Nitric Oxide Mediation in Hydroxyurea and Nitric Oxide Metabolites’ Inhibition of Erythroid Progenitor Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colony Forming Assays
2.2. Hematologic Parameters
2.3. Measurement of Nitrite Levels
2.4. Immunocytochemistry
2.5. Statistical Analysis
3. Results
3.1. Plasma NO Derivative Levels Following In Vivo Treatment of Mice by Hydroxyurea
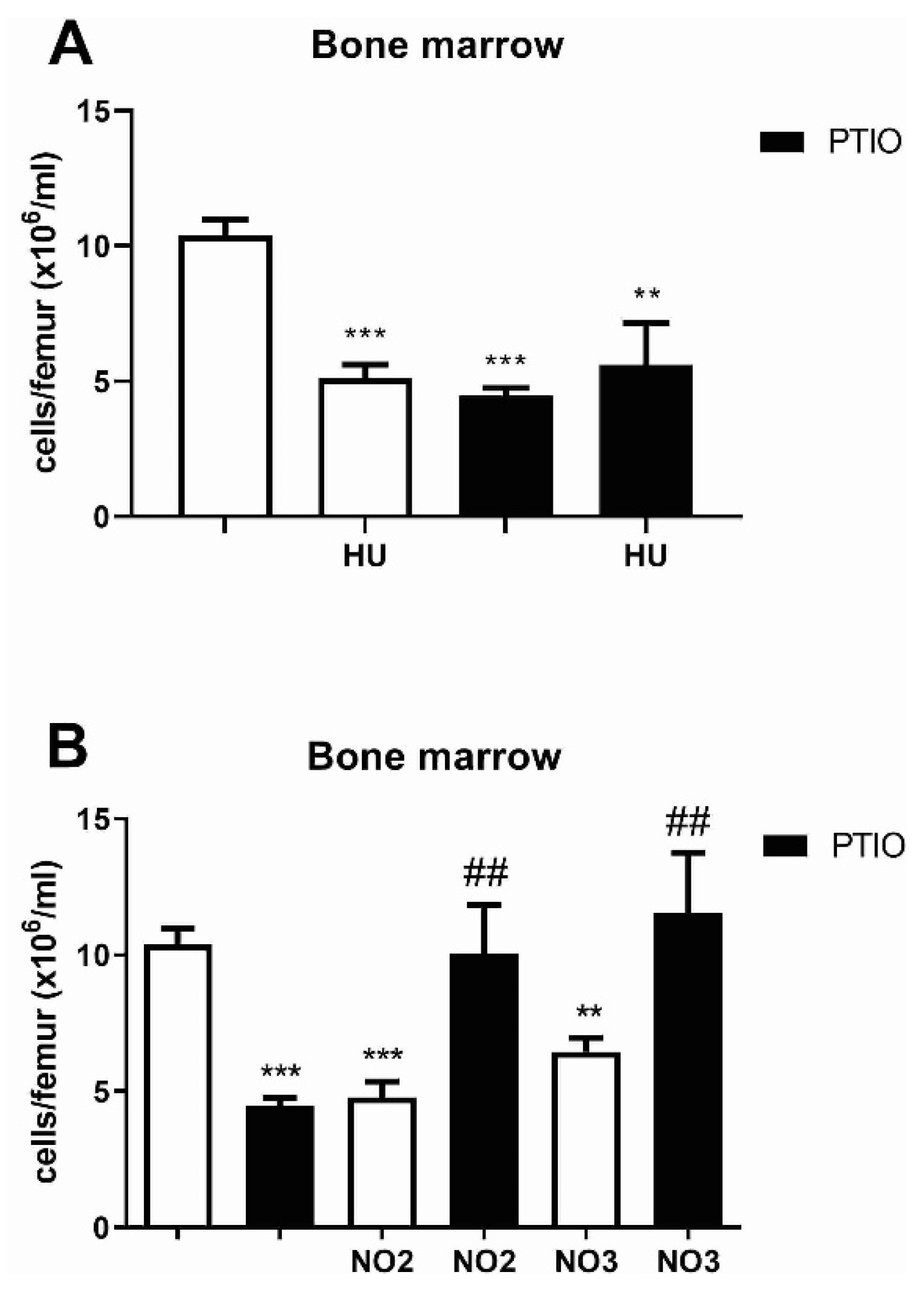
3.2. Hydroxyurea and NO Effects on Bone Marrow and Peripheral Blood Cells of Mice
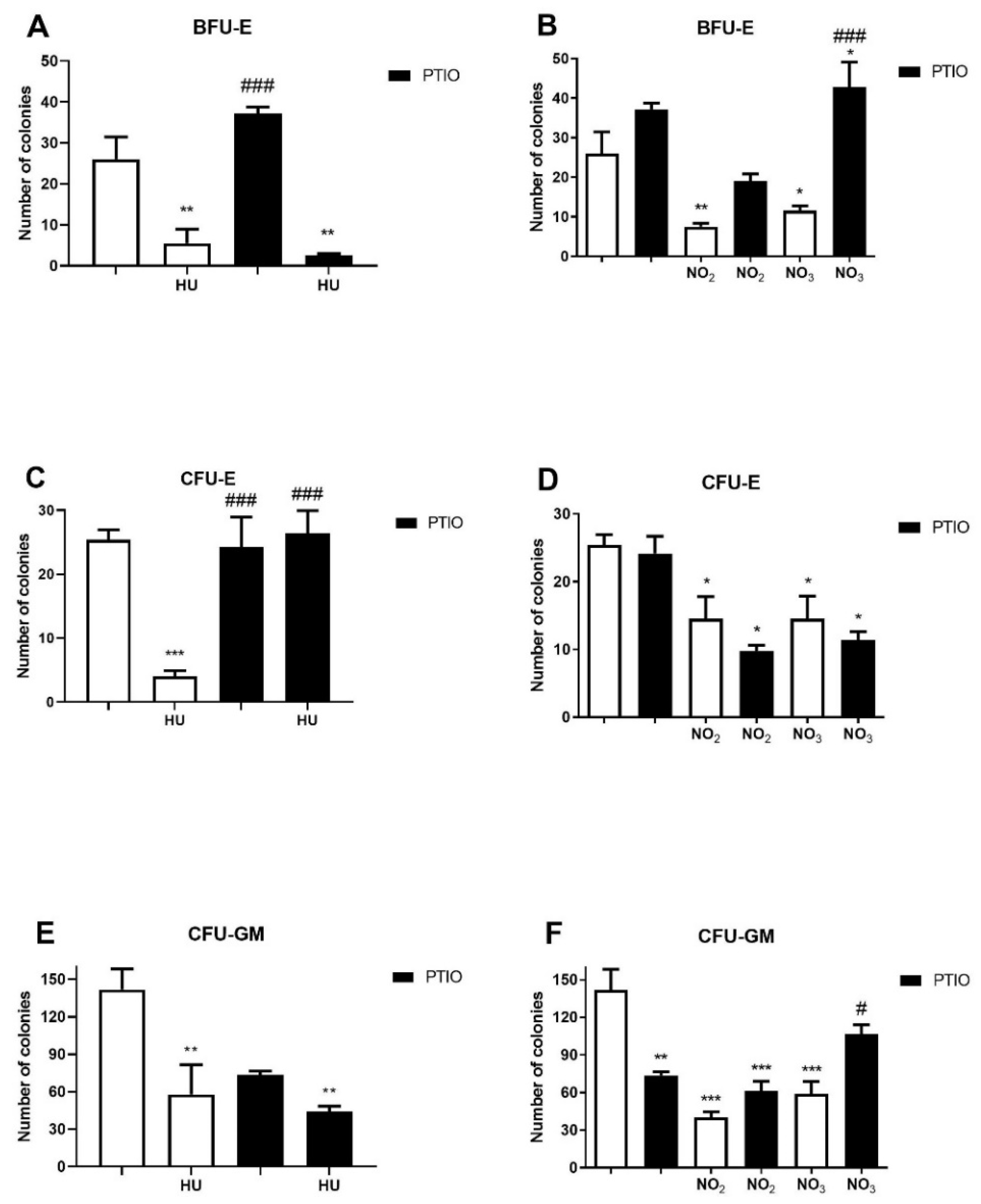
3.3. Effects of Hydroxyurea and NO Metabolites on the Myeloid Progenitor Growth
3.4. The Level of NOS Isoforms in Bone Marrow Cells during Hydroxyurea and NO Metabolites Treatment
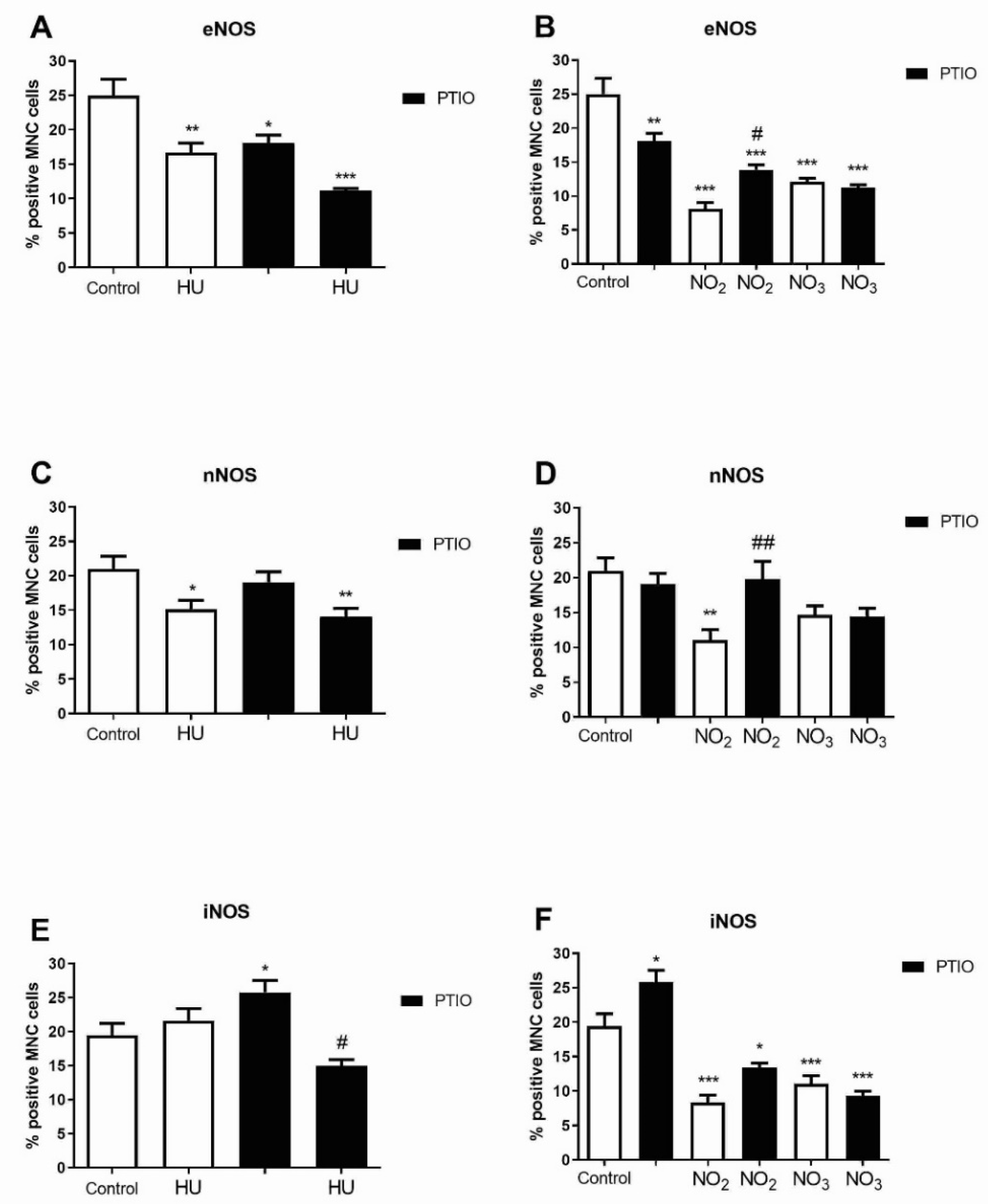
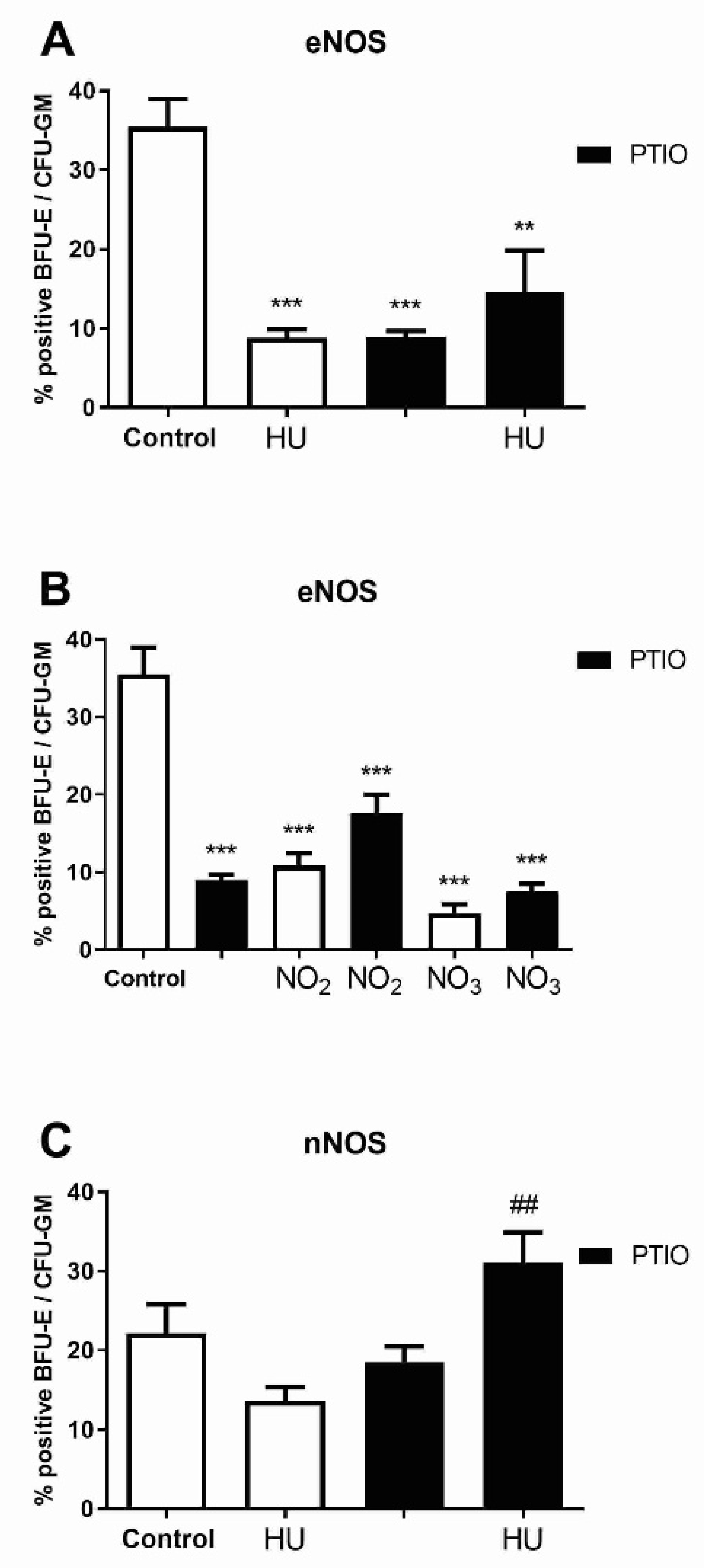
3.5. Level of NOS Isoforms in Mature Erythroid Progenitors after Hydroxyurea and NO Metabolites Treatment
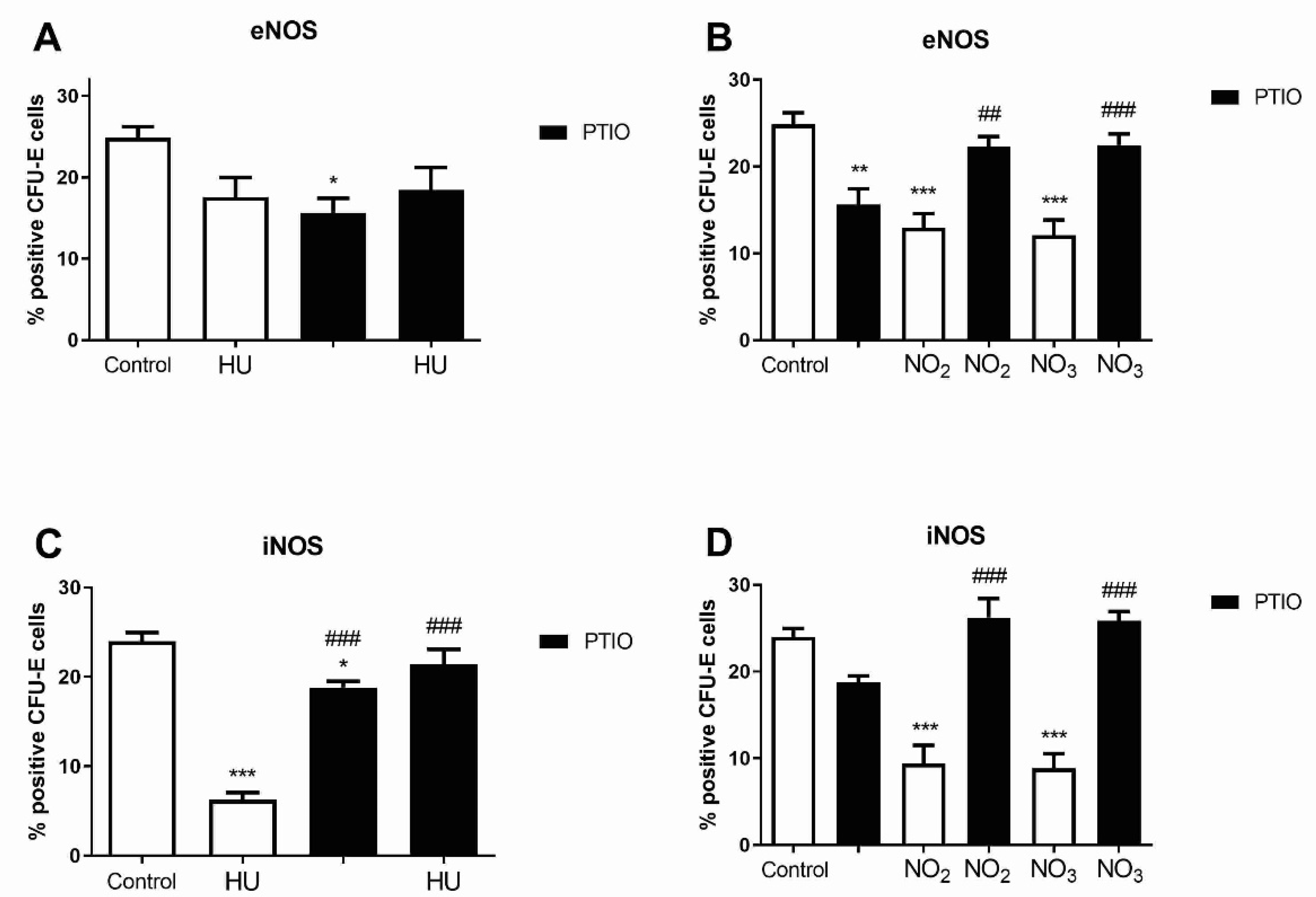
3.6. Level of NOS Isoforms in Early Myeloid Progenitors after Hydroxyurea and Nitrite/Nitrate Treatment
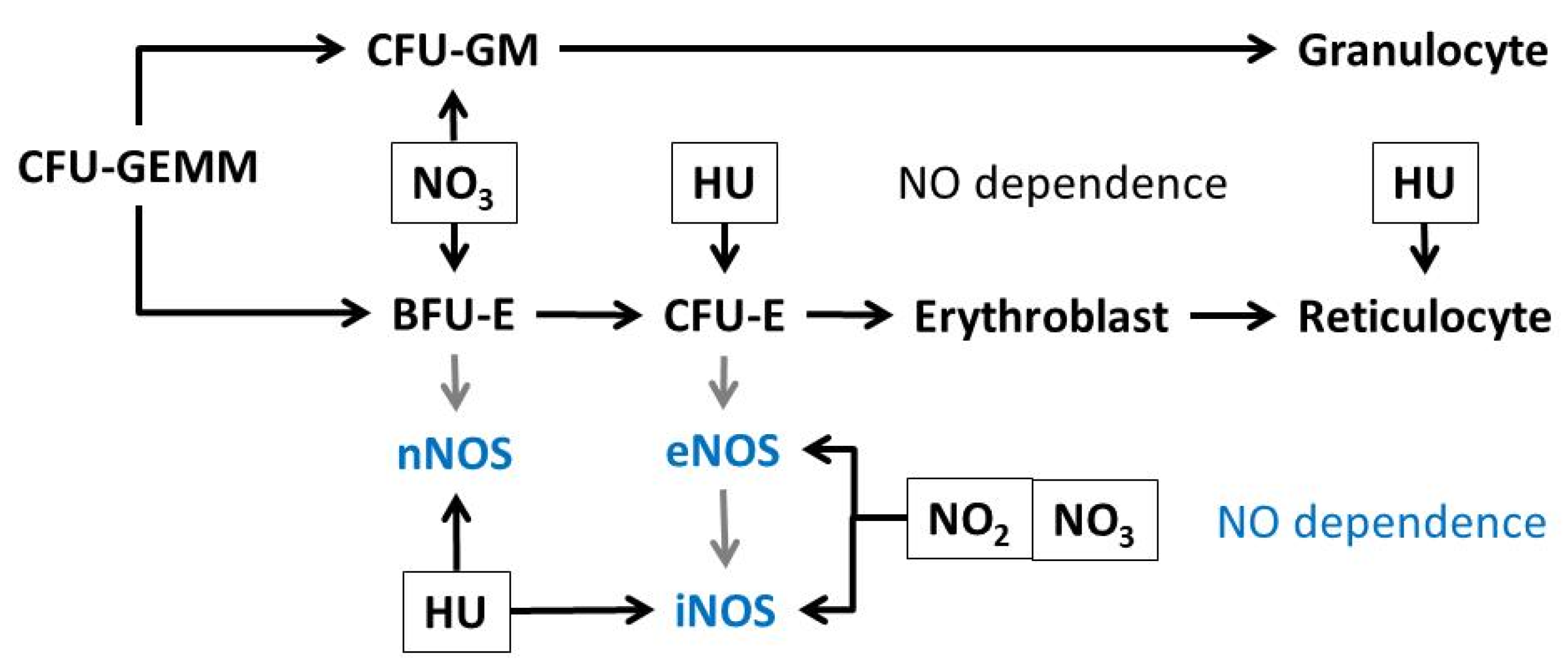
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gladwin, M.T.; Shelhamer, J.H.; Ognibene, F.P.; Pease-Fye, M.E.; Nichols, J.S.; Link, B.; Patel, D.B.; Jankowski, M.A.; Pannell, L.K.; Schechter, A.N.; et al. Nitric oxide donor properties of hydroxyurea in patients with sickle cell disease. Br. J. Haematol. 2002, 116, 436–444. [Google Scholar] [CrossRef]
- Piccin, A.; Murphy, C.; Eakins, E.; Rondinelli, M.B.; Daves, M.; Vecchiato, C.; Wolf, D.; Mc Mahon, C.; Smith, O.P. Insight into the complex pathophysiology of sickle cell anaemia and possible treatment. Eur. J. Haematol. 2019, 102, 319–330. [Google Scholar] [CrossRef]
- Čokić, V.P.; Beleslin-Čokić, B.B.; Tomić, M.; Stojilković, S.S.; Noguchi, C.T.; Schechter, A.N. Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood 2006, 108, 184–191. [Google Scholar] [CrossRef] [Green Version]
- da Guarda, C.C.; Santiago, R.P.; Pitanga, T.N.; Santana, S.S.; Zanette, D.L.; Borges, V.M.; Goncalves, M.S. Heme changes HIF-α, eNOS and nitrite production in HUVECs after simvastatin, HU, and ascorbic acid therapies. Microvasc. Res. 2016, 106, 128–136. [Google Scholar] [CrossRef]
- Subotički, T.; Ajtić, O.M.; Đikić, D.; Santibanez, J.F.; Tošić, M.; Čokić, V.P. Nitric Oxide Synthase Dependency in Hydroxyurea Inhibition of Erythroid Progenitor Growth. Genes 2021, 12, 1145. [Google Scholar] [CrossRef]
- Yang, Y.M.; Pace, B.; Kitchens, D.; Ballas, S.K.; Shah, A.; Baliga, B.S. BFU-E colony growth in response to hydroxyurea: Correlation between in vitro and in vivo fetal hemoglobin induction. Am. J. Hematol. 1997, 56, 252–258. [Google Scholar] [CrossRef]
- Baliga, B.S.; Haynes, J., Jr.; Obiako, B.; Mishra, N. Combined effects of arginine and hydroxyurea on BFU-E derived colony growth and HbF synthesis in erythroid progenitors isolated from sickle cell blood. Cell Mol. Biol. 2010, 56, OL1290–OL1298. [Google Scholar] [PubMed]
- Reykdal, S.; Abboud, C.; Liesveld, J. Effect of nitric oxide production and oxygen tension on progenitor preservation in ex vivo culture. Exp. Hematol. 1999, 27, 441–450. [Google Scholar] [CrossRef]
- Shami, P.J.; Weinberg, J.B. Differential effects of nitric oxide on erythroid and myeloid colony growth from CD34+ human bone marrow cells. Blood 1996, 87, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.B.; Scheiermann, C.; Jang, J.E.; Prophete, C.; Costa, F.F.; Conran, N.; Frenette, P.S. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood. 2012, 120, 2879–2888. [Google Scholar] [CrossRef]
- Cella, G.; Marchetti, M.; Vianello, F.; Panova-Noeva, M.; Vignoli, A.; Russo, L.; Barbui, T.; Falanga, A. Nitric oxide derivatives and soluble plasma selectins in patients with myeloproliferative neoplasms. Thromb. Haemost. 2010, 104, 151–156. [Google Scholar] [CrossRef]
- Morris, C.R.; Vichinsky, E.P.; van Warmerdam, J.; Machado, L.; Kepka-Lenhart, D.; Morris, S.M., Jr.; Kuypers, F.A. Hydroxyurea and arginine therapy: Impact on nitric oxide production in sickle cell disease. J. Pediatr. Hematol. Oncol. 2003, 25, 629–634. [Google Scholar] [CrossRef]
- Nahavandi, M.; Tavakkoli, F.; Millis, R.M.; Wyche, M.Q.; Habib, M.J.; Tavakoli, N. Effects of hydroxyurea and L-arginine on the production of nitric oxide metabolites in cultures of normal and sickle erythrocytes. Hematology 2006, 11, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Weitzberg, E.; Hezel, M.; Lundberg, J.O. Nitrate-nitrite-nitric oxide pathway: Implications for anesthesiology and intensive care. Anesthesiology 2010, 113, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Dejam, A.; Hunter, C.J.; Tremonti, C.; Pluta, R.M.; Hon, Y.Y.; Grimes, G.; Partovi, K.; Pelletier, M.M.; Oldfield, E.H.; Cannon, R.O.; et al. Nitrite infusion in humans and nonhuman primates. Circulation 2007, 116, 1821–1831. [Google Scholar] [CrossRef]
- Mayhew, C.N.; Phillips, J.D.; Greenberg, R.N.; Birch, N.J.; Elford, H.L.; Gallicchio, V.S. In vivo and in vitro comparison of the short-term hematopoietic toxicity between hydroxyurea and trimidox or didox, novel ribonucleotide reductase inhibitors with potential anti-HIV-1 activity. Stem Cells 1999, 17, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Amdahl, M.; DeMartino, A.W.; Gladwin, M.T. Inorganic nitrite bioactivation and role in physiological signaling and therapeutics. Biol. Chem. 2019, 401, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Cokic, V.P.; Smith, R.D.; Beleslin-Cokic, B.B.; Njoroge, J.M.; Miller, J.L.; Gladwin, M.T.; Schechter, A.N. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J. Clin. Investig. 2003, 111, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, T.H.; Hyduke, D.R.; Vaughn, M.W.; Fukuto, J.M.; Liao, J.C. Nitric oxide reaction with red blood cells and hemoglobin under heterogeneous conditions. Proc. Natl. Acad. Sci. USA 2002, 99, 7763–7768. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.M.; Kasztan, M.; Sedaka, R.; Molina, P.A.; Dunaway, L.S.; Pollock, J.S.; Pollock, D.M. Hydroxyurea improves nitric oxide bioavailability in humanized sickle cell mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R630–R640. [Google Scholar] [CrossRef]
- Nader, E.; Grau, M.; Fort, R.; Collins, B.; Cannas, G.; Gauthier, A.; Walpurgis, K.; Martin, C.; Bloch, W.; Poutrel, S.; et al. Hydroxyurea therapy modulates sickle cell anemia red blood cell physiology: Impact on RBC deformability, oxidative stress, nitrite levels and nitric oxide synthase signalling pathway. Nitric Oxide 2018, 81, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Jordan, S.J.; Barr, D.P.; Gunther, M.R.; Maeda, H.; Mason, R.P. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol. Pharmacol. 1997, 52, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with *NO, *NO2, and O2-*. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.; Kamimura, S.; de Souza Batista, C.M.; Spornick, N.; Nettleton, M.Y.; Walek, E.; Smith, M.L.; Finkel, J.C.; Darbari, D.S.; Wakim, P.; et al. Sickle cell disease subjects and mouse models have elevated nitrite and cGMP levels in blood compartments. Nitric Oxide 2020, 94, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Mozar, A.; Charlot, K.; Lamarre, Y.; Weyel, L.; Suhr, F.; Collins, B.; Jumet, S.; Hardy-Dessources, M.D.; Romana, M.; et al. High red blood cell nitric oxide synthase activation is not associated with improved vascular function and red blood cell deformability in sickle cell anaemia. Br. J. Haematol. 2015, 168, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Eleutério, R.M.N.; Nascimento, F.O.; Araújo, T.G.; Castro, M.F.; Filho, T.P.A.; Filho, P.A.M.; Eleutério, J., Jr.; Elias, D.B.D.; Lemes, R.P.G. Double-Blind Clinical Trial of Arginine Supplementation in the Treatment of Adult Patients with Sickle Cell Anaemia. Adv. Hematol. 2019, 2019, 4397150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, T.F.; Singh, M.; Mackie, A.; Li, W.; Pace, B.S. Hydroxyurea generates nitric oxide in human erythroid cells: Mechanisms for gamma-globin gene activation. Exp. Biol. Med. 2009, 234, 1374–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment (n = 7) | Leukocytes (×109/L) | Erythrocytes (×1012/L) | Hemoglobin % | Hematocrit (L/L) | Reticulocytes % 1 | Thrombocytes (×105) | Weight: Treatment | |
|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||
| 2 Control | 7.6 ± 1.9 | 6.3 ± 0.7 | 17.5 ± 2.2 | 46.3 ± 2.7 | 67.5 ± 8 | 10.8 ± 1 | 24.1 ± 1.3 | 25.2 ± 1.8 |
| 2 HU | 6 ± 1.8 * | 5.3 ± 1.5 | 16.5 ± 2.2 | 44.8 ± 2.1 | 21.5 ± 1 *** | 2.3 ± 0.6 *** | 22.3 ± 1.5 | 21.8 ± 2.2 |
| PTIO | 4.5 ± 0.2 *** | 3.7 ± 0.4 *** | 17.3 ± 3 | 37.4 ± 1.8 *** | 63 ± 5 | 3.4 ± 0.3 *** | 23.6 ± 0.9 | 22.9 ± 1.8 |
| HU + PTIO | 4.8 ± 0.6 ** | 5.3 ± 0.5 | 20.1 ± 1.1 | 40.2 ± 1.1 ** | 47.2 ± 6 | 3.8 ± 0.3 *** | 23 ± 0.7 | 23.2 ± 0.6 |
| 2 NO2 | 7.7 ± 1.5 | 5.6 ± 0.6 | 19.1 ± 1.4 | 45.1 ± 1.7 | 90.5 ± 10 * | 9.1 ± 0.6 | 23.3 ± 1.2 | 24.1 ± 1.6 |
| NO2 + PTIO | 3.3 ± 0.5 ** | 4.3 ± 0.5 ** | 17 ± 4.6 | 38.1 ± 0.9 *** | 27.8 ± 4 *** | 3.3 ± 0.6 *** | 23.6 ± 0.5 | 23.5 ± 1.4 |
| 2 NO3 | 6.5 ± 1.1 | 5.5 ± 0.6 | 18.8 ± 1.4 | 42.7 ± 3.4 | 167 ± 23 *** | 8 ± 0.3 | 22.7 ± 0.9 | 23.6 ± 1.3 |
| NO3 + PTIO | 5.3 ± 1 * | 4.1 ± 0.1 *** | 19.6 ± 6.1 * | 38.8 ± 0.8 *** | 41.8 ± 6 ** | 3.2 ± 0.3 *** | 24 ± 1.2 | 24.7 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subotički, T.; Mitrović Ajtić, O.; Djikić, D.; Kovačić, M.; Santibanez, J.F.; Tošić, M.; Čokić, V.P. Nitric Oxide Mediation in Hydroxyurea and Nitric Oxide Metabolites’ Inhibition of Erythroid Progenitor Growth. Biomolecules 2021, 11, 1562. https://doi.org/10.3390/biom11111562
Subotički T, Mitrović Ajtić O, Djikić D, Kovačić M, Santibanez JF, Tošić M, Čokić VP. Nitric Oxide Mediation in Hydroxyurea and Nitric Oxide Metabolites’ Inhibition of Erythroid Progenitor Growth. Biomolecules. 2021; 11(11):1562. https://doi.org/10.3390/biom11111562
Chicago/Turabian StyleSubotički, Tijana, Olivera Mitrović Ajtić, Dragoslava Djikić, Marijana Kovačić, Juan F. Santibanez, Milica Tošić, and Vladan P. Čokić. 2021. "Nitric Oxide Mediation in Hydroxyurea and Nitric Oxide Metabolites’ Inhibition of Erythroid Progenitor Growth" Biomolecules 11, no. 11: 1562. https://doi.org/10.3390/biom11111562
APA StyleSubotički, T., Mitrović Ajtić, O., Djikić, D., Kovačić, M., Santibanez, J. F., Tošić, M., & Čokić, V. P. (2021). Nitric Oxide Mediation in Hydroxyurea and Nitric Oxide Metabolites’ Inhibition of Erythroid Progenitor Growth. Biomolecules, 11(11), 1562. https://doi.org/10.3390/biom11111562








