Quercetin Completely Ameliorates Hypoxia–Reoxygenation-Induced Pathophysiology Severity in NY1DD Transgenic Sickle Mice: Intrinsic Mild Steady State Pathophysiology of the Disease in NY1DD Is Also Reversed
Abstract
:1. Introduction
2. Methods
2.1. Animal Model
2.2. Preparation of Quercetin
2.3. Experimental Design
2.4. Intravital Microscopy Studies
2.5. Estimation of Lipid Peroxidation Product in Plasma
2.6. Assay of Circulating Plasma Levels of Cell Adhesion Molecules (CAM)
2.7. Statistical Analysis
3. Results
3.1. Selection of Quercetin Dose
3.2. Qualitative Video Microscopic Evaluation of SCD Therapeutic Activity of Quercetin
3.3. Influence on Vaso-Occlusion
3.4. Influence on Microcirculation
3.4.1. Effect of Quercetin on Vrbc in Post-Capillary Venules
3.4.2. Effect of Quercetin on Wall Shear Rate and Q in Post-Capillary Venules
3.5. Influence on Biomarkers
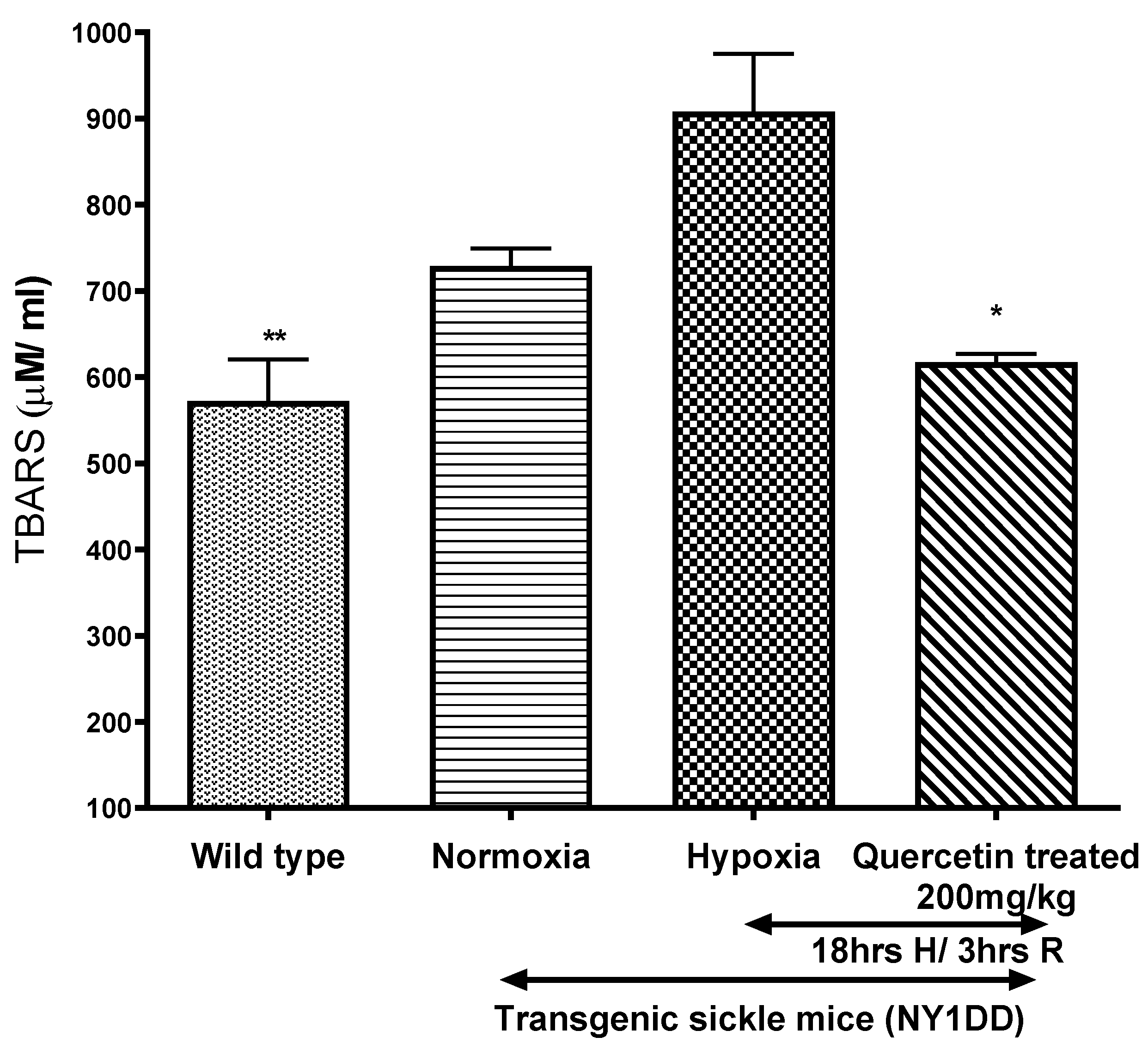
3.5.1. Effect of Quercetin on Lipid Peroxidation
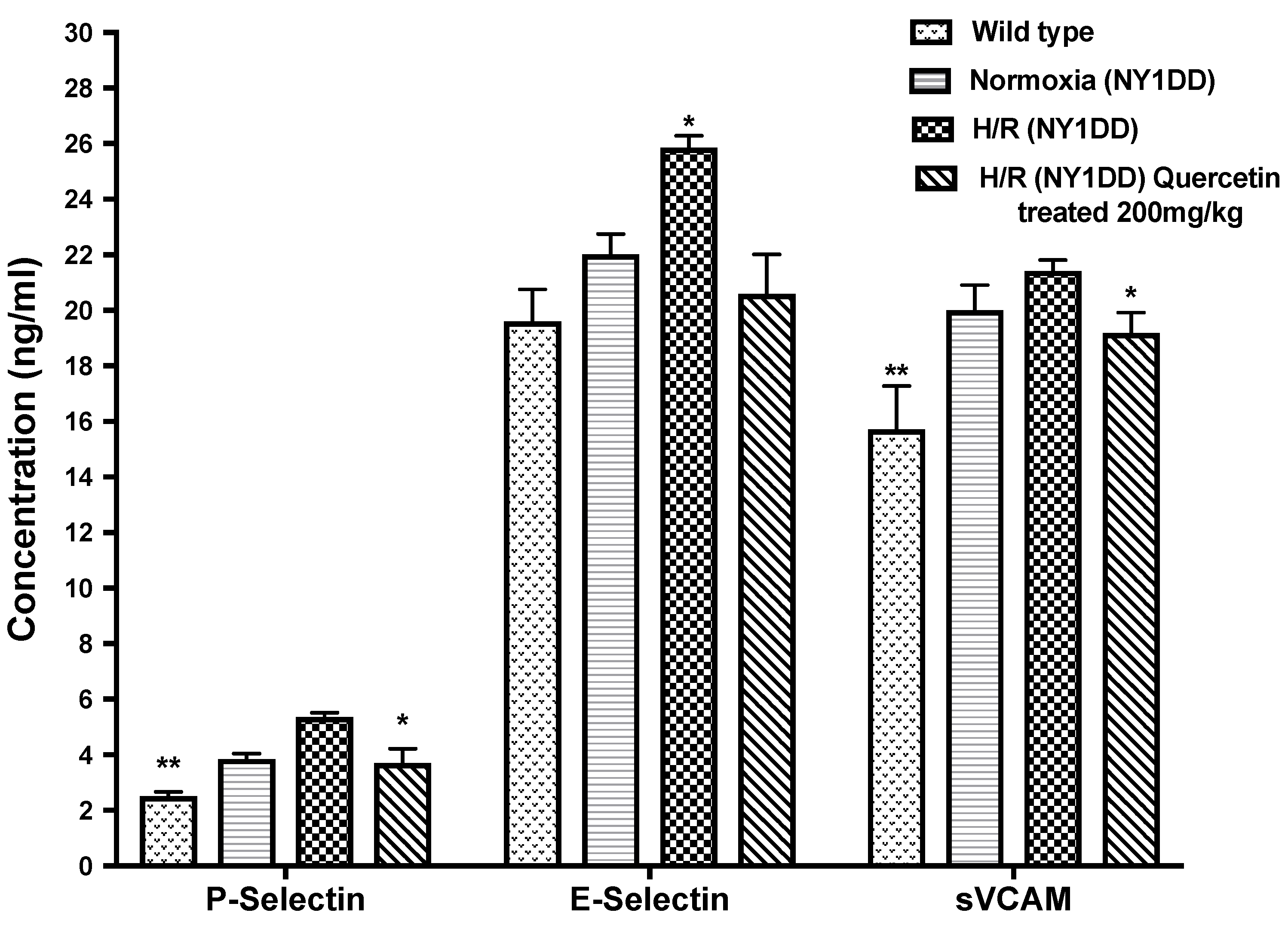
3.5.2. Effect of Quercetin on Soluble Cell Adhesion Molecules (CAM)
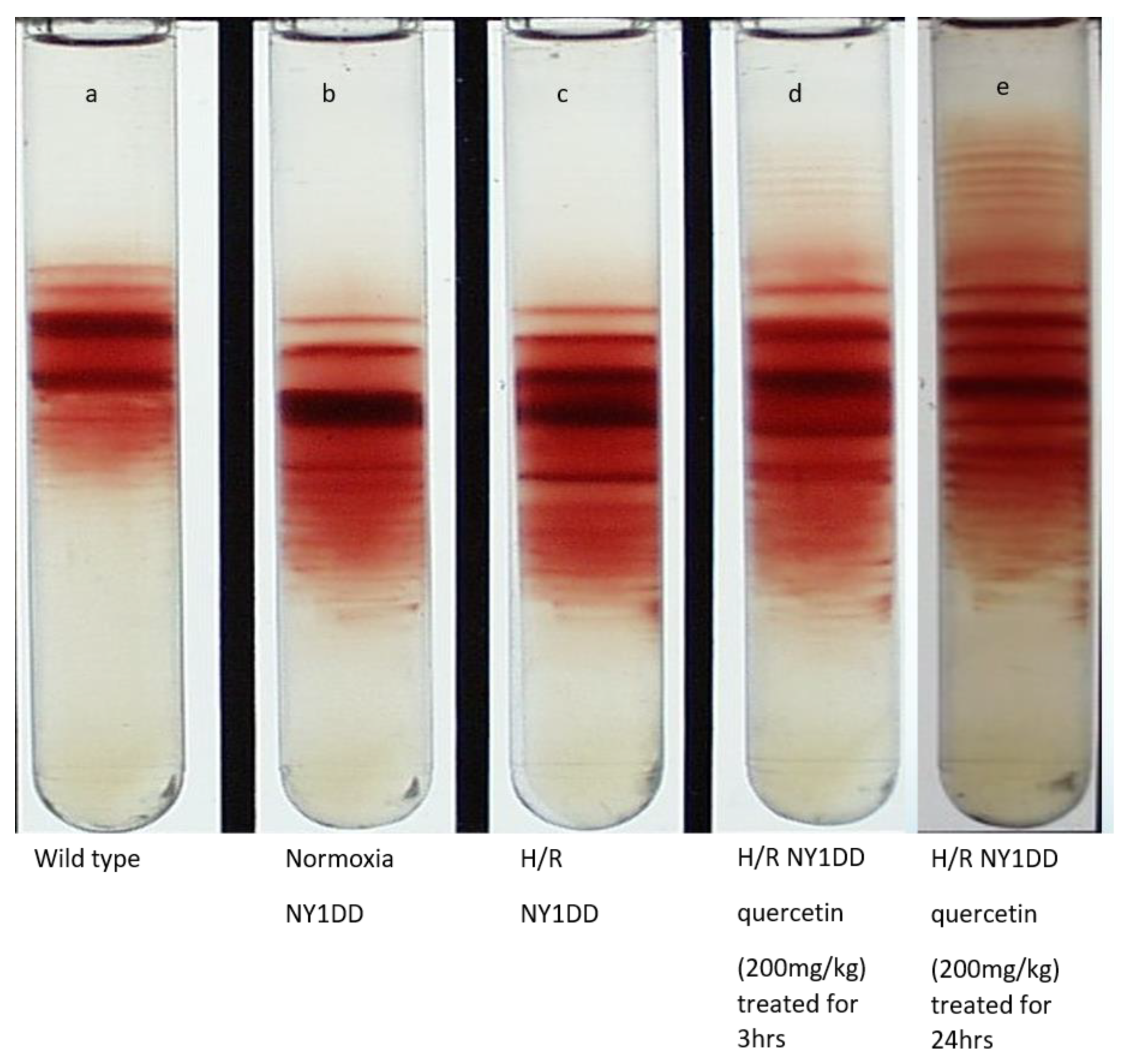
3.6. Influence on Red Cell Density Pattern
3.7. The Efficacy of H/R to Induce Vaso-Occlusion in Control C57BL/6J Mice and Therapeutic Efficacy of Quercetin to Ameliorate the Induced Vaso-Occlusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conran, N.; Costa, F.F. Hemoglobin disorders and endothelial cell interactions. Clin. Biochem. 2009, 42, 1824–1838. [Google Scholar] [CrossRef]
- Chiu, D.; Lubin, B. Abnormal vitamin E and glutathione peroxidase levels in sickle cell anemia: Evidence for increased susceptibility to lipid peroxidation in vivo. J. Lab. Clin. Med. 1979, 94, 542–548. [Google Scholar]
- Hebbel, R.P.; Eaton, J.; Balasingam, M.; Steinberg, M.H. Spontaneous oxygen radical generation by sickle erythrocytes. J. Clin. Investig. 1982, 70, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Shohet, S.B. A novel phospholipid in irreversibly sickled cells: Evidence for in vivo peroxidative membrane damage in sickle cell disease. Blood. 1984, 63, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Lachant, N.A.; Davidson, W.D.; Tanaka, K.R. Impaired pentose phosphate shunt function in sickle cell disease: A potential mechanism for increased Heinz body formation and membrane lipid peroxidation. Am. J. Hematol. 1983, 15, 1–13. [Google Scholar] [CrossRef]
- Rank, B.H.; Carlsson, J.; Hebbel, R.P. Abnormal redox status of membrane-protein thiols in sickle erythrocytes. J. Clin. Investig. 1985, 75, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Omorphos, S.C.; Baysal, E. Sickle cell membranes and oxidative damage. J. Biochem. 1986, 237, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Wetterstroem, N.; Brewer, G.J.; Warth, J.A.; Mitchinson, A.; Near, K. Relationship of glutathione levels and Heinz body formation to irreversibly sickled cells in sickle cell anemia. J. Lab. Clin. Med. 1984, 103, 589–596. [Google Scholar]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front Physiol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Nair, R.C. Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br. J. Haematol. 1980, 44, 87–92. [Google Scholar] [CrossRef]
- Belcher, J.D.; Beckman, J.D.; Balla, G.; Balla, J.; Vercellotti, G. Heme degradation and vascular injury. Antioxid. Redox Signal. 2010, 12, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Kaul, D.K.; Hebbel, R.P. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J. Clin. Investig. 2000, 106, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.E.; Hart, E.; Kirkham, F.J.; Stotesbury, H. L-Glutamine in sickle cell disease. Drugs of Today. 2020, 56, 257. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Lee, K.W.; Kim, B.H.; Bode, A.M.; Lee, H.J.; Heo, Y.S.; Boardman, L.; Limburg, P.; Lee, H.J.; Dong, Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis. 2011, 32, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012, 6, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal Phenolic Phytochemicals in Selected California Wines and Their Antioxidant Activity in Inhibiting Oxidation of Human Low-Density Lipoproteins. J. Agric. Food. Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Takizawa, S.; Fukuyama, N.; Hirabayashi, H.; Kohara, S.; Kazahari, S.; Shinohara, Y.; Nakazawa, H. Quercetin, a natural flavonoid, attenuates vacuolar formation in the optic tract in rat chronic cerebral hypoperfusion model. Brain Res. 2003, 980, 156–160. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food. Chem. 2004, 15, 7514–7517. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; Van Erk, M.J.; Wielinga, P.Y.; Kooistra, Y. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011, 218, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Luangaram, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food. Chem. Toxicol. 2007, 45, 448–455. [Google Scholar]
- Fabry, M.E.; Nagel, R.L.; Pachnis, A.; Suzuka, S.M.; Costantini, F. High expression of human beta S- and alpha-globins in transgenic mice: Hemoglobin composition and hematological consequences. Proc. Natl. Acad. Sci. USA 1992, 89, 12150–12154. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.P.; Nagel, R.L.; Acharya, A.S. Molecular basis of the inhibition of beta s-chain-dependent polymerization by mouse alpha-chain. Semisynthesis of chimeras of human and mouse alpha-chains. J. Biol. Chem. 1993, 22, 16406–16412. [Google Scholar] [CrossRef]
- Kaul, D.K.; Liu, X.D.; Choong, S.; Belcher, J.D.; Vercellotti, G.M.; Hebbel, R.P. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. Am. J. Physiol. Heart. Circ. Physiol. 2004, 287, H293–H301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesquini, M.; Torsoni, M.A.; Stoppa, G.R.; Ogo, S.H. t-BOOH-induced oxidative damage in sickle red blood cells and the role of flavonoids. Biomed. Pharmacother. 2003, 57, 124–129. [Google Scholar] [CrossRef]
- Kaul, D.K.; Thangaswamy, S.; Suzuka, S.M.; Fabry, M.E.; Wanderer, A.A.; Gram, H. Anti-Interleukin-1β Antibody-Based Therapy Ameliorates Endothelial Activation and Inflammation in Sickle Mice. Blood 2011, 118, 848. [Google Scholar] [CrossRef]
- Baez, S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvas. Res. 1973, 5, 384–394. [Google Scholar] [CrossRef]
- Kaul, D.K.; Fabry, M.E.; Costantini, F.; Rubin, E.M.; Nagel, R.L. In vivo demonstration of red cell-endothelial interaction, sickling and altered microvascular response to oxygen in the sickle transgenic mouse. J. Clin. Investig. 1995, 96, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Intaglietta, M. The correlation of photometric signals derived from in vivo red blood cell flow in microvessels. Microvas. Res. 1974, 7, 156–169. [Google Scholar] [CrossRef]
- Wayland, H.; Johnson, P.C. Erythrocyte velocity measurement in microvessels by a two-slit photometric method. J. Appl. Physiol. 1967, 22, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Wayland, H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc. Res. 1974, 7, 131–143. [Google Scholar] [CrossRef]
- Lipowsky, H.; Usami, H.S.; Chien, S. In vivo measurements of "apparent viscosity" and microvessel hematocrit in the mesentery of the cat. Microvasc. Res. 1980, 19, 297–319. [Google Scholar] [CrossRef]
- Romero, J.R.; Suzuka, S.M.; Alami, R.; Feiring, S.N.; Bouhassira, E.E.; Nagel, R.L.; Fabry, M.E. Positively Charged Alpha-Chains Can Stimulate K-Cl Cotransport in Transgenic Mouse Red Cells. Blood 2005, 106, 2326. [Google Scholar] [CrossRef]
- Cain, D.M.; Vang, D.; Simone, D.A.; Hebbel, R.P.; Gupta, K. Mouse models for studying pain in sickle disease: Effects of strain, age, and acuteness. Br. J. Haematol. 2012, 156, 535–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.J.; Acharya, A.S.; Manjula, B.N.; Kumar, R. Chimeric hemoglobins—hybrids of human and swine hemoglobin: Assembly and stability of interspecies hybrids Protein. Science 1996, 5, 956–965. [Google Scholar]
- Mchedlishvili, G. Disturbed blood flow structuring as critical factor of hemorheological disorders in microcirculation. Clin. Hemorheol. Microcirc. 1998, 19, 315–325. [Google Scholar]
- Reiter, C.D.; Wang, X.; Tanus-Santos, J.E.; Hogg, N.; Cannon, R.O.; Schechter, A.N.; Gladwin, M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002, 8, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Suzuki, M.; Granger, D.N. Nitric oxide:an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 1991, 11, 4651–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nur, E.; Verwijs, M.; Waart, D.R.; Schnog, J.J.; Otten, M.; Brandjes, D.P.; Biemond, B.J.; Elferink, R.P. CURAMA Study Group. Increased efflux of oxidized glutathione (GSSG) causes glutathione depletion and potentially diminishes antioxidant defense in sickle erythrocytes. Biochim. Biophys. Acta. 2011, 1812, 1412–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogeeta, S.K.; Gnanapragasam, A.; Kumar, S.S.; Subhashini, R.; Sathivel, A.; Devaki, T. Synergistic interactions of ferulic acid with ascorbic acid: Its cardioprotective role during isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 2006, 283, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Osarogiagbon, U.R.; Choong, S.; Belcher, J.D.; Vercellotti, G.M.; Paller, M.S.; Hebbel, R.P. Reperfusion injury pathophysiology in sickle transgenic mice. Blood 2000, 96, 314–320. [Google Scholar] [CrossRef]
- Dasgupta, T.; Hebbel, R.P.; Kaul, D.K. Protective Effect of Arginine on Oxidative Stress in Transgenic Sickle Mouse Models. Free Radic. Biol. Med. 2006, 41, 1771–1780. [Google Scholar]
- Ozlem, Y.; Pedro, C. Increased hemoglobin O2 affinity protects during acute hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H271–H281. [Google Scholar]
- Simon, W.; Simon, R.; Mathias, S.; Hannes, G. Modulation of Hb-O 2 affinity to improve hypoxemia in COVID-19 patients. Clin. Nutr. 2021, 40, 38–39. [Google Scholar]
- Peter, C.H.H.; Jeanne, H.M.; Devaris, D.L.; Marcel, J.B.M.; Martijin, B.K. Absorption of dietary Quercetin glycosides and Quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar]
- Johnson, M.K.; Loo, G. Effect of epigallocatechin gallate and Quercetin on oxidative damage to cellular DNA. Mutat. Res. 2000, 459, 211–218. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, N.M.; Wods, J.A.; Aherne, S.A.; O’Callaghan, Y.C. Cytotoxicity, genotoxicity, and oxidative reactions in cell-culture models: Modulatory effects of photochemicals. Biochem. Soc. Trans. 2000, 28, 22–26. [Google Scholar] [CrossRef]
- Spiecker, M.; Darius, H.; Kaboth, K.; Hubner, F.; Liao, J.K. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J. Leukoc. Biol. 1998, 63, 732–739. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, J.H.; Yang, W.S.; Kim, S.B.; Park, S.K.; Park, J.S. Exogenous nitric oxide inhibits VCAM-1 expression in human peritoneal mesothelial cells. Role of cyclic GMP and NF-kappa B. Nephron 2000, 90, 447–454. [Google Scholar] [CrossRef]
- Belcher, J.D.; Mahaseth, H.; Welch, T.E.; Vilback, A.E.; Sonbol, K.M.; Kalambur, V.S.; Bowlin, P.R.; Bischof, J.C.; Hebbel, R.P.; Vercellotti, G.M. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am. J. Physiol. Heart. Circ. Physiol. 2005, 288, H2715–H2725. [Google Scholar] [CrossRef]
- Clarissa, J.; Telen, M.T. Adhesion Molecules and Hydroxyurea in the Pathophysiology of Sickle Cell Disease. Haematologica 2008, 93, 481–485. [Google Scholar]
- Egert, S.; Wolffram, S.; Westphal, A.S.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily Quercetin Supplementation Dose-Dependently Increases Plasma Quercetin Concentrations in Healthy Humans. J. Nutr. 2008, 9, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Kaul, D.K.; Thangaswamy, S.; Bhutoria, S.; Gerfen, G.; Branch, C.M.; Intaglietta, M.; Acharya, S.A. Semisynthetic supra plasma expanders: A new class of therapeutics to improve microcircualtion in sickle cell anaemia. Artif. Cells Nanomed. Biotechnol. 2019, 47, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Nivaldo, V.R.; Pedro, C.; Amy, T.G.; Intaglietta, M. Microcirculatory effects of changing blood hemoglobin oxygen affinity during hemorrhagic shock resuscitation in an experimental model. Shock 2009, 31, 646–653. [Google Scholar]
- Glaros, A.K.; Razvi, R.; Shah, N.; Zaidi, A.U. Voxelotor: Alteration of sickle cell disease pathophysiology by a first-in-class polymerization inhibitor. Ther. Adv. Hematol. 2021, 12. [Google Scholar] [CrossRef]
- Hebbel, R.P.; Hedlund, P.E. Sickle hemoglobin oxygen affinity-shifting strategies have unequal cerebrovascular risks. Am. J. Hematol. 2018, 3, 321–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, W.A.; Bunn, H.F. Treating sickle cell disease by targeting HbS polymerization. Blood 2017, 129, 2719–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinsheye, I.; Alsultan, A.; Solovieff, N.; Ngo, D.; Baldwin, C.T.; Sebastiani, P.; Chui, D.H.K.; Steinberg, M.H. Fetal hemoglobin in sickle cell anemia. Blood 2011, 1, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, D.P.; Khattar, J.; Lappin, S.L. Physiology, Fetal Hemoglobin; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar] [PubMed]
- Nkya, S.; Mgaya, J.; Urio, F.; Makubi, A.; Thein, S.L.; Menzel, S.; Cox, S.E.; Newton, C.R.; Kirkham, F.J.; Mmbando, B.P.; et al. Fetal Hemoglobin is Associated with Peripheral Oxygen Saturation in Sickle Cell Disease in Tanzania. Bio Medicine 2017, 23, 146–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
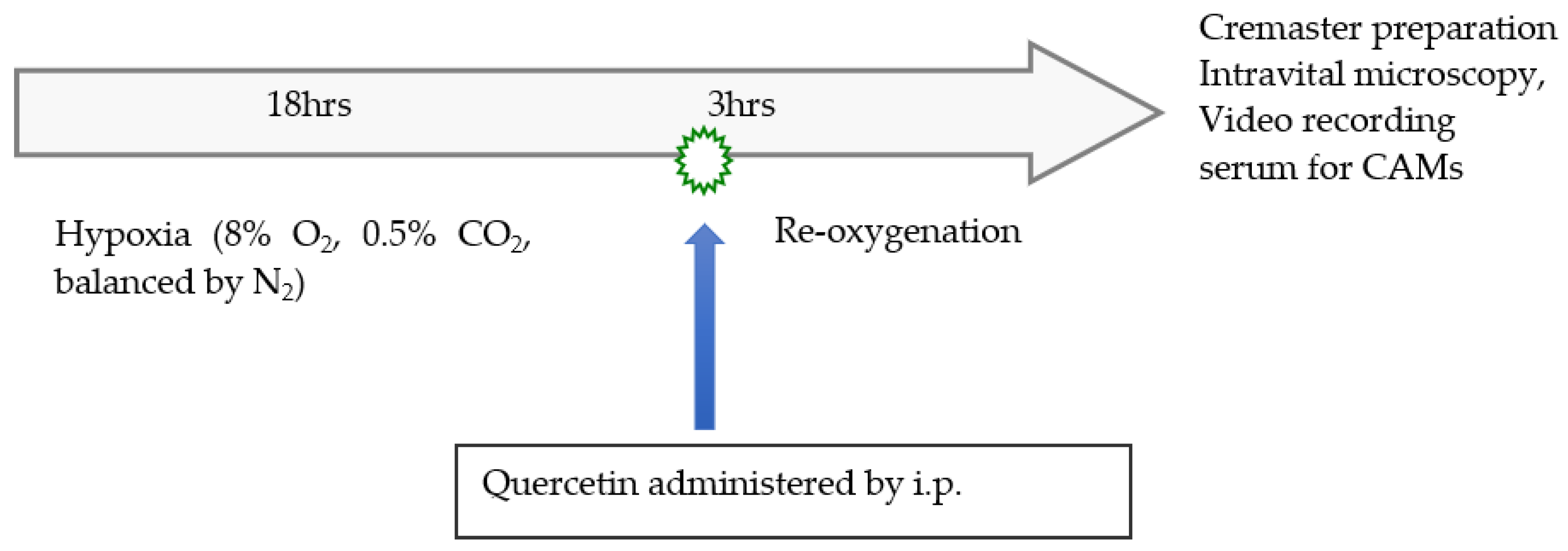

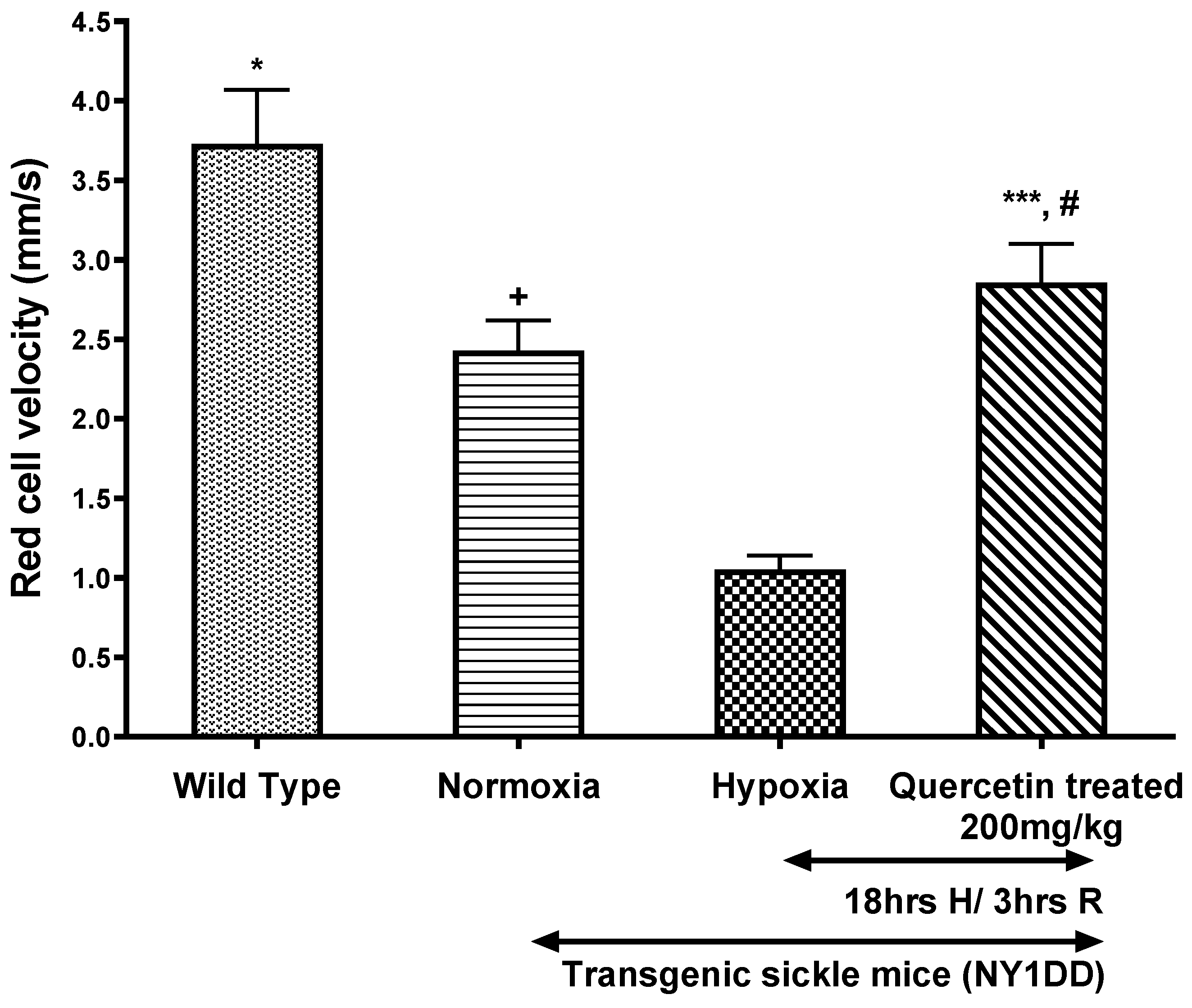
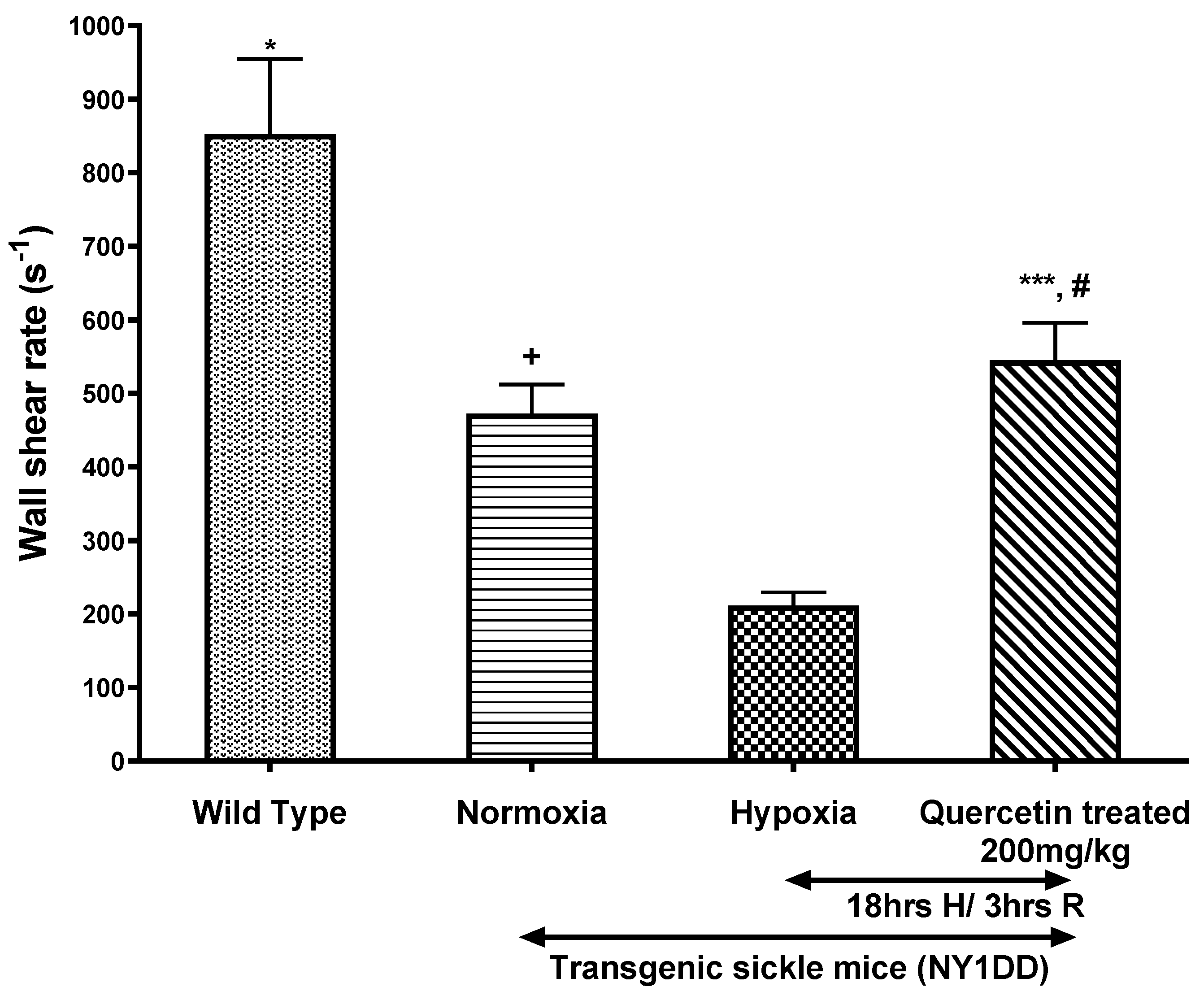
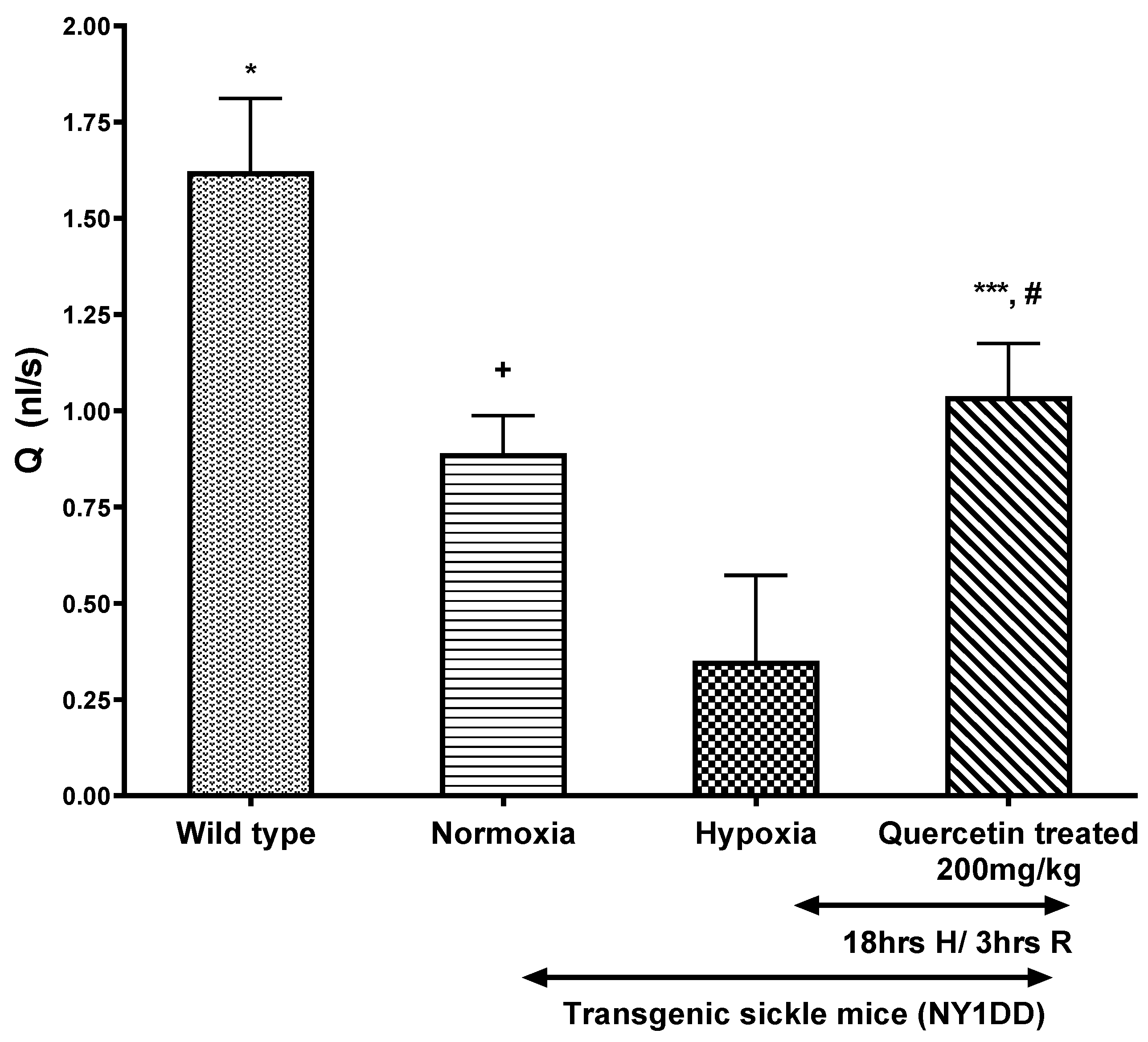

| Parameter | Normoxia | Hypoxia Reoxygenation (NY1DD Mice) | ||
|---|---|---|---|---|
| Wild Type (C57BL/6J) | Sickle Mice (NY1DD) | Untreated | Quercetin (200 mg/kg) | |
| Diameter (μm) | 27.18 ± 0.99 | 25.66 ±0.81 | 24.76 ± 0.73 | 26.02 ± 1.24 |
| Leukocyte adhesion (Cells/100 μm) | 0.5 ± 0.15 | 1.7 ± 0.26 + | 5.8 ± 0.57 * | 0.47 ± 0.12 *** |
| Leukocyte emigration (Cells/field) | 0.11 ± 0.07 | 0.74 ±0.12 + | 1.7 ±0.28 * | 0.22 ± 0.09 *** |
| Leukocyte rolling flux (Cells/min) | 16.0 ± 5.11 | 36.71 ± 1.44 ++ | 47.17 ± 2.8 * | 30.19 ±1.96 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaswamy, S.; Branch, C.A.; Ambadipudi, K.; Acharya, S.A. Quercetin Completely Ameliorates Hypoxia–Reoxygenation-Induced Pathophysiology Severity in NY1DD Transgenic Sickle Mice: Intrinsic Mild Steady State Pathophysiology of the Disease in NY1DD Is Also Reversed. Biomolecules 2021, 11, 1473. https://doi.org/10.3390/biom11101473
Thangaswamy S, Branch CA, Ambadipudi K, Acharya SA. Quercetin Completely Ameliorates Hypoxia–Reoxygenation-Induced Pathophysiology Severity in NY1DD Transgenic Sickle Mice: Intrinsic Mild Steady State Pathophysiology of the Disease in NY1DD Is Also Reversed. Biomolecules. 2021; 11(10):1473. https://doi.org/10.3390/biom11101473
Chicago/Turabian StyleThangaswamy, Sangeetha, Craig A. Branch, Kamalakar Ambadipudi, and Seetharama A. Acharya. 2021. "Quercetin Completely Ameliorates Hypoxia–Reoxygenation-Induced Pathophysiology Severity in NY1DD Transgenic Sickle Mice: Intrinsic Mild Steady State Pathophysiology of the Disease in NY1DD Is Also Reversed" Biomolecules 11, no. 10: 1473. https://doi.org/10.3390/biom11101473
APA StyleThangaswamy, S., Branch, C. A., Ambadipudi, K., & Acharya, S. A. (2021). Quercetin Completely Ameliorates Hypoxia–Reoxygenation-Induced Pathophysiology Severity in NY1DD Transgenic Sickle Mice: Intrinsic Mild Steady State Pathophysiology of the Disease in NY1DD Is Also Reversed. Biomolecules, 11(10), 1473. https://doi.org/10.3390/biom11101473






