Abstract
Pyrroloquinoline quinone (PQQ) is associated with biological processes such as mitochondriogenesis, reproduction, growth, and aging. In addition, PQQ attenuates clinically relevant dysfunctions (e.g., those associated with ischemia, inflammation and lipotoxicity). PQQ is novel among biofactors that are not currently accepted as vitamins or conditional vitamins. For example, the absence of PQQ in diets produces a response like a vitamin-related deficiency with recovery upon PQQ repletion in a dose-dependent manner. Moreover, potential health benefits, such as improved metabolic flexibility and immuno-and neuroprotection, are associated with PQQ supplementation. Here, we address PQQ’s role as an enzymatic cofactor or accessory factor and highlight mechanisms underlying PQQ’s actions. We review both large scale and targeted datasets demonstrating that a neonatal or perinatal PQQ deficiency reduces mitochondria content and mitochondrial-related gene expression. Data are reviewed that suggest PQQ’s modulation of lactate acid and perhaps other dehydrogenases enhance NAD+-dependent sirtuin activity, along with the sirtuin targets, such as PGC-1α, NRF-1, NRF-2 and TFAM; thus, mediating mitochondrial functions. Taken together, current observations suggest vitamin-like PQQ has strong potential as a potent therapeutic nutraceutical.
Keywords:
pyrroloquinoline quinone; PQQ; cell signaling; nutrition; vitamins; inflammation; antioxidant 1. Introduction
Pyrroloquinoline quinone (PQQ) was first reported as a cofactor for bacterial dehydrogenases in the late 1960s [1]. PQQ’s recognition as a cofactor was important because, at the time, only nicotinamide cofactors and flavins were considered cofactors for bacterial dehydrogenases. Over the next fifteen years, the structure of PQQ was fully elucidated [2,3,4,5,6,7,8]. As a result, PQQ is now appreciated as part of a family of quinone cofactors, classified as quinoproteins, that are utilized by dehydrogenases and oxidases. The other quinoprotein cofactors include 2,4,5–trihydroxyphenylalanine quinone or topaquinone (TPQ), lysine tyrosylquinone (LTQ), cysteine tryptophylquinone (CTQ) and tryptophan phyloquinone (TTQ) [7,8].
Through nutritive and environmental exposures, PQQ affects essential biological processes, influencing mitochondriogenesis, reproduction, growth and aging. Multiple lines of evidence, in organisms ranging from fungi to mammals, indicate that the absence of PQQ from nutrient sources produces a broad assortment of abnormalities. Moreover, as a nutraceutical, PQQ attenuates clinically relevant conditions such as ischemia, inflammation, and lipotoxicity, and also has nootropic properties [9,10]. In this regard, genes essential for fatty acid metabolism and mitochondrial function are particularly targeted by PQQ.
Throughout this review, PQQ’s role in cell signaling will be a focus. PQQ serves as a catalytic accessory factor for lactate and other dehydrogenases in the oxidation of NADH to NAD+ which has emerged as a significant finding [11]. PQQ enhances NAD+-dependent sirtuin activity and the expression of sirtuins targets, such as PGC–1α, NRF–1 and 2, and TFAM [10]. Notably, few bioactive food components reduce the levels of reactive oxidative species as efficiently as PQQ [9].
2. Chemistry and Biologic Mechanisms of Action
2.1. General Properties
Pyrroloquinoline quinone (PQQ; 4,5-dihydro-4,5-dioxo-1H-pyrrolo [2,3-f]quinoline-2,7,9-tricarboxylic acid) is an aromatic water-soluble quinone whose chemical properties are analogous to combining the chemical features of ascorbic acid, riboflavin and pyridoxal-5-phosphate into one molecule. For example, both the oxidized (PQQox) and reduced (PQQH2) forms of PQQ carry out redox cycling. PQQ also serves as a dehydrogenase cofactor, a free radical scavenger, and an amine oxidase catalyst [12,13]. PQQ is highly electrophilic and reacts with many substances (e.g., various carbonyl reagents, such as hydrazine, hydroxylamine and semicarbazides). Moreover, PQQ forms complexes with acetone, aminoguanidine, urea, various diamines, and divalent metals [12,13]. In addition, PQQ reacts with ammonia to produce an iminoquinone, and under acidic conditions, internal lactones are formed, in contrast to the formation of dihydrates under neutral and basic conditions [14].
PQQ does not easily self-oxidize or condense into inactive forms. Accordingly, when compared on a molar basis, PQQ can be 100 to 1000 times more efficient in redox cycling assays than other enediols, such as ascorbic acid and menadione, as well as many isoflavonoids, phytoalexins and polyphenolic compounds [12]. As a redox cofactor, PQQ is capable of catalyzing continuous redox reactions involving oxidation of thiols [12], riboflavin [12], ubiquinone, terminal cytochromes [15], α–tocopheroxyl radicals [16] and nicotinamide adenine dinucleotide cofactors [17]. As an antioxidant, PQQH2 can act as an aroxyl radical scavenger, even more so than α-tocopherol, against peroxyl radicals [16]. Moreover, in combination with α-tocopherol, PQQH2 can protect α-tocopherol by reducing α-tocopheroxyl radicals generated in the oxidation of polyunsaturated lipids. Figure 1 summarizes relationships seminal to PQQ’s role in redox processes and as an antioxidant.
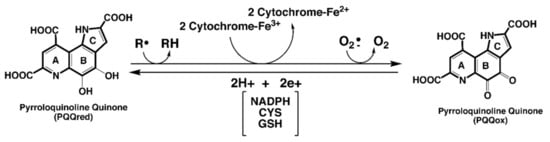
Figure 1.
PQQ and electron transfer reactions. PQQ, in its reduced state, efficiently catalyzes electron transfer reactions. Potential substrates range from organic radicals to radical forms of oxygen. For many of the PQQ—dependent dehydrogenase systems, electron transfer may involve a heme-associated component. PQQ is reduced in reactions, utilizing either NAD[P]H, thiols such as cysteine and glutathione, enediols such as ascorbic acid, or aroxyl radical reductants such as the tocopherols.
Oxidized PQQ does not exert appreciable antioxidant activity in the presence of metals like copper; however, PQQox can catalyze the non-enzymatic oxidation of the epsilon amine function of lysyl groups to aldehydic functions in proteins, such as elastin and collagen, and the oxidation of pyridoxamine and pyridoxamine-5-phosphate to pyridoxal and pyridoxal-5-phosphate [12].
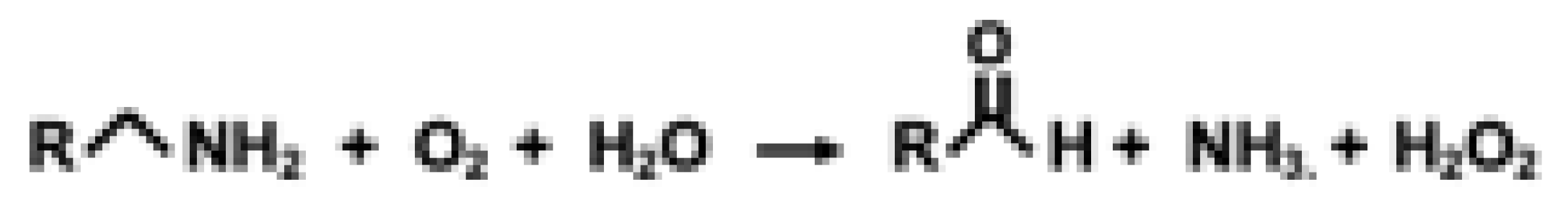
2.2. PQQ as an Enzyme Cofactor
Of the bacterial enzymes for which PQQ serves as a cofactor, most are glucose or alcohol dehydrogenases. The one exception is lupinine hydroxylase, from the genus Pseudomonas [13]. This PQQ—dependent quinoenzyme serves as an amine dehydrogenase, with the quinolizidine alkaloid from lupin plants, lupinine, as the substrate. Mechanisms involving hydride ion transfers are proposed for the oxidation of glucose and alcohol substrates. Metals ions, such as K+, Mg2+, Li+ or Ca2+ also serve catalytic roles [13].
Of fundamental importance, Akagawa and coworkers [18,19] have reported that PQQ serves a functional role in mammalian dehydrogenases such as lactate dehydrogenase (LDH). PQQ bound to LDH enhances NADH oxidation to NAD+ and pyruvate production [18,19]. Optimizing oxidative metabolism is inextricably linked with improved antioxidant defense and enhanced immune function. In this regard, NAD+ is a substrate for the deacylation reactions catalyzed by sirtuins, particularly sirtuin 1 and 3 (SIRT1 and SIRT3). The sirtuins are a family of signaling proteins essential to metabolic regulation related to oxidative processes. Some of the relationships are outlined in Figure 2.
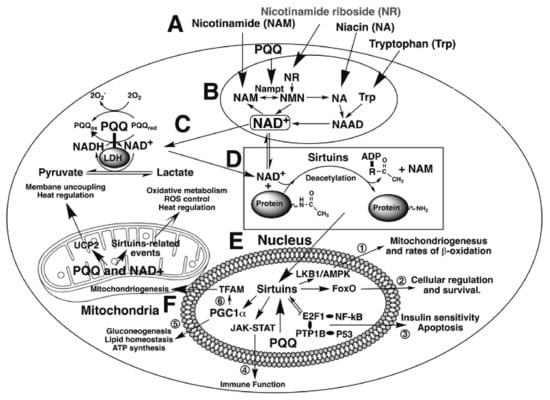
Figure 2.
PQQ and cellular NAD+. Events elicited by PQQ and NAD+ are highlighted. At the top of the figure, A, niacin-related serum components are depicted that are important in the production of NAD+. Each interacts to generate NAD+ as indicated in the section encircled as B. PQQ plays a role in the process by enhancing the expression of nicotinamide phosphoribosyltransferase (designated as Nampt}. Increasing Nampt activity increases NAD+ cellular levels. NAD+ performs two principal functions; first, as a cofactor for dehydrogenases and reductases, such as lactic acid dehydrogenase (LDH), C, and second, as a co-substrate for sirtuin-catalyzed protein deacetylations as noted in D. Likewise, PQQ is a catalytic cofactor for LDH, C. PQQ facilitates the conversion of lactate to pyruvate. In the nucleus, E, NAD+, as a co-substrate for sirtuins, promotes targeted acetylation or deacetylation of proteins involved in cell signaling. Six examples are enumerated: (1) the LKBl/AMP-Kinase-pathway, important in the regulation of rnitochondriogenesis and rates of P-oxidation, (2) Forkhead box 0 transcription factors (FoxO) important to cellular proliferation and survival, (3) transcription factors involving NF-KB and P53 proteins, which regulate multiple aspects of innate and adaptive immune responsiveness, (4) the Janus kinase-signal transducer and activator of transcription JAK-STAT) pathway essential for processes related to hematopoiesis, lactation, and immune responsiveness, (5) peroxisome proliferator-activated receptor-gamma coactivator (PGC-lα), which plays a central role in cellular metabolism and ATP production and (6) the regulation of the mitochondrial transcription factor (TFAM) and other factors essential for mitochondrial genome replication. Finally, in the mitochondria, F. PQQ and NAD+ profoundly impact oxidative metabolism, ROS control, and heat regulation through events controlled by mitochondrial sirtuins and uncoupling proteins, such as UCP2.
To elaborate further, SIRT1 is primarily found in the nucleus and lesser amounts in the cytoplasm [11,20], whereas SIRT3 is generally localized in the mitochondria and associated with mitochondrial DNA transcription [11,21]. Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-lα is one of the most critical factors controlled by sirtuin activity and is activated through deacetylation by SIRT1. PGC-lα acts as a transcriptional coactivator by enabling the assembly of other transcriptional enhancers, such as nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2). Such factors are necessary elements in the cell required for maintaining energy homeostasis and engaging antioxidant response genes [22]. Moreover, Tchaparian et al. [23] have reported on the identification of other transcriptional networks responding to dietary PQQ supplementation. Among these are the 5′-adenosine monophosphate-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK), and the Janus kinase signal transducer and activator of transcription JAK-STAT) pathways, which aid in the control of cellular proliferation, mitosis, apoptosis and most aspects of the immune response [24,25,26,27,28,29,30,31,32].
3. PQQ’s Role in Prokaryotes and Fungi
3.1. PQQ Synthesis
Over one hundred prokaryotes have been identified as capable of PQQ synthesis and use PQQ as an enzymatic cofactor [33]. Most are Gram—negative and range in function from plant symbionts (e.g., root-associated rhizobacteria) to insect pathogens. The PQQ-requiring alcohol and glucose dehydrogenases in bacteria catalyze the oxidation of a wide variety of primary and secondary alcohols as well as catabolism of acyclic terpenes [13]. The glucose dehydrogenases (GDHs) exist in both membrane-bound and soluble forms [34,35]. Their DNA-derived protein sequences, however, are dissimilar, and there is little to no immunological cross-reactivity. Most attention has been given to the membrane bound GDHs localized to the periplasmic surface of cytoplasmic membranes [35], which are essential to bacterial respiration, oxidative metabolism, and growth. Moreover, their regulation is sensitive to changes in oxygen concentration and factors linked to cell growth. It is also important to note that some organisms that do not make PQQ will utilize it when available. A good example is the enteric bacterium Escherichia coli (E. coli), which has a membrane-bound PQQ-dependent GOH and an NADH-dependent GDH. The PQQ-dependent GDH is used preferentially when PQQ is available [13].
For those bacteria that make PQQ, five protein gene products, designated PqqA, PqqB, PqqC, PqqD, and PqqE, are required for PQQ production (Figure 3). Three gene products in the pqq operon, PqqB, PqqC, and PqqE, are members of large protein superfamilies. The roles for each gene product have been described by Zhu and Klinman [36]. Following synthesis, PQQ next binds to quinoenzymes, such as the bacterial glucose and alcohol dehydrogenases, at binding domains with the properties of β-propeller sequences. β-propeller sequences are characterized by 4 to 8 highly symmetrical blade-shaped beta-sheets arranged toroidally around a central axis. β-Propellers are found in all kingdoms of life. They function mainly as scaffolds for macromolecular interactions and ligand binding at catalytic sites [37].
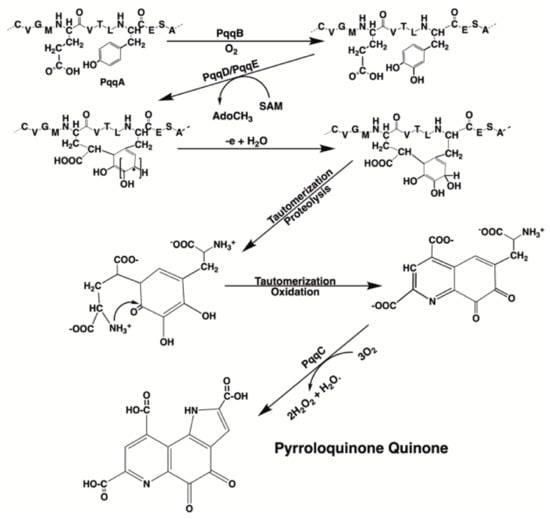
Figure 3.
PQQ synthesis. The scheme shown for PQQ synthesis was adapted from the PQQ biosynthetic pathway in Methylobacterium extorquens. Multiple gene products (designated PqqA, PqqB, PqqC, etc.) are required for PQQ synthesis. PQQ synthesis is unusual in that PQQ is derived from the enzymatic annulation of peptidyl tyrosine and glutamic acid found in PqqA. The annulation of the glutamyl and tyrosyl residues is catalyzed by PqqD and PqqE. PqqD is a novel peptide chaperone that forms a ternary complex with the radical S-adenosylmethionine-requiring protein, Pqq E. The annulation step is then followed by oxidation, tautomerization, and the eventual proteolytic release of PQQ [36].
3.2. PQQ and Fungi
The first to demonstrate a PQQ-containing enzyme in a eukaryote were Taketa, Matsumura, and coworkers [38,39,40,41], who isolated and characterized a novel PQQ-dependent sugar oxidoreductase from the basidiomycete, Coprinopsis cinerea (Cc). Basidiomycota are model organisms for studying fungal sex and mating types, mushroom development, and processes essential to multicellularity. The enzyme, an extracellular PQQ-dependent sugar dehydrogenase (CcSDH), comprises a signaling domain for extracellular secretion and domains for cellulose adsorption, PQQ binding, and cytochrome binding. Subsequently, Varnai and coworkers provided similar observations [15]. Although there are reports that yeast contains PQQ no specific PQQ-containing enzymes have yet been identified [42]. However, as Matsumura et al. [38] have emphasized, BLAST searches indicate the existence of many genes encoding homologous proteins to CcSDH in bacteria, archaea, amoebozoa, and fungi. Furthermore, phylogenetic analysis suggests those identified as quinoproteins are widely distributed in prokaryotes and the fungal family of eukaryotes.
4. PQQ and Plant Growth
There is no evidence that plants produce PQQ; however, PQQ exposure promotes plant growth [43,44,45,46,47,48,49,50,51]. The sources of PQQ for plants are primarily the soil and plant root (rhizome)-associated bacteria. Mechanisms for PQQ—mediated growth stimulation range from increasing soil mineral bioavailability to improving defenses against reactive oxidant species [44,49,50]. For example, rhizobacteria aid in the solubilization of mineral phosphates [43,44,45,46,47,48], whereas Pseudomonas mutants, lacking genes responsible for PQQ synthesis, do not promote growth [47]. Treatment of leaf discs with PQQ or wild-type Pseudomonas fluorescens results in scavenging of ROS and reductions in hydrogen peroxide. As little as 100 nM of PQQ stimulates growth of Cucumis sativus (cucumber) seedlings [44]. PQQ also improves the tolerance of phosphate-solubilizing rhizobacteria to ultraviolet radiation, even when grown under phosphate—free conditions [50]. Improved bacterial tolerance to mitomycin C is also apparent, i.e., fewer DNA strand breaks are observed following PQQ exposure [50]. As a final point, growth promotion may occur by decreasing the harmful effects of plant pathogenic microorganisms. Many rhizobacteria that depend on PQQ for replication produce antibacterial compounds that protect against plant root pathogens such as Agrobacterium tumefaciens, which can cause cancerous proliferation of the stem tissue, or Ralstonia solanacearum, an aerobic, plant pathogenic bacterium [51].
5. PQQ and Insects (Drosophila Melanogaster and Nematodes)
5.1. Drosophila Melanogaster
Drosophila melanogaster and nematodes are often used as models in studies focused on gut microbiology and longevity [52] and surrogates to assess disease vectors at the cell signaling and gene levels [52]. For example, in D. melanogaster models of Parkinson’s disease, genetic or pharmacological activation of the D. melanogaster homolog of mammalian PGC-1α. rescues the disease phenotype by stimulating D. melanogaster PGC-1α. [53]. In turn, silencing D. melanogaster PGC-1α results in the expression of Parkinsonian phenotypes [54]. Drosophila also depend on commensal bacteria to maintain normal microbiome function. Interestingly, Shin et al. [55] reported that the PQQ-dependent alcohol dehydrogenase (PQQ-ADH) activity of Acetobacter pomorum, a commensal acetic acid-producing Drosophila bacterium, modulates insulin/insulin-like growth factor (I/IL-GF) signaling. In germ-free D. melanogaster, I/IL-GF signaling-related defects are reversed by enhancing host I/IL-GF signaling or supplementing the diet with acetic acid. I/IL-GF signaling influences D. melanogaster development, body size, energy metabolism, and intestinal stem cell activity.
5.2. Nematodes
In nematodes (Caenorhabditis elegans), PQQ exposure enhances nematode antioxidant capacity and extends lifespan by increasing the transcriptional activities of DAF-16/FOXO, SKN-1/NRF2 and SOD-3, factors involved in life extension [56]. DAF-16 is the ortholog of the FOXO family of transcription factors in C. elegans and is responsible for activating genes involved in survival (e.g., those associated with telomere extension and oxidative stress). The SKN-1 gene encodes a transcription factor that resembles mammalian NRF-2, which is associated with protection against oxidative damage and inflammation. Finally, Sasakura et al. [57] reported that PQQ activates DUOX protein BLI-3. BLI-3 controls the levels of reactive oxidant species, ensuring the levels are appropriate for optimal growth yet below those that promote inflammation.
6. Nutritional Importance in Animal Models and Human Subjects
6.1. Murine and Rodents
Diet is the apparent source of PQQ in animals given that little, if any, PQQ appears to be synthesized by the intestinal microflora (cf., 3. PQQ’s role in Prokaryotes and Fungi; [58,59]). In nutritional studies, delayed growth and neonatal development are features in mice and rats fed diets devoid of PQQ (Figure 4; [60,61]). The neonatal growth rates for mice [60,61] and rats [62] respond to PQQ in a dose-dependent manner. Optimal neonatal growth is achieved at approximately one μmol of PQQ/kg added to basal diets void of PQQ. Moreover, when PQQ exposure is initiated prior to pregnancy, the mice most deprived of PQQ have fewer litters [61].
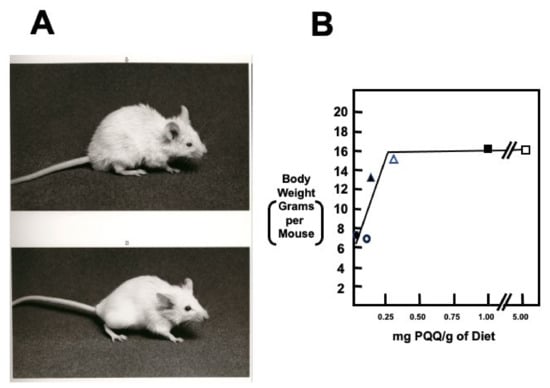
Figure 4.
Growth and appearance of PQQ-deprived and -supplemented BALB/c mice. (A,B) are typical of a PQQ-deprived mouse and control mouse, respectively. Mice fed chemically defined diets devoid of PQQ grow poorly. Severely affected mice have friable skin, hair loss, and a kyphotic appearance. The graph indicates the growth of first-generation mouse pups born from BALB/c dams fed diets containing varying amounts of PQQ. The data suggests that a maternal and subsequent neonatal intake of approximately 300 ng PQQ/g of diet is required for optimal neonatal growth. Values are the means for a minimum of ten 6-week-old mice per. group. Additional details may be found in Steinberg et al. [60,61].
Importantly, PQQ derivatives are readily absorbed from diets. For example, Smidt et al. [58] administered a dose of C14—PQQ (0.42 μCi/μmol) by gavage to Swiss-Webster mice. Sixty percent of the dose was absorbed, and over 80 percent of the absorbed dose was excreted renally. In addition, most of the C14—PQQ in the blood (>95%) was associated with the blood cell fraction rather than plasma.
6.2. Pigs
Regarding other animal species, Yin et al. [63] measured growth, intestinal morphology, redox status, and the levels of selected cytokines using weanling pigs. Without added PQQ the average daily weight gain was 336 g/day for the 28-day observational period. At 4.5 mg PQQ/kg diet, the rate of weight gain was −380 g/day. SOD and glutathione peroxidase (GPX) levels were also increased in the duodenum, jejunum, and ileum. In addition, PQQ exposure decreased inflammatory cytokines such as interleukin (IL)-1β, IL-2 and interferon-Y. Likewise, Wang et al. [64] examined the offspring of sows fed diets with or without PQQ supplementation. The diets were fed throughout gestation and lactation. The activity and mRNA expression levels of heme oxygenase 1 (HMOXl), SOD 1 and 2, catalase (CAT), GPX 1 and 4, and glutamate-cysteine ligase (GCL), the first-rate limiting enzyme of glutathione synthesis, were all increased in the intestine of piglets exposed to PQQ. Additional observations reported by Zhang et al. [65] are also complementary to those of Wang et al. [64] and Yin et al. [63].
6.3. Chickens
Similarly, PQQ elicits responses in avian species consistent with improvements in redox and immune status. Using a basal diet containing approximately 20 μg PQQ/kg of diet, Samuel et al. [66] reported that the incremental addition of PQQ to 800μg/Kg improved antioxidant defenses, decreased inflammation, and had positive effects on growth in young 220 chickens. Moreover, Zheng et al. [67] reported that PQQ at 10 mg/kg of diet countered the inflammatory effects elicited by lipopolysaccharide (LPS). On a molar basis, PQQ was 10 to 100 times more effective than anti-inflammatory drugs such as acetylsalicylic acid and meclofenamate [68], or biofactors such as melatonin [69], in attenuating the effects of LPS.
6.4. Humans
In college—age human subjects, Harris et al. [70] reported that PQQ supplementation (5–10 mg per day) reduced C-reactive protein, interleukin-6 levels, and plasma malonaldehyde levels in plasma. In addition, the ratio of blood lactate to pyruvate and the profile of urinary metabolites (estimated by 1H-nuclear magnetic resonance) were consistent with enhanced mitochondrial oxidation. Likewise, Hwang et al. [71] show daily supplementation with 20 mg PQQ optimizes mitochondrial biogenesis in human subjects. Notably, cognitive function and memory also are improved in human subjects, following PQQ supplementation (10–20 mg per day) [72,73,74].
7. PQQ and Its Derivatives in Diets and Supplements
The assays used for PQQ determination, both spectrophotometric and enzymatic, are sensitive and precise but typically measure only PQQ [75,76,77,78]. Regrettably, precise quantitation of PQQ plus its derivatives is difficult. Using mass spectrometry-based analytical approaches is challenging because of the complex gas-phase fragmentation of PQQ [75]. PQQ-derived ions can arise through acid-catalyzed tautomeric lactonization of PQQ and subsequent oxidation of the PQQ lactone (PQQL) (cf. Figure 5). Decarboxylation of PQQ also occurs easily. Accordingly, the quantitation of total PQQ, including derivatives, is usually qualitative at best. When mass spectrometric analysis has been successfully used to assay PQQ and related derivatives, the levels can be more than an order of magnitude greater than for PQQ alone [75]. For example, in human milk, the concentration of PQQ is estimated to be 20-30 μg/L. Whereas PQQ plus imidazolopyrroloquinoline (IPQ), a derivative easily formed when PQQ reacts with non-branched chain amino acids, is in the range of 140-180 μg/L (~0.5 μM) or 500 to 750 μg/kg of milk solids (~2 μM). PQQ added to aqueous suspensions of semi-purified diet forms derivatives and adducts rapidly, as observed by Steinberg et al. [60,61] and Stites et al. [79]. For example, the half-life for the rate of PQQ adduct formation or disappearance is 45 min at pH 7.0 and ~60 min at pH 2.5 [60].
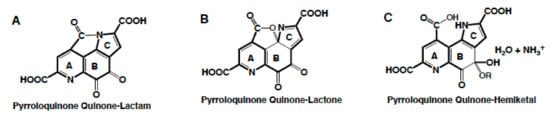
Figure 5.
PQQ derivatives. PQQ can tautomerize under acidic conditions to form lactones (B), lactams (A) and hemiketals (C). In addition, PQQ readily engages in nucleophilic additions and substitutions.
Nevertheless, as reported in Section 6, strong inferences may be made regarding the need for PQQ. For example, it is possible to produce an apparent PQQ deficiency using experimental diets in animal models. This is novel in that deficiency signs have not been described for other organic biofactors except for those currently viewed conditional vitamins. Moreover, when the effects of PQQ dietary supplements are reported, they are often observed in the low mg/kg/diet or per 1000 kcal range. Except for anthocyanins [80], other biofactors (quercetin, resveratrol, catechins) require intakes of 100–400 mg/kg diet for measurable antioxidant or immune protection-related effects in animal models and humans.
Moreover, IPQ is often as effective as PQQ in bioassays. IPQ is an intermediate when redox cycling is driven by the imidization of amino acids such as L-glycine [81,82,83]; therefore, it can be a potential source of PQQ (Figure 6). Naito et al. [84] measured [3H]thymidine incorporation into cultured fibroblast DNA. PQQ significantly enhanced thymidine incorporation in the nM range and IPQ in the μM range. Yamada et al. [85] also compared PQQ and IPQ using human neuroblastoma and hepatocellular carcinoma cell lines, and assays designed to test neuroprotection from 6-hydroxydopamine exposure. Their findings also indicated that IPQ’s biological activity was like that of PQQ. In addition, Tsuchida et al. [86] demonstrated that both PQQ and IPQ are protective in vivo against carbon tetrachloride-induced liver injury in rats.
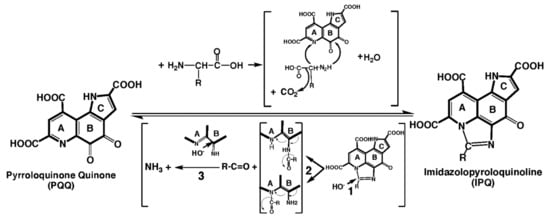
Figure 6.
Imidazolopyrroloquinoline (IPQ). PQQ reacts with non−branched chain amino acids to form imidazole derivatives (IPQ), with or without retention of the amino acid sidechain (R). The reverse reaction (IPQ → PQQ) most likely results from a base-catalyzed opening of the imidazolium ring as a first step (1). Next, two intermediate forms are possible with the release of the imidazole carbon atom as an aldehyde moiety with or without an attached R group (2). Finally, there is a loss of the imine function that results from nucleophilic addition of OH−, isomerization and oxidation (3).
As a final point, PQQ as a supplement is safe. PQQ meets all the USA-FDA “generally regarded as safe” (GRAS) requirements [87]. Liang et al. [88] have reported that the No-observed-adverse-effect-level (NOAEL) for disodium PQQ is 0.4 g/kg body weight/day in rats for both sexes. The median lethal dose for PQQ in rats is 1.0–2.0 g/kg body weight in males and 0.5–1.0 g/kg body weight in females [89,90].
8. Selected Clinical and Organ-Specific Dysfunctions Responsive to PQQ
8.1. Kidney and Liver
Chronic kidney disease (CKD) affects almost 15% of the global population [91]. Complications include cardiovascular diseases (CVDs), diabetes, hypertension, anemia, renal disease progression, acute kidney injury, mineral and bone disorders [92], and cognitive decline [93,94,95,96]. The kidney controls body fluid composition by filtering and reabsorbing materials. Reabsorption requires ATP, most of which is generated by mitochondria [97]. The high oxidative activity of mitochondria in the kidney can elevate oxidative stress, resulting in renal dysfunction and CKD progression [98,99]. In both human and animal models, depletion of antioxidant defense activity and increased production of ROS induces inflammatory damage, predominantly targeting renal tubular epithelial cells. The nuclear factor erythroid 2-related factor 2 (NFE2L2/NRF2)—antioxidant responsive element (ARE) prevents the progression of acute kidney injury (AKI) to CKD [100,101] by regulating cellular antioxidant defenses [102]. In multiple studies, a mechanism underlying NFE2L2’s protective role is its action on its target genes, including SOD2 and heme oxygenase (HMOX1), thereby decreasing ROS in the intracellular environment. In experimental CKD models, natural bioactive compounds with kidney protective potential are associated with NFE2L2 activation [103,104]. In this regard, recent work in vivo [105] and in vitro [106] suggests that PQQ appears to target the NFE2L2 pathway. In a mouse model of nephrotoxicity induced with the chemotherapeutic cyclophosphamide (CTX, an immunosuppressive agent), Lin et al. [105] showed PQQ supplementation of CTX-treated mice ameliorates nephrotoxicity by rescuing CTX-mediated 2inhibition of NFE2L2 signaling, characterized by altered expression of NFE2L2 target genes [105].
When treated with high glucose, the HK-1 human proximal tubular epithelial cell line models the progression of diabetes in the kidney. High glucose treatment of HK-1 cells results in elevated ROS and expression of pro-inflammatory genes, with concomitant inhibition of NFE2L2 and its targets. Wang et al. [106] found that PQQ supplementation of HK-1 cells treated with high glucose activates NFE2L2 signaling by increasing NFE2L2 translocation to the nucleus. Furthermore, PQQ mitigates pro-inflammatory signaling both in vivo and in vitro, which Lin et al. showed is regulated through the NLRP3 inflammasome [105].
Factors critical to the programming of neonatal development (e.g., maternal obesity or early exposure to high fat diets) also promote hepatic inflammation. Such processes can lead to nonalcoholic fatty liver disease progression to steatohepatitis in murine models [cf., POO, intestinal barrier functions and the microbiome]. Like the kidney, the expression of NFE2L2 and its target HMOX1, along with NLRP3, is diminished in liver from the offspring of mice fed a Western-style diet and treated with PQQ [107]. In addition, supplementation of PQQ attenuates hepatic inflammation in obese rats [108]. In rats, the amelioration of cadmium- and mercury-induced liver and kidney damage has also been reported following PQQ exposure [109].
8.2. Intestinal Barrier Functions and the Microbiome
An intact intestinal barrier is now recognized as an integral regulator of health [110,111,112]. Bacteria and their products are vital contributors to impairment and permeability of the gut barrier, resulting in an increased influx of bacteria, endotoxin, bacterial DNA, and metabolites into the host circulation. Recent reviews show that microbial dysbiosis and impaired barrier function are associated with gastrointestinal disease [113,114,115], neurodegenerative disease [116,117,118,119], autoimmune disease [120,121,122,123,124,125], and an impaired metabolic status in the host manifested by obesity, insulin resistance and cardiovascular complications [126,127,128,129,130,131,132,133,134,135]. In several animal models [63,64,107,136,137], exposure to PQQ increases mRNA expression levels of tight junction proteins and improves jejunal barrier function, suggesting PQQ may act through the gut to affect tissues in the periphery. In this regard, recent evidence has emerged suggesting a short-chain fatty acid, butyrate, is a crucial modulator of barrier function and colonic homeostasis [138,139]. Interestingly, exposure to PQQ leads to increased butyrate levels [137,140], most likely because it is used preferentially as a cofactor for membrane-bound hexose and alcohol dehydrogenases in E. coli [141]. As noted previously, membrane-bound hexose and alcohol dehydrogenases are involved in the synthesis of gluconic acid, which is fermented by lactic acid bacteria into lactate and acetate products used by acid-utilizing bacteria to form butyrate [142]. Butyrate exerts a broad spectrum of positive effects, both in the intestine and systemically, including promoting energy expenditure, stimulating peroxisome proliferator-activated receptor coactivator activity, improving insulin resistance, and enhancing mitochondrial function [138,143]. Furthermore, butyrate acts as an inhibitor of histone deacetylase activity [144]; therefore, PQQ-mediated alterations in butyrate levels might result in epigenetic change. Indeed, studies in pigs [137] and rodents [107,136] demonstrated improved barrier function in offspring following supplementation with PQQ. Although studies directly investigating the effects of PQQ on microbiota composition or function are sparse, a current supposition is that some gut bacteria may require PQQ for optimal function.
Using a mouse model of developmental programming of nonalcoholic fatty liver disease, Jonscher and colleagues [107,145] were the first to detect differences in gut bacteria composition in response to PQQ exposure. PQQ supplementation to the dams significantly reversed the diet-induced decrease in Bacteroidetes and increases in Firmicutes observed in offspring bacterial composition (Figure 7). Genera within both phyla, such as Bacteroides and Allobaculum, increased in abundance while Akkermansia, from the phylum Verrucomicrobiae, decreased. Notably, PQQ attenuates the WD-induced bloom of Clostridiales spp. In aged rats fed a lard-based high fat diet, Clostridiales was the order most affected by diet, and its decrease appeared responsible for gut microbial dysfunction [146]. Whether age or type of fat affects Clostridiales abundance remains to be determined, but it is suggestive that this order may be a dynamic, longitudinal biomarker for obesity or lipotoxicity.
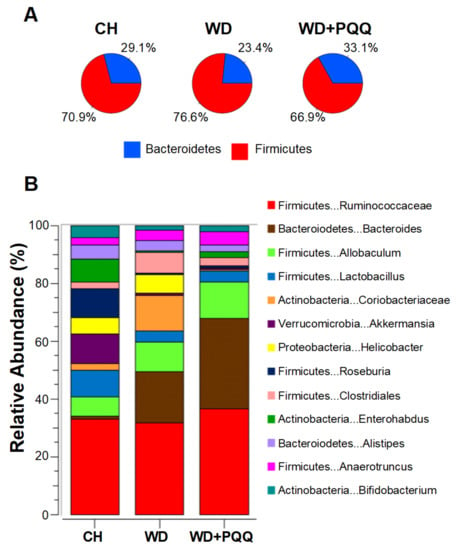
Figure 7.
PQQ-associated changes in the gut microbiota of offspring of obese mouse dams. Obesity in dams was induced by feeding a chow (CH) or Western-style diet (WD), with and without PQQ supplementation. Bacterial compositional differences were measured using 16S sequencing of offspring cecal contents at postnatal day 21 [107]. (A) The ratio of Bacteroidetes to Firmicutes was diminished by WD exposure and rescued by PQQ. (B) WD exposure altered relative abundances of the 13 most abundant genera, some of which returned to near control levels in offspring exposed to maternal PQQ.
8.3. Cardiac and Skeletal Muscle Protection
Skeletal and cardiac muscles require a high rate of ATP turnover for efficient contraction. In this regard, rats deficient in PQQ are markedly compromised when subjected to cardiac ischemia/reperfusion injury protocols. For example, Bauerly et al. [147] reported that 20% of rats fed a PQQ deficient diet did not survive ischemia reperfusion, whereas all PQQ supplemented rats survived. These studies were an extension of previous work [148,149,150] in which the cardioprotective effectiveness of PQQ was compared with metoprolol, a beta(l)—selective adrenoceptor antagonist. Rats underwent 30 min of left anterior descending coronary artery occlusion, followed by 2 h of reperfusion. Although both PQQ and metoprolol improved indices of cardiac function, PQQ was superior to metoprolol in protecting mitochondria from ischemia/reperfusion oxidative damage. Whether PQQ supplementation influences cardiac and skeletal muscle function in healthy individuals and animal models remains unclear. For example, Hwang et al. [71] investigated the effects of PQQ supplementation on aerobic exercise performance and indices of mitochondrial biogenesis in men following a six-week endurance training/supplementation program. No differences in aerobic performance were observed; however, there were improvements in peak oxygen consumption and total exercise test duration and recovery. In addition, the PQQ group had a significant increase in PGC—lα protein levels from baseline to post endurance training compared to non-supplemented subjects. In mice, Lui et al. [151] demonstrated that PQQ protects against exercise-induced fatigue and reduced oxidative damage, presumably by improving mitochondrial function.
From a marketing perspective, there are numerous claims made regarding PQQ and exercise performance. The claims are often made without considering that oxygen is the most limiting nutrient influencing exercise performance. Although exposure to compounds with biologic properties like those of PQQ increases mitochondrial content, regular exercise may have similar (or even greater) effects. Body heat regulation is also not often considered. The surface temperature of well-functioning mitochondria is estimated to be 50 °C [152]. Cardiac cell mitochondria appear to decrease ATP production and increase inner-mitochondrial membrane permeability when heat dispersal is compromised, or temperatures are sustained at greater than 40 °C [153]. Thus, it is too soon to conclude that PQQ supplementation can independently improve exercise performance. Indeed, Hwang et al. [71] have recently reported that PQQ supplementation in men undergoing endurance exercise training has little effect on performance apart from an elevation of PGC-lα.
8.4. PQQ and Neuroprotection
PQQ is protective in cases and models of brain aging and neurodegeneration, including Parkinson’s disease, stroke, and traumatic brain injury. Perhaps the best of current examples are PQQ’s ability to protect against neuronal agents, such as rotenone [154,155,156,157,158,159,160]. Moreover, the ability of PQQ to protect against neurodegeneration may go beyond mitochondriogenesis, given that PQQ can reduce α—synuclein fibril formation [161]. PQQ also confers resistance to neurocognitive loss in rodent models of stroke and brain injury. Jensen and associates [162] were among the first to use carotid ligation to assess neuroprotective properties following intra peritoneal injection of PQQ. The interruption of blood flow resulted in most of the cortex displaying signs of infarction. Rats without PQQ supplementation had infarctions across −95 percent of cortices compared to −70 percent in rats pretreated with PQQ. In a similar study, Zhang et al. [163] used reversible middle cerebral artery occlusion to simulate interruption of blood circulation to adult rat brains. When PQQ was injected into the jugular vein, either at the same time as occlusion or 3 h after the start of ischemia, markedly improved neurobehavioral scores were observed, along with a reduction in infarct size. Regarding traumatic brain injury, Zhang and associates [155,163,164] assessed spatial memory in rats using the Morris water maze test and demonstrated that PQQ administered intraperitoneally three days before injury improves spatial memory.
In human clinical trials, PQQ was reported to promote cognitive function and improved regional blood flow in older adults [74]. In a randomized placebo-controlled, double-blinded clinical trial, PQQ administered orally (20 mg PQQ/day) to elderly adults resulted in improved cognitive measures. The PQQ dosage was based on a previous animal study showing that PQQ improves spatial memory in rats, as measured by Morris water maze [164].
9. Conclusions
As outlined in Table 1, many aspects of PQQ as a biofactor are novel. It is one of the few biofactors for which a nutritional deficiency and a potential nutritional requirement are definable. PQQ acts as an accessory factor for lactate acid and potentially other dehydrogenases in the oxidation of NADH to NAD+. Relationships important to aging, immunity, ROS defense and neurologic integrity involve maintaining optimal NAD+ levels. The effects of PQQ exposure, both in vivo and in vitro, mimic those of cellular NAD+ augmentation [165]. In addition, PQQ is associated with the attenuation of clinically relevant dysfunctions such as ischemia, inflammation, and lipotoxicity. An important observation is that levels of PQQ needed for such effects are usually in the nM to μM range, in contrast to the mM concentrations needed for other popular biofactors. Accordingly, PQQ has strong potential as a therapeutic nutraceutical. A strong case is also evolving, suggesting that PQQ may serve as an essential vitamin-like factor.

Table 1.
Pyrroloquinoline Quinone: Novel Attributes.
Funding
This work was funded by NIH/NIDDK, grant number R01DK121951 (to KRJ) and a grant from the UCD Emeriti Association (to RBR).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hauge, J.G. Glucose dehydrogenase of Bacterium anitratum: An enzyme with a novel prosthetic group. J. Biol. Chem. 1964, 239, 3630–3639. [Google Scholar] [CrossRef]
- Frank, J.; Dijkstra, M.; Duine, J.A.; Balny, C.; Jzn, J.F. Kinetic and spectral studies on the redox forms of methanol dehydrogenase from Hyphomicrobium X. JBIC J. Biol. Inorg. Chem. 1988, 174, 331–338. [Google Scholar] [CrossRef]
- Duine, J.A.; Jzn, J.F.; Verwiel, P.E.J. Structure and activity of the prosthetic group of methanol dehydrogenase. JBIC J. Biol. Inorg. Chem. 1980, 108, 187–192. [Google Scholar] [CrossRef]
- Duine, J.A. Quinoproteins: Enzymes containing the quinonoid cofactor pyrroloquinoline quinone, topaquinone or tryptophan-tryptophan quinone. JBIC J. Biol. Inorg. Chem. 1991, 200, 271–284. [Google Scholar] [CrossRef]
- Anthony, C.; Ghosh, M. The structure and function of the PQQ-containing quinoprotein dehydrogenases. Prog. Biophys. Mol. Biol. 1998, 69, 1–21. [Google Scholar] [CrossRef]
- Salisbury, S.A.; Forrest, H.S.; Cruse, W.B.T.; Kennard, O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nat. Cell Biol. 1979, 280, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Klinman, J.P.; Mu, D. Quinoenzymes in biology. Annu. Rev. Biochem. 1994, 63, 299–344. [Google Scholar] [CrossRef]
- Anthony, C. Pyrroloquinoline quinone (PQQ) and quinoprotein enzymes. Antioxid. Redox Signal. 2001, 3, 757–774. [Google Scholar] [CrossRef]
- Jonscher, K.R.; Rucker, R.B. Pyrroloquinoline quinone: Its profile. Effects on the liver and implications for health and disease prevention. In Dietary Interventions in Liver Disease; Watson, R.M., Preedy, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 157–168. [Google Scholar]
- Zhao, L.; Gong, N.; Liu, M.; Pan, X.; Sang, S.; Sun, X.; Yu, Z.; Fang, Q.; Zhao, N.; Fei, G.; et al. Beneficial synergistic effects of microdose lithium with pyrroloquinoline quinone in an Alzheimer’s disease mouse model. Neurobiol. Aging 2014, 35, 2736–2745. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- Stites, T.E.; Mitchell, A.E.; Rucker, R.B. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J. Nutr. 2000, 130, 719–727. [Google Scholar] [CrossRef]
- McIntire, W.S. Newly discovered redox cofactors: Possible nutritional, medical, and pharmacological relevance to higher animals. Annu. Rev. Nutr. 1998, 18, 145–177. [Google Scholar] [CrossRef]
- Jayagobi, M.; Raghunathan, R.; Sainath, S.; Raghunathan, M. Synthesis and antibacterial property of pyrrolopyrano quinolinones and pyrroloquinolines. Eur. J. Med. Chem. 2011, 46, 2075–2082. [Google Scholar] [CrossRef]
- Várnai, A.; Umezawa, K.; Yoshida, M.; Eijsink, V.G.H. The Pyrroloquinoline-quinone-dependent pyranose dehydrogenase from Coprinopsis cinerea drives lytic polysaccharide monooxygenase action. Appl. Environ. Microbiol. 2018, 84, 00156–18. [Google Scholar] [CrossRef]
- Ouchi, A.; Ikemoto, K.; Nakano, M.; Nagaoka, S.I.; Mukai, K. Kinetic study of aroxyl radical scavenging and alpha-tocopheroxyl regeneration rates of pyrroloquinoline quinol (PQQH2, a reduced form of pyrroloquinoline quinone) in dimethyl sulfoxide solution: Finding of synergistic effect on the reaction rate due to the coexistence of alpha-tocopherol and PQQH2. J. Agric. Food Chem. 2013, 61, 11048–11060. [Google Scholar] [PubMed]
- Chan, S.I.; Chuankhayan, P.; Nareddy, P.K.R.; Tsai, I.-K.; Tsai, Y.-F.; Chen, K.H.-C.; Yu, S.S.-F.; Chen, C.-J. Mechanism of pyrroloquinoline quinone-dependent hydride transfer chemistry from spectroscopic and high-resolution X-ray structural studies of the methanol dehydrogenase from Methylococcus capsulatus (Bath). J. Am. Chem. Soc. 2021, 143, 3359–3372. [Google Scholar] [CrossRef]
- Akagawa, M.; Minematsu, K.; Shibata, T.; Kondo, T.; Ishii, T.; Uchida, K. Identification of lactate dehydrogenase as a mammalian pyrroloquinoline quinone (PQQ)-binding protein. Sci. Rep. 2016, 6, 26723. [Google Scholar] [CrossRef]
- Akagawa, M.; Nakano, M.; Ikemoto, K. Recent progress in studies on the health benefits of pyrroloquinoline quinone. Biosci. Biotechnol. Biochem. 2016, 80, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic Shuttling of the NAD+-dependent Histone Deacetylase SIRT1. J. Biol. Chem. 2007, 282, 6823–6832. [Google Scholar] [CrossRef]
- Onyango, P.; Celic, I.; McCaffery, J.M.; Boeke, J.D.; Feinberg, A.P. SIRT3, a human SIR2 homologue, is an NAO-dependent deacetylase localized to mitochondria. Proc. Natl. Acad. Sci. USA 2002, 99, 13653–13658. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kerins, M.J.; Tian, W.; Neupane, D.; Zhang, D.D.; Ooi, A. Differential and overlapping targets of the transcriptional regulators NRF1, NRF2, and NRF3 in human cells. J. Biol. Chem. 2019, 294, 18131–18149. [Google Scholar] [CrossRef]
- Tchaparian, E.; Marshal, L.; Cutler, G.; Bauerly, K.; Chowanadisai, W.; Satre, M.; Harris, C.; Rucker, R.B. Identification of transcriptional networks responding to pyrroloquinoline quinone dietary supplementation and their influence on thioredoxin expression, and the JAK/STAT and MAPK pathways. Biochem. J. 2010, 429, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- Lyons, C.L.; Roche, H.M. Nutritional modulation of AMPK-impact upon metabolic-inflammation. Int. J. Mol. Sci. 2018, 19, 3092. [Google Scholar] [CrossRef] [PubMed]
- Peti, W.; Page, R. Molecular basis of MAP kinase regulation. Protein Sci. 2013, 22, 1698–1710. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. AMPK activation inhibits the functions of myeloid-derived suppressor cells (MDSC): Impact on cancer and aging. J. Mol. Med. 2019, 97, 1049–1064. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAP Kl ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Shen, Y.-Q.; Bonnot, F.; Imsand, E.M.; RoseFigura, J.M.; Sjölander, K.; Klinman, J.P. Distribution and properties of the genes encoding the biosynthesis of the bacterial cofactor, pyrroloquinoline quinone. Biochemistry 2012, 51, 2265–2275. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Naoki, K.; Kataoka, N.; Matsutani, M.; Ano, Y.; Adachi, O.; Matsushita, K.; Yakushi, T. Characterization of a cryptic, pyrroloquinoline quinone-dependent dehydrogenase of Gluconobacter sp. strain CHM43. Biosci. Biotechnol. Biochem. 2021, 85, 998–1004. [Google Scholar] [CrossRef]
- Yakushi, T.; Terada, Y.; Ozaki, S.; Kataoka, N.; Akakabe, Y.; Adachi, O.; Matsutani, M.; Matsushita, K. Aldopentoses as new substrates for the membrane-bound, pyrroloquinoline quinone-dependent glycerol (polyol) dehydrogenase of Gluconobacter sp. Appl. Microbiol. Biotechnol. 2018, 102, 3159–3171. [Google Scholar] [CrossRef]
- Zhu, W.; Klinman, J.P. Biogenesis of the peptide-derived redox cofactor pyrroloquinoline quinone. Curr. Opin. Chem. Biol. 2020, 59, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Lupas, A.N. The VCBS superfamily forms a third supercluster of beta-propellers that includes tachylectin and integrins. Bioinformatics 2021, 36, 5618–5622. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Umezawa, K.; Takeda, K.; Sugimoto, N.; Ishida, T.; Samejima, M.; Ohno, H.; Yoshida, M.; Igarashi, K.; Nakamura, N. Discovery of a eukaryotic pyrroloquinoline quinone-dependent oxidoreductase belonging to a new auxiliary activity family in the database of carbohydrate-active enzymes. PLoS ONE 2014, 9, e104851. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Ishida, T.; Yoshida, M.; Samejima, M.; Ohno, H.; Igarashi, K.; Nakamura, N. Crystal structure of the catalytic and cytochrome b domains in a eukaryotic pyrroloquinoline quinone-dependent dehydrogenase. Appl. Environ. Microbiol. 2019, 85, e01692–19. [Google Scholar] [CrossRef]
- Takeda, K.; Matsumura, H.; Ishida, T.; Samejima, M.; Ohno, H.; Yoshida, M.; Igarashi, K.; Nakamura, N. Characterization of a novel pqq-dependent quinohemoprotein pyranose dehydrogenase from Coprinopsis cinerea Classified into auxiliary activities family 12 in carbohydrate-active enzymes. PLoS ONE 2015, 10, e0115722. [Google Scholar] [CrossRef]
- Takeda, K.; Umezawa, K.; Várnai, A.; Eijsink, V.G.; Igarashi, K.; Yoshida, M.; Nakamura, N. Fungal PQQ-dependent dehydrogenases and their potential in biocatalysis. Curr. Opin. Chem. Biol. 2019, 49, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ohsugi, M.; Noda, H.; Muro, K.; Ishiba, A.; Kondo, Y.; Nakao, S. Effects of the yeast extract components pyrroloquinoline quinone and aspartic acid on vitamin B12 production in Klebsiella pneumoniae IFO 13541. J. Nutr. Sci. Vitaminol. 1989, 35, 661–665. [Google Scholar] [CrossRef]
- Bharwad, K.; Rajkumar, S. Modulation of PQQ-dependent glucose dehydrogenase (mGDH and sGDH) activity by succinate in phosphate solubilizing plant growth promoting Acinetobacter sp. SK2. 3 Biotech 2020, 10, 5. [Google Scholar] [CrossRef]
- Choi, O.; Kim, J.; Kim, J.G.; Jeong, Y.; Moon, J.S.; Park, C.S.; Hwang, I. Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol. 2008, 146, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Bhowmik, A.; Chakdar, H.; Khan, M.A.; Selvaraj, C.; Singh, S.K.; Murugan, K.; Kumar, S.; Saxena, A.K. Understanding the biological role of PqqB in Pseudomonas stutzeri using molecular dynamics simulation approach. J. Biomol. Struct. Dyn. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.M.; Boiardi, J.L.; Luna, M.F. Mineral phosphate solubilization activity of Gluconacetobacter diazotrophicus under P-limitation and plant root environment. Agric. Sci. 2011, 2, 16–22. [Google Scholar] [CrossRef][Green Version]
- Eotieno, N.; Lally, R.D.; Ekiwanuka, S.; Elloyd, A.; Eryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef]
- You, M.; Fang, S.; MacDonald, J.; Xu, J.; Yuan, Z.-C. Isolation and characterization of Burkholderia cenocepacia CR318, a phosphate solubilizing bacterium promoting corn growth. Microbiol. Res. 2019, 233, 126395. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Sohail, Y.; Khalid, N.; Ahmed, I.; Mumtaz, A.S. Evaluation of glucose dehydrogenase and pyrroloquinoline quinine (pqq) mutagenesis that renders functional inadequacies in host plants. J. Microbiol. Biotechnol. 2015, 25, 1349–1360. [Google Scholar] [CrossRef]
- Shrivastava, M.; Rajpurohit, Y.S.; Misra, H.S.; D’Souza, S.F. Survival of phosphate-solubilizing bacteria against DNA damaging agents. Can. J. Microbiol. 2010, 56, 822–830. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Jiao, Z.; Hale, L.; Wu, W.; Guo, Y. Disruption of gene pqqA or pqqb reduces plant growth promotion activity and biocontrol of crown gall disease by Rahnella aquatilis HX2. PLoS ONE 2014, 9, e115010. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Twyman, R.M.; Vilcinskas, A. Insects as models to study the epigenetic basis of disease. Prog. Biophys. Mol. Biol. 2015, 118, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Basil, A.H.; Hang, L.; Tan, R.; Goh, K.L.; O’Neill, S.; Zhang, X.; Yu, F.; Lim, K.L. Genetic or pharmacological activation of the Drosophila PGC-1alpha ortholog spargel rescues the disease phenotypes of genetic models of Parkinson’s disease. Neurobiol. Aging 2017, 55, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Puschmann, A. Monogenic Parkinson’s disease and parkinsonism: Clinical phenotypes and frequencies of known mutations. Parkinsonism Relat. Disord. 2013, 19, 407–415. [Google Scholar] [CrossRef]
- Shin, S.C.; Kim, S.-H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.-A.; Yoon, J.-H.; Ryu, J.-H.; Lee, W.-J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef]
- Wu, J.; Huang, J.; Khanabdali, R.; Kalionis, B.; Xia, S.; Cai, W. Pyrroloquinoline quinone enhances the resistance to oxidative stress and extends lifespan upon DAF-16 and SKN-1 activities in C. elegans. Exp. Gerontol. 2016, 80, 43–50. [Google Scholar] [CrossRef]
- Sasakura, H.; Moribe, H.; Nakano, M.; Ikemoto, K.; Takeuchi, K.; Mori, I. Lifespan extension by peroxidase/dual oxidase-mediated ROS signaling through pyrroloquinoline quinone in C. elegans. J. Cell Sci. 2017, 130, 2631–2643. [Google Scholar] [CrossRef] [PubMed]
- Smidt, C.R.; Unkefer, C.J.; Houck, D.R.; Rucker, R.B. Intestinal absorption and tissue distribution of [14C]pyrroloquinoline quinone in mice. Exp. Biol. Med. 1991, 197, 27–31. [Google Scholar] [CrossRef]
- Smidt, C.R.; Bean-Knudsen, D.; Kirsch, D.G.; Rucker, R.B. Does the intestinal microflora synthesize pyrroloquinoline quinone? BioFactors 1991, 3, 53–59. [Google Scholar]
- Steinberg, F.; Stites, T.E.; Anderson, P.; Storms, D.; Chan, I.; Eghbali, S.; Rucker, R. pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp. Biol. Med. 2003, 228, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, F.M.; Gershwin, M.E.; Rucker, R.B. Dietary pyrroloquinoline quinone: Growth and immune response in BALB/c mice. J. Nutr. 1994, 124, 744–753. [Google Scholar] [CrossRef]
- Rucker, R.; Killgore, J.; Duich, L.; Romero-Chapman, N.; Smidt, C.; Tinker, D. Nutritional essentiality of pyrroloquinoline quinone. In PQQ and Quinoproteins: Proceedings of the First International Symposium on PQQ and Quinoprotein, Delft, The Netherlands; Jongejan, J.A., Duine, J.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 159–161. [Google Scholar]
- Yin, X.; Ming, D.; Bai, L.; Wu, F.; Liu, H.; Chen, Y.; Sun, L.; Wan, Y.; Thacker, P.A.; Wu, G.; et al. Effects of pyrroloquinoline quinone supplementation on growth performance and small intestine characteristics in weaned pigs. J. Anim. Sci. 2019, 97, 246–256. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, B.; Zhang, H.; Yang, W.; Meng, Q.; Shi, B.; Shan, A. Effect of dietary pyrroloquinoline quinone disodium in sows on intestinal health of the offspring. Food Funct. 2020, 11, 7804–7816. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Cao, C.; Zhang, B.; Yang, W.; Shi, B.; Shan, A. Pyrroloquinoline quinone inhibits the production of inflammatory cytokines via the SIRTl/NF-κΒ signal pathway in weaned piglet jejunum. Food Funct. 2020, 11, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Samuel, K.; Zhang, H.; Wang, J.; Wu, S.; Yue, H.; Sun, L.; Qi, G. Effects of dietary pyrroloquinoline quinone disodium on growth performance, carcass yield and antioxidant status of broiler chicks. Animal 2015, 9, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, J.; Zhou, H.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide. Poult. Sci. 2020, 99, 5389–5398. [Google Scholar] [CrossRef]
- Wideman, R.; Bowen, O.; Erf, G. Broiler pulmonary hypertensive responses during lipopolysaccharide-induced tolerance and cyclooxygenase inhibition. Poult. Sci. 2009, 88, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Zhang, S.; Mi, Y.; Zhang, C. Attenuating effect of melatonin on lipopolysaccharide-induced chicken small intestine inflammation. Poult. Sci. 2018, 97, 2295–2302. [Google Scholar] [CrossRef]
- Harris, C.B.; Chowanadisai, W.; Mishchuk, D.O.; Satre, M.A.; Slupsky, C.M.; Rucker, R.B. Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. J. Nutr. Biochem. 2013, 24, 2076–2084. [Google Scholar] [CrossRef]
- Hwang, P.S.; Machek, S.B.; Cardaci, T.D.; Wilburn, D.; Kim, C.S.; Suezaki, E.S.; Willoughby, D.S. Effects of pyrroloquinoline quinone (PQQ) supplementation on aerobic exercise performance and indices of mitochondrial biogenesis in untrained men. J. Am. Coll. Nutr. 2019, 39, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; He, Y.; Zhang, K.; Yang, X.; Hao, D.; Jiang, Y.; He, B. Mini-review: Functions and action mechanisms of PQQ in osteoporosis and neuro injury. Curr. Stem Cell Res. Ther. 2020, 15, 32–36. [Google Scholar] [CrossRef]
- Shiojima, Y.; Takahashi, M.; Takahashi, R.; Moriyama, H.; Bagchi, D.; Bagchi, M.; Akanuma, M. Effect of dietary pyrroloquinoline quinone disodium salt on cognitive function in healthy volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. J. Am. Coll. Nutr. 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Nakano, M.; Murayama, Y.; Hu, L.; Ikemoto, K.; Uetake, T.; Sakatani, K. Effects of antioxidant supplements (BioPQQ™) on cerebral blood flow and oxygen metabolism in the prefrontal cortex. Adv. Exp. Med. Biol. 2016, 923, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Jones, A.; Mercer, R.; Rucker, R. Characterization of pyrroloquinoline quinone amino acid derivatives by electrospray ionization mass spectrometry and detection in human milk. Anal. Biochem. 1999, 269, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.; Kawai, E.; Shimizu, N.; Mikekado, T.; Kimura, F.; Miyazawa, T.; Nakagawa, K. Determination of pyrroloquinoline quinone by enzymatic and LC-MS/MS methods to clarify its levels in foods. PLoS ONE 2018, 13, e0209700. [Google Scholar] [CrossRef]
- Stites, T.E.; Sih, T.R.; Rucker, R.B. Synthesis of [14C]pyrroloquinoline quinone (PQQ) in E. coli using genes for PQQ synthesis from K. pneumoniae. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2000, 1524, 247–252. [Google Scholar] [CrossRef]
- Misset-Smits, M.; Oltshoorn, A.; Dewanti, A.; Duine, J. [11] Production, assay, and occurrence of pyrroloquinoline quinone. Methods Enzymol. 1997, 280, 89–98. [Google Scholar] [CrossRef]
- Stites, T.; Storms, D.; Bauerly, K.; Mah, J.; Harris, C.; Fascetti, A.; Rogers, Q.; Tchaparian, E.; Satre, M.; Rucker, R.B. Pyrroloquinoline quinone modulates mitochondrial quantity and function in mice. J. Nutr. 2006, 136, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef]
- Flückiger, R.; Apaz, M.; Gallop, P.M. [11] Redox-cycling detection of dialyzable pyrroloquinoline quinone and quinoproteins. Methods Enzymol. 1995, 258, 140–149. [Google Scholar] [CrossRef]
- Fluckiger, R.; Paz, M.; Mah, J.; Bishop, A.; Gallop, P. Characterization of the glycine-dependent redox-cycling activity in animal fluids and tissues using specific inhibitors and activators: Evidence for presence of PQQ. Biochem. Biophys. Res. Commun. 1993, 196, 61–68. [Google Scholar] [CrossRef]
- Rucker, R.; Chowanadisai, W.; Nakano, M. Potential physiological importance of pyrroloquinoline quinone. Altern. Med. Rev. J. Clin. Ther. 2009, 14, 268–277. [Google Scholar]
- Naito, Y.; Kumazawa, T.; Kino, I.; Suzuki, O. Effects of pyrroloquinoline quinone (PQQ) and PQQ-oxazole on DNA synthesis of cultured human fibroblasts. Life Sci. 1993, 52, 1909–1915. [Google Scholar] [CrossRef]
- Yamada, Y.; Nishii, K.; Kuwata, K.; Nakamichi, M.; Nakanishi, K.; Sugimoto, A.; Ikemoto, K. Effects of pyrroloquinoline quinone and imidazole pyrroloquinoline on biological activities and neural functions. Heliyon 2020, 6, e03240. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Yasuyama, T.; Higuchi, K.; Watanabe, A.; Urakami, T.; Akaike, T.; Sato, K.; Maeda, H. The protective effect of pyrroloquinoline quinone and its derivatives against carbon tetrachloride-induced liver injury of rats. J. Gastroenterol. Hepatol. 1993, 8, 342–347. [Google Scholar] [CrossRef]
- GRAS Notice for Pyrroloquinoline Quinone (PQQ) Disodium Salt; (GRN) No. 694; Office of Food Additive Safety (FHS-200) Center for Food Safety and Applied Nutrition Food and Drug Administration: Washinton, DC, USA, 2017.
- Liang, C.; Zhang, X.; Wang, W.; Song, Y.; Jia, X. A subchronic oral toxicity study on pyrroloquinoline quinone (PQQ) disodium salt in rats. Food Chem. Toxicol. 2015, 75, 146–150. [Google Scholar] [CrossRef]
- Nakano, M.; Suzuki, H.; Imamura, T.; Lau, A.; Lynch, B. Genotoxicity of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™). Regul. Toxicol. Pharmacol. 2013, 67, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Takahashi, H.; Koura, S.; Chung, C.; Tafazoli, S.; Roberts, A. Acute and subchronic toxicity studies of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™) in rats. Regul. Toxicol. Pharmacol. 2014, 70, 107–121. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.; O’Callaghan, C.A.; Lasserson, D.; Hobbs, R. Global prevalence of chronic kidney—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Pendse, S.; Singh, A.K. Complications of chronic kidney disease: Anemia, mineral metabolism, and cardiovascular disease. Med. Clin. N. Am. 2005, 89, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Crouzet, C.; Lau, W.L.; Cribbs, D.H.; Fisher, M.J. Cerebral blood flow in chronic kidney disease. J. Stroke Cerebrovasc. Dis. 2021, 30, 105702. [Google Scholar] [CrossRef]
- Koren, M.J.; Blumen, H.M.; Ayers, E.I.; Verghese, J.; Abramowitz, M.K. Cognitive dysfunction and gait abnormalities in CKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 694–704. [Google Scholar] [CrossRef]
- Lee, Y.J.; Yoon, E.; Park, S.; Kim, Y.W.; Kim, S.E.; Ko, J.; Park, J.H.; Park, K.M.; Kim, I.H.; Park, B.S. Alteration of brain connectivity in neurologically asymptomatic patients with chronic kidney disease. Medicine 2021, 100, e25633. [Google Scholar] [CrossRef]
- Miller, L.M.; Rifkin, D.; Lee, A.K.; Tamura, M.K.; Pajewski, N.M.; Weiner, D.E.; Al-Rousan, T.; Shlipak, M.; Ix, J.H. Association of urine biomarkers of kidney tubule injury and dysfunction with frailty index and cognitive function in persons with CKD in SPRINT. Am. J. Kidney Dis. 2021, 78, 530–540. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive oxygen species and redox signaling in chronic kidney disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Renal. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am. J. Nephrol. 2017, 45, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, P.; Qiao, Y.; Jiang, C.; Ge, Y.; Flickinger, B.; Malhotra, D.K.; Dworkin, L.D.; Liu, Z.; Gong, R. GSK3β-mediated Keap1-independent regulation of Nrf2 antioxidant response: A molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 2019, 26, 101275. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Kundu, J.K.; Na, H.-K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Medica 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, K.; Kanda, H.; Shimazaki, R. Nrf2 activator for the treatment of kidney diseases. Toxicol. Appl. Pharmacol. 2018, 360, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-H.; Kang, K.-S.; Kwak, M.-K. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules 2014, 19, 12727–12759. [Google Scholar] [CrossRef]
- Lin, X.; Yang, F.; Huang, J.; Jiang, S.; Tang, Y.; Li, J. Ameliorate effect of pyrroloquinoline quinone against cyclophosphamide-induced nephrotoxicity by activating the Nrf2 pathway and inhibiting the NLRP3 pathway. Life Sci. 2020, 256, 117901. [Google Scholar] [CrossRef]
- Wang, Z.; Han, N.; Zhao, K.; Li, Y.; Chi, Y.; Wang, B. Protective effects of pyrroloquinoline quinone against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway. Int. Immunopharmacol. 2019, 72, 445–453. [Google Scholar] [CrossRef]
- Friedman, J.E.; Dobrinskikh, E.; Alfonso-Garcia, A.; Fast, A.; Janssen, R.C.; Soderborg, T.K.; Anderson, A.L.; Reisz, J.A.; D’Alessandro, A.; Frank, D.N.; et al. Pyrroloquinoline quinone prevents developmental programming of microbial dysbiosis and macrophage polarization to attenuate liver fibrosis in offspring of obese mice. Hepatol. Commun. 2018, 2, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Devasani, K.; Kaul, R.; Majumdar, A. Supplementation of pyrroloquinoline quinone with atorvastatin augments mitochondrial biogenesis and attenuates low grade inflammation in obese rats. Eur. J. Pharmacol. 2020, 881, 173273. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Chaudhari, A.; Kumar, G.N. Amelioration of cadmium- and mercury-induced liver and kidney damage in rats by genetically engineered probiotic Escherichia coli Nissle 1917 producing pyrroloquinoline quinone with oral supplementation of citric acid. Nutrition 2016, 32, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Triantos, C.; Maroulis, I.; Gogos, C. The role of the gut barrier function in health and disease. Gastroenterol. Res. 2018, 11, 261–263. [Google Scholar] [CrossRef]
- Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired intestinal barrier and tissue bacteria: Pathomechanisms for metabolic diseases. Front. Endocrinol. 2021, 12, 616506. [Google Scholar] [CrossRef] [PubMed]
- Pat, Y.; Ogulur, I. The epithelial barrier hypothesis: A 20-year journey. Allergy 2021. [Google Scholar] [CrossRef] [PubMed]
- Aldars-García, L.; Chaparro, M.; Gisbert, J. Systematic review: The gut microbiome and its potential clinical application in inflammatory bowel disease. Microorganisms 2021, 9, 977. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Cadwell, K.H.; Colombel, J.-F.; Shah, S.C. The role of gastrointestinal pathogens in inflammatory bowel disease: A systematic review. Ther. Adv. Gastroenterol. 2021, 14, 17562848211004493. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High fat diet, gut microbiome and gastrointestinal cancer. Theranostics 2021, 11, 5889–5910. [Google Scholar] [CrossRef] [PubMed]
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Snyder, M.P.; Segal, E.; Elinav, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Picón, M.; Laguna, A. New avenues for Parkinson’s Disease therapeutics: Disease-modifying strategies based on the gut microbiota. Biomolecules 2021, 11, 433. [Google Scholar] [CrossRef]
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.-H. Disorders of the enteric nervous system—A holistic view. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 393–410. [Google Scholar] [CrossRef]
- Ojeda, J.; Ávila, A.; Vidal, P. Gut Microbiota interaction with the central nervous system throughout life. J. Clin. Med. 2021, 10, 1299. [Google Scholar] [CrossRef]
- Snethlage, C.M.F.; Nieuwdorp, M.; van Raalte, D.H.; Rampanelli, E.; Verchere, B.C.; Hanssen, N.M. Auto-immunity and the gut microbiome in type 1 diabetes: Lessons from rodent and human studies. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101544. [Google Scholar] [CrossRef]
- Takewaki, D.; Yamamura, T. Gut microbiome research in multiple sclerosis. Neurosci. Res. 2021, 168, 28–31. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 1–13. [Google Scholar] [CrossRef]
- Li, R.; Meng, X.; Chen, B.; Zhao, L.; Zhang, X. Gut Microbiota in Lupus: A Butterfly Effect? Curr. Rheumatol. Rep. 2021, 23, 27. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Borro, M.; Gangemi, S. Hygiene hypothesis and autoimmune diseases: A narrative review of clinical evidences and mechanisms. Autoimmun. Rev. 2021, 20, 102845. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the skin and gut microbiome in psoriasis and psoriatic arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Sobarzo-Sanchez, E.; Bungau, S. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. 2021, 273, 119311. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Role of gut microbiota and their metabolites on atherosclerosis, hypertension and human blood platelet function: A review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef]
- He, L. Alterations of gut microbiota by overnutrition impact gluconeogenic gene expression and insulin signaling. Int. J. Mol. Sci. 2021, 22, 2121. [Google Scholar] [CrossRef]
- Liébana-García, R.; Olivares, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Romaní-Pérez, M.; Sanz, Y. The gut microbiota as a versatile immunomodulator in obesity and associated metabolic disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101542. [Google Scholar] [CrossRef]
- Massey, W.; Brown, J.M. The gut microbial endocrine organ in type 2 diabetes. Endocrinology 2021, 162, 235. [Google Scholar] [CrossRef]
- Petraroli, M.; Castellone, E.; Patianna, V.; Esposito, S. Gut microbiota and obesity in adults and children: The state of the art. Front. Pediatr. 2021, 9, 657020. [Google Scholar] [CrossRef]
- Régnier, M.; Van Hul, M.; Knauf, C.; Cani, P.D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. 2021, 248, R67–R82. [Google Scholar] [CrossRef]
- Rovella, V.; Rodia, G.; Di Daniele, F.; Cardillo, C.; Campia, U.; Noce, A.; Candi, E.; Della-Morte, D.; Tesauro, M. Association of gut hormones and microbiota with vascular dysfunction in obesity. Nutrients 2021, 13, 613. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Van Son, J.; Koekkoek, L.; La Fleur, S.; Serlie, M.; Nieuwdorp, M. The role of the gut microbiota in the gut–brain axis in obesity: Mechanisms and future implications. Int. J. Mol. Sci. 2021, 22, 2993. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, W.; Zhang, H.; He, S.; Meng, Q.; Chen, Z.; Shan, A. Effect of pyrroloquinoline quinone disodium in female rats during gestating and lactating on reproductive performance and the intestinal barrier functions in the progeny. Br. J. Nutr. 2019, 121, 818–830. [Google Scholar] [CrossRef]
- Huang, C.; Ming, D.; Wang, W.; Wang, Z.; Hu, Y.; Ma, X.; Wang, F. Pyrroloquinoline quinone alleviates jejunal mucosal barrier function damage and regulates colonic microbiota in piglets challenged with enterotoxigenic Escherichia coli. Front. Microbiol. 2020, 11, 1754. [Google Scholar] [CrossRef]
- Gasaly, N.; Hermoso, M.; Gotteland, M. Butyrate and the fine-tuning of colonic homeostasis: Implication for inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Huang, X.; Oshima, T.; Tomita, T.; Fukui, H.; Miwa, H. Butyrate alleviates cytokine-induced barrier dysfunction by modifying claudin-2 levels. Biology 2021, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pandey, S.K.; Kumar, G.N. Pyrroloquinoline quinone-secreting probiotic Escherichia coli Nissle 1917 ameliorates ethanol-induced oxidative damage and hyperlipidemia in rats. Alcohol. Clin. Exp. Res. 2014, 38, 2127–2137. [Google Scholar] [CrossRef]
- Yamada, M.; Elias, M.D.; Matsushita, K.; Migita, C.T.; Adachi, O. Escherichia coli PQQ-containing quinoprotein glucose dehydrogenase: Its structure comparison with other quinoproteins. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2003, 1647, 185–192. [Google Scholar] [CrossRef]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–28. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Q.; Sun, H.; Qiao, Y. Inhibition of histone deacetylase by butyrate protects rat liver from chemic reperfusion injury. Int. J. Mol. Sci. 2014, 15, 21069–21079. [Google Scholar] [CrossRef] [PubMed]
- Jonscher, K.R.; Stewart, M.S.; Alfonso-Garcia, A.; De Felice, B.C.; Wang, X.X.; Luo, Y.; Levi, M.; Heerwagen, M.J.R.; Janssen, R.C.; de la Houssaye, B.A.; et al. Early PQQ supplementation has persistent long-term protective effects on developmental programming of hepatic lipotoxicity and inflammation in obese mice. FASEB J. 2017, 31, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Guirro, M.; Costa, A.; Gual-Grau, A.; Herrero, P.; Torrell, H.; Canela, N.; Arola, L. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: A multiomics approach. PLoS ONE 2019, 14, e0218143. [Google Scholar] [CrossRef]
- Bauerly, K.; Harris, C.; Chowanadisai, W.; Graham, J.; Havel, P.J.; Tchaparian, E.; Satre, M.; Karliner, J.S.; Rucker, R.B. Altering pyrroloquinoline quinone nutritional status modulates mitochondrial, lipid, and energy metabolism in rats. PLoS ONE 2011, 6, e21779. [Google Scholar] [CrossRef]
- Tao, R.; Karliner, J.S.; Simonis, U.; Zheng, J.; Zhang, J.; Honbo, N.; Alano, C.C. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem. Biophys. Res. Commun. 2007, 363, 257–262. [Google Scholar] [CrossRef][Green Version]
- Zhu, B.Q.; Simonis, U.; Cecchini, G.; Zhou, H.Z.; Li, L.; Teerlink, J.R.; Karliner, J.S. Comparison of pyrroloquinoline quinone and/or metoprolol on myocardial infarct size and mitochondrial damage in a rat model of ischemialreperfusion injury. J. Cardiovasc. Pharmacol. Ther. 2006, 11, 119–128. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Zhou, H.Z.; Teerlink, J.R.; Karliner, J.S. Pyrroloquinoline quinone (PQQ) decreases myocardial infarct size and improves cardiac function in rat models of ischemia and ischemial reperfusion. Cardiovasc. Drugs Ther. 2004, 18, 421–431. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Liu, T.; Ke, C.; Huang, J.; Fu, Y.; Lin, Z.; Chen, F.; Wu, X.; Chen, Q. Pyrroloquinoline quinone protects against exercise-induced fatigue and oxidative damage via improving mitochondrial function in mice. FASEB J. 2021, 35, e21394. [Google Scholar] [CrossRef]
- Chrétien, D.; Bénit, P.; Ha, H.H.; Keipert, S.; El-Khoury, R.; Chang, Y.T.; Jastroch, M.; Jacobs, H.T.; Rustin, P.; Rak, M. Mitochondria are physiologically maintained at close to 50 degrees C. PLoS Biol. 2018, 16, e2003992. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, A.; Rüb, C.; Sylvester, M.; Voos, W. Analysis of heat-induced protein aggregation in human mitochondria. J. Biol. Chem. 2018, 293, 11537–11552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, J.; Shen, M.; Xu, H.; Yu, S.; Cheng, Q.; Ding, F. Pyrroloquinoline quinone inhibits rotenone-induced microglia inflammation by enhancing autophagy. Molecules 2020, 25, 4359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.I.; Chen, S.; Yu, S.; Qin, J.; Zhang, J.; Cheng, Q.; Ke, K.; Ding, F. Neuroprotective effects of pyrroloquinoline quinone against rotenone injury in primary cultured midbrain neurons and in a rat model of Parkinson’s disease. Neuropharmacology 2016, 108, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, M.; Yu, S.; Gao, X.; Zhang, J.; Dong, X.; Ji, J.; Zhang, Y.; Zhou, L.; Zhang, Q.; et al. Pyrroloquinoline quinone-conferred neuroprotection in rotenone models of Parkinson’s disease. Toxicol. Lett. 2015, 238, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, S.; Shen, M.; He, Q.; Zhang, Y.; Shi, Y.; Ding, F.; Zhang, Q. Mitochondrial regulation by pyrroloquinoline quinone prevents rotenone-induced neurotoxicity in Parkinson’s disease models. Neurosci. Lett. 2018, 687, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Bauerly, K.A.; Tchaparian, E.; Wong, A.; Cortopassi, G.A.; Rucker, R.B. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1α expression. J. Biol. Chem. 2010, 285, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Chen, J.; Guo, H.; Lu, J.-L.; Zhou, J.; Guo, X.-Y.; Shi, Y.; Zhang, Y.; Yu, S.; Zhang, Q.; et al. Pyrroloquinoline quinone promotes mitochondrial biogenesis in rotenone-induced Parkinson’s disease model via AMPK activation. Acta Pharmacol. Sin. 2021, 42, 665–678. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Kim, J.; Harada, R.; Kobayashi, M.; Kobayashi, N.; Sode, K. The inhibitory effect of pyrroloquinoline quinone on the amyloid formation and cytotoxicity of truncated alpha-synuclein. Mol. Neurodegener. 2010, 5, 20. [Google Scholar] [CrossRef]
- Jensen, F.E.; Gardner, G.J.; Williams, A.P.; Gallop, P.M.; Aizenman, E.; Rosenberg, P.A. The putative essential nutrient pyrroloquinoline quinone is neuroprotective in a rodent model of hypoxiclischemic brain injury. Neuroscience 1994, 62, 399–406. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Cheng, C.; Yuan, Y.; Yu, B.; Shen, A.; Yan, M. The neuroprotective effect of pyrroloquinoline quinone on traumatic brain injury. J. Neurotrauma 2012, 29, 851–864. [Google Scholar] [CrossRef]
- Zhang, Y.; Feustel, P.; Kimelberg, H.K. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 2006, 1094, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ralto, K.M.; Rhee, E.P.; Parikh, S.M. NAD+ homeostasis in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 99–111. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).