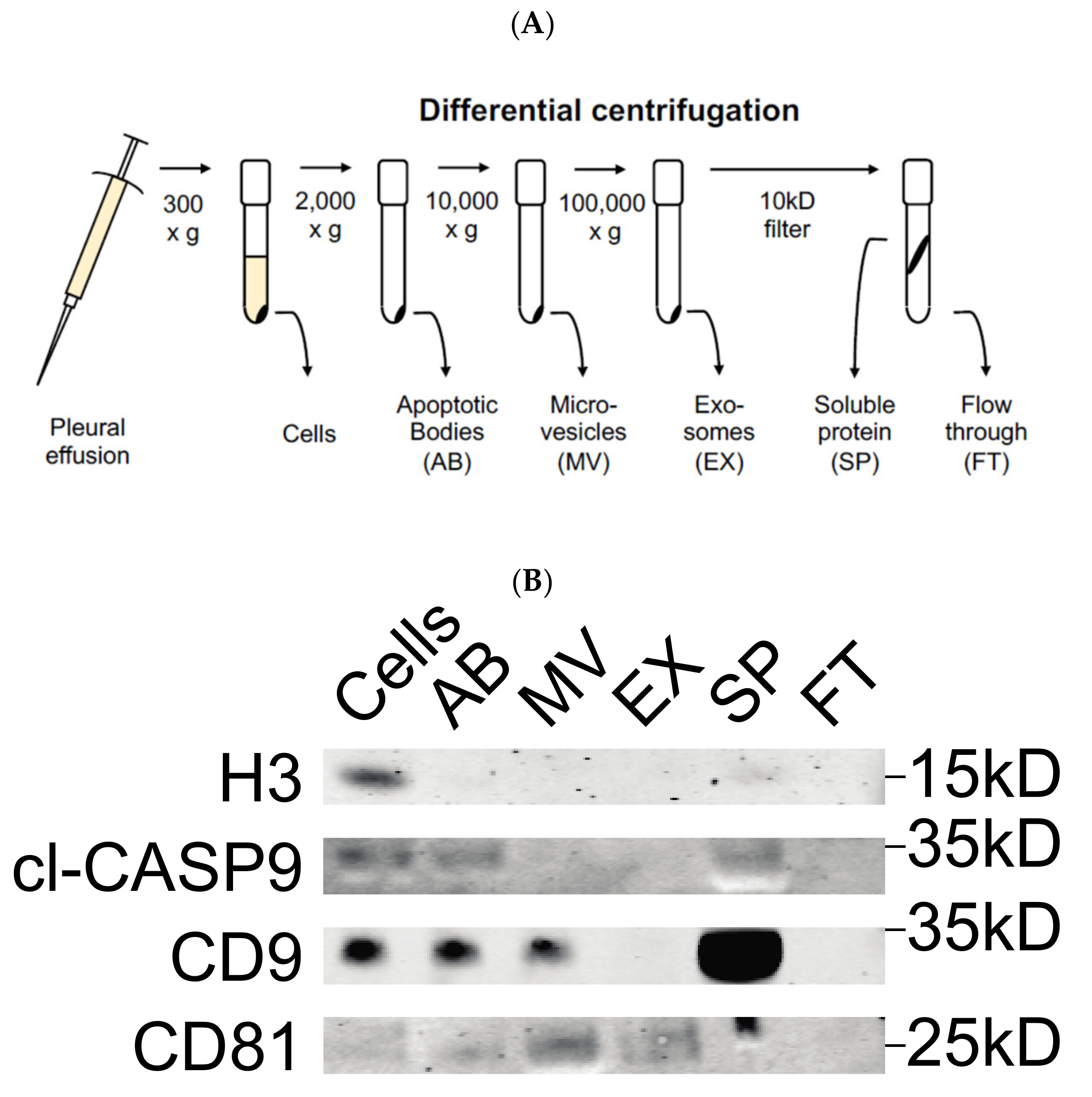
3.1. Validation of Differential Centrifugation Fraction of Pleural Effusion
3.2. Measurement of Exosome Particle Size and Concentration
3.3. Detection of Surface Protein Markers
3.4. Quantification of Biomarkers in Different Pleural Effusion Fractions
3.5. Presence of Proteins in Exosomes vs. Supernatant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, L.L.; Yang, Y.; Wang, Z.; Wang, X.J.; Tong, Z.H.; Shi, H.Z. Malignant pleural mesothelioma: Diagnostic value of medical thoracoscopy and long-term prognostic analysis. BMC Pulm. Med. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondola, S.; Manners, D.; Nowak, A.K. Malignant pleural mesothelioma: An update on diagnosis and treatment options. Ther. Adv. Respir. Dis. 2016, 10, 275–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjerpe, A.; Abd-Own, S.; Dobra, K. Cytopathologic Diagnosis of Epithelioid and Mixed-Type Malignant Mesothelioma: Ten Years of Clinical Experience in Relation to International Guidelines. Arch. Pathol. Lab. Med. 2018, 142, 893–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcel, J.M. Diagnosis and characterization of malignant effusions through pleural fluid cytological examination. Curr. Opin. Pulm. Med. 2019, 25, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Mundt, F.; Heidari-Hamedani, G.; Nilsonne, G.; Metintas, M.; Hjerpe, A.; Dobra, K. Diagnostic and prognostic value of soluble syndecan-1 in pleural malignancies. Biomed Res. Int. 2014, 2014, 419853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjerpe, A.; Ascoli, V.; Bedrossian, C.W.; Boon, M.E.; Creaney, J.; Davidson, B.; Dejmek, A.; Dobra, K.; Fassina, A.; Field, A.; et al. Guidelines for the cytopathologic diagnosis of epithelioid and mixed-type malignant mesothelioma: Complementary Statement from the International Mesothelioma Interest Group, Also Endorsed by the International Academy of Cytology and the Papanicolaou Society of Cytopathology. Diagn. Cytopathol. 2015, 43, 563–576. [Google Scholar]
- Halimi, M.; BeheshtiRouy, S.; Salehi, D.; Rasihashemi, S.Z. The Role of Immunohistochemistry Studies in Distinguishing Malignant Mesothelioma from Metastatic Lung Carcinoma in Malignant Pleural Effusion. Iran. J. Pathol. 2019, 14, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Watabe, S.; Kikuchi, Y.; Morita, S.; Komura, D.; Numakura, S.; Kumagai-Togashi, A.; Watanabe, M.; Matsutani, N.; Kawamura, M.; Yasuda, M.; et al. Clinicopathological significance of microRNA-21 in extracellular vesicles of pleural lavage fluid of lung adenocarcinoma and its functions inducing the mesothelial to mesenchymal transition. Cancer Med. 2020, 9, 2879–2890. [Google Scholar] [CrossRef]
- Stok, U.; Blokar, E.; Lenassi, M.; Holcar, M.; Frank-Bertoncelj, M.; Erman, A.; Resnik, N.; Sodin-Semrl, S.; Cucnik, S.; Pirkmajer, K.P.; et al. Characterization of Plasma-Derived Small Extracellular Vesicles Indicates Ongoing Endothelial and Platelet Activation in Patients with Thrombotic Antiphospholipid Syndrome. Cells 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Rilla, K.; Mustonen, A.M.; Arasu, U.T.; Harkonen, K.; Matilainen, J.; Nieminen, P. Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biol. 2019, 75, 201–219. [Google Scholar] [CrossRef]
- Lee, J.S.; Hur, J.Y.; Kim, I.A.; Kim, H.J.; Choi, C.M.; Lee, J.C.; Kim, W.S.; Lee, K.Y. Liquid biopsy using the supernatant of a pleural effusion for EGFR genotyping in pulmonary adenocarcinoma patients: A comparison between cell-free DNA and extracellular vesicle-derived DNA. BMC Cancer 2018, 18, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samir, E.L.A.; Mager, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar]
- Guo, M.; Wu, F.; Hu, G.; Chen, L.; Xu, J.; Xu, P.; Wang, X.; Li, Y.; Liu, S.; Zhang, S.; et al. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.A.; Karunakaran, D.; Geoffrion, M.; Cheng, H.S.; Tandoc, K.; Perisic Matic, L.; Hedin, U.; Maegdefessel, L.; Fish, J.E.; Rayner, K.J. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, F.; Takahashi, T.; Nakamura, K.; Takahashi, Y.; Kobayashi, T.; Shida, S.; Kameyama, T.; Mekada, E. Domain analysis of the tetraspanins: Studies of CD9/CD63 chimeric molecules on subcellular localization and upregulation activity for diphtheria toxin binding. Cell Struct. Funct. 2000, 25, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greening, D.W.; Xu, R.; Gopal, S.K.; Rai, A.; Simpson, R.J. Proteomic insights into extracellular vesicle biology—Defining exosomes and shed microvesicles. Expert Rev. Proteom. 2017, 14, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Bleckmann, A.; Schulz, M.; Ries, L.; Binder, C. Isolation and Characterization of Microvesicles from Peripheral Blood. J. Vis. Exp. 2017, 119, e55057. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A.; et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Huang, X.; Woodcock, M.; Du, M.; Dittmar, R.; Wang, Y.; Tsai, S.; Kohli, M.; Boardman, L.; Patel, T.; et al. Plasma extracellular RNA profiles in healthy and cancer patients. Sci. Rep. 2016, 6, 19413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noppen, M.; De Waele, M.; Li, R.; Gucht, K.V.; D’Haese, J.; Gerlo, E.; Vincken, W. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am. J. Respir. Crit. Care Med. 2000, 162, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Gjomarkaj, M.; Pace, E.; Melis, M.; Spatafora, M.; Toews, G.B. Mononuclear cells in exudative malignant pleural effusions: Characterization of pleural phagocytic cells. Chest 1994, 106, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotvall, J.; Valadi, H. Cell to cell signalling via exosomes through esRNA. Cell Adh. Migr. 2007, 1, 156–158. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javadi, J.; Dobra, K.; Hjerpe, A. Multiplex Soluble Biomarker Analysis from Pleural Effusion. Biomolecules 2020, 10, 1113. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Bostancioglu, R.B.; Welsh, J.A.; Zickler, A.M.; Murke, F.; Corso, G.; Felldin, U.; Hagey, D.W.; Evertsson, B.; Liang, X.M.; et al. Systematic Methodological Evaluation of a Multiplex Bead-Based Flow Cytometry Assay for Detection of Extracellular Vesicle Surface Signatures. Front. Immunol. 2018, 9, 1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagey, D.W.; Kordes, M.; Gorgens, A.; Mowoe, M.O.; Nordin, J.Z.; Moro, C.F.; Lohr, J.M.; El Andaloussi, S. Extracellular vesicles are the primary source of blood-borne tumour-derived mutant KRAS DNA early in pancreatic cancer. J. Extracell. Vesicles 2021, 10, e12142. [Google Scholar] [CrossRef] [PubMed]
- Carnell-Morris, P.; Tannetta, D.; Siupa, A.; Hole, P.; Dragovic, R. Analysis of Extracellular Vesicles Using Fluorescence Nanoparticle Tracking Analysis. Methods Mol. Biol. 2017, 1660, 153–173. [Google Scholar]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Rabinovich, G.A.; Griffioen, A.W. Vascular galectins: Regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev. 2013, 24, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Qian, M.; Ho, M. The role of mesothelin in tumor progression and targeted therapy. Anticancer Agents Med. Chem. 2013, 13, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Einama, T.; Kamachi, H.; Nishihara, H.; Homma, S.; Kanno, H.; Takahashi, K.; Sasaki, A.; Tahara, M.; Okada, K.; Muraoka, S.; et al. Co-expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma. Pancreas 2011, 40, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.A.; Purushothaman, A.; Ramani, V.C.; Vlodavsky, I.; Sanderson, R.D. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 2013, 288, 10093–10099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, A.N.; Colby, T.V.; Ordóñez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 142, 89–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Angiopoietin-1 | HGF | Osteopontin | TIMP-1 | Galectin-1 | ||||||
| Patient Group | Exosomes | Supernatant | Exosomes | Supernatant | Exosomes | Supernatant | Exosomes | Supernatant | Exosomes | Supernatant |
| AD | 49.60% | 50.40% | 32.50% | 67.50% | 1.70% | 98.30% | 48.20% | 51.80% | 6.70% | 93.30% |
| MPM | 33.50% | 66.50% | 17.20% | 82.80% | 5.50% | 94.50% | 49.50% | 50.50% | 9.30% | 90.70% |
| Benign | 81% | 19% | 32.60% | 67.40% | 2.10% | 97.90% | 41.90% | 58.10% | 5.10% | 94.90% |
| Mesothelin | NRG1-b1 | SDC-1 | VEGF | MMP-7 | ||||||
| Patient Group | Exosomes | Supernatant | Exosomes | Supernatant | Exosomes | Supernatant | Exosomes | Supernatant | Exosomes | Supernatant |
| AD | 7.50% | 92.50% | 6.50% | 93.50% | 12.20% | 87.80% | 0.00% | 100% | 2.90% | 96.90% |
| MPM | 25.40% | 74.60% | 10.50% | 89.50% | 0.00% | 100% | 12.30% | 87.70% | 2.90% | 93.10% |
| Benign | 10.30% | 89.70% | 8.10% | 91.90% | 0.00% | 100% | 0.00% | 100% | 8.90% | 91% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javadi, J.; Görgens, A.; Vanky, H.; Gupta, D.; Hjerpe, A.; EL-Andaloussi, S.; Hagey, D.; Dobra, K. Diagnostic and Prognostic Utility of the Extracellular Vesicles Subpopulations Present in Pleural Effusion. Biomolecules 2021, 11, 1606. https://doi.org/10.3390/biom11111606
Javadi J, Görgens A, Vanky H, Gupta D, Hjerpe A, EL-Andaloussi S, Hagey D, Dobra K. Diagnostic and Prognostic Utility of the Extracellular Vesicles Subpopulations Present in Pleural Effusion. Biomolecules. 2021; 11(11):1606. https://doi.org/10.3390/biom11111606
Chicago/Turabian StyleJavadi, Joman, André Görgens, Hanna Vanky, Dhanu Gupta, Anders Hjerpe, Samir EL-Andaloussi, Daniel Hagey, and Katalin Dobra. 2021. "Diagnostic and Prognostic Utility of the Extracellular Vesicles Subpopulations Present in Pleural Effusion" Biomolecules 11, no. 11: 1606. https://doi.org/10.3390/biom11111606
APA StyleJavadi, J., Görgens, A., Vanky, H., Gupta, D., Hjerpe, A., EL-Andaloussi, S., Hagey, D., & Dobra, K. (2021). Diagnostic and Prognostic Utility of the Extracellular Vesicles Subpopulations Present in Pleural Effusion. Biomolecules, 11(11), 1606. https://doi.org/10.3390/biom11111606









