Insight into the Lubrication and Adhesion Properties of Hyaluronan for Ocular Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Sodium Dodecanoyl Hyaluronate (HA-C12)
2.3. Structural Characterization
2.4. Size Exclusion Chromatography–Multiangle Laser Light Scattering (SEC-MALLS)
2.5. Nuclear Magnetic Resonance Spectroscopy
2.6. Tribological Setup
2.7. Mucoadhesive Index
2.8. Evaluation of Protective Efficacies of Different Lubricants on HaCaT Cells against Dehydration
3. Results and Discussion
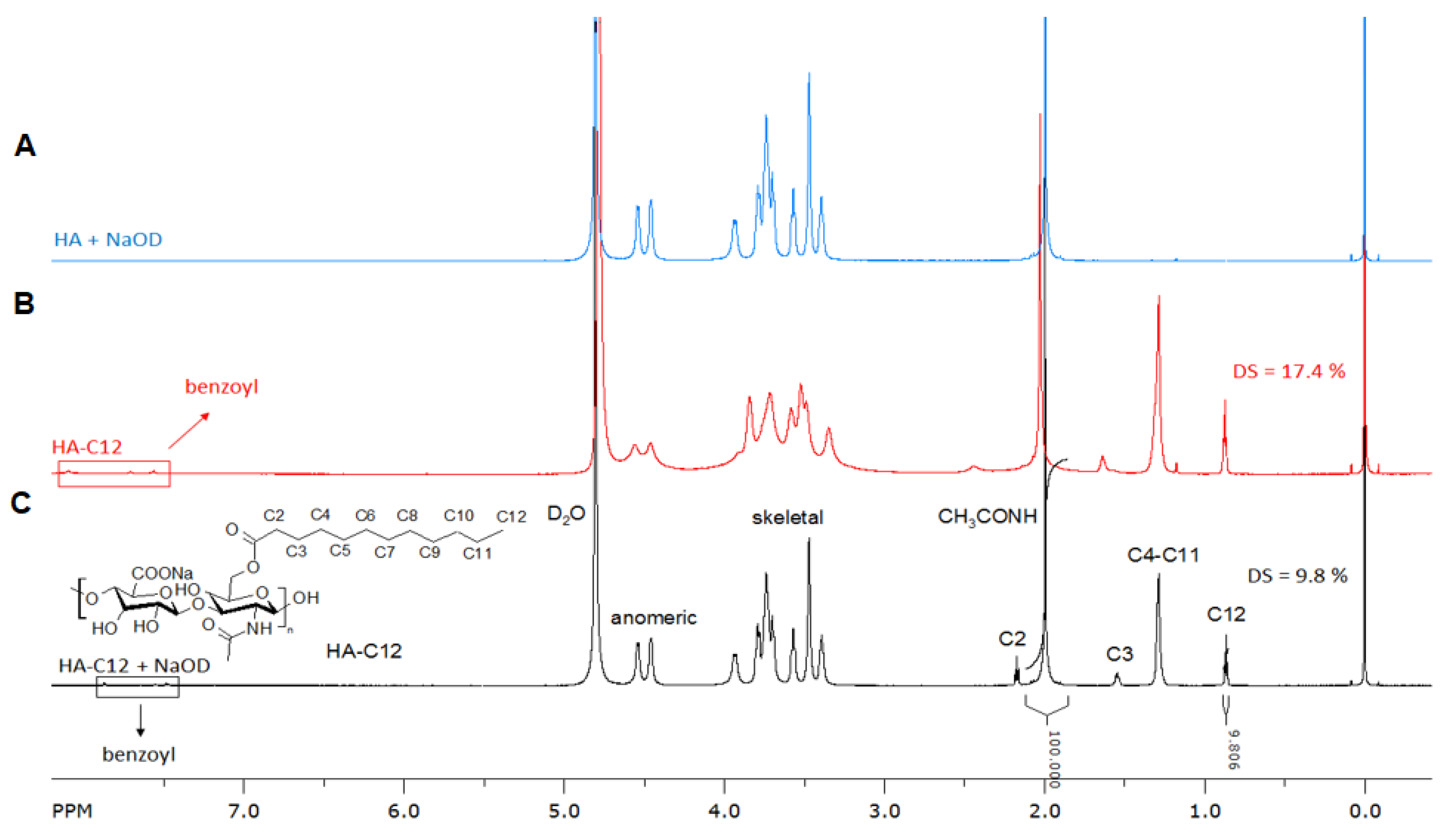
3.1. Synthesis and Characterization of Amphiphilic Hyaluronan (HA-C12)
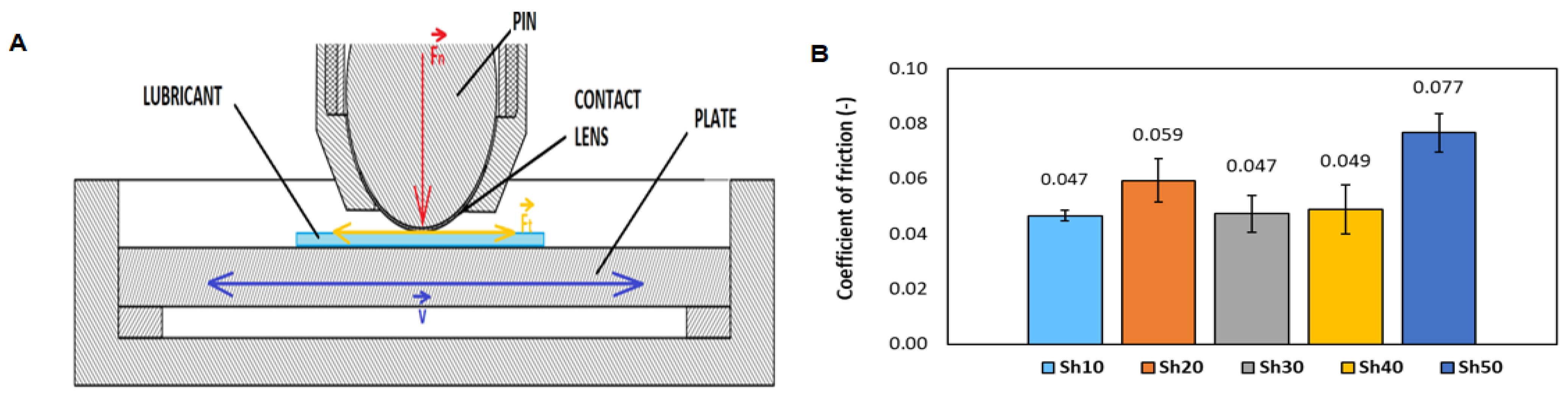
3.2. Tribological Model of the Eye
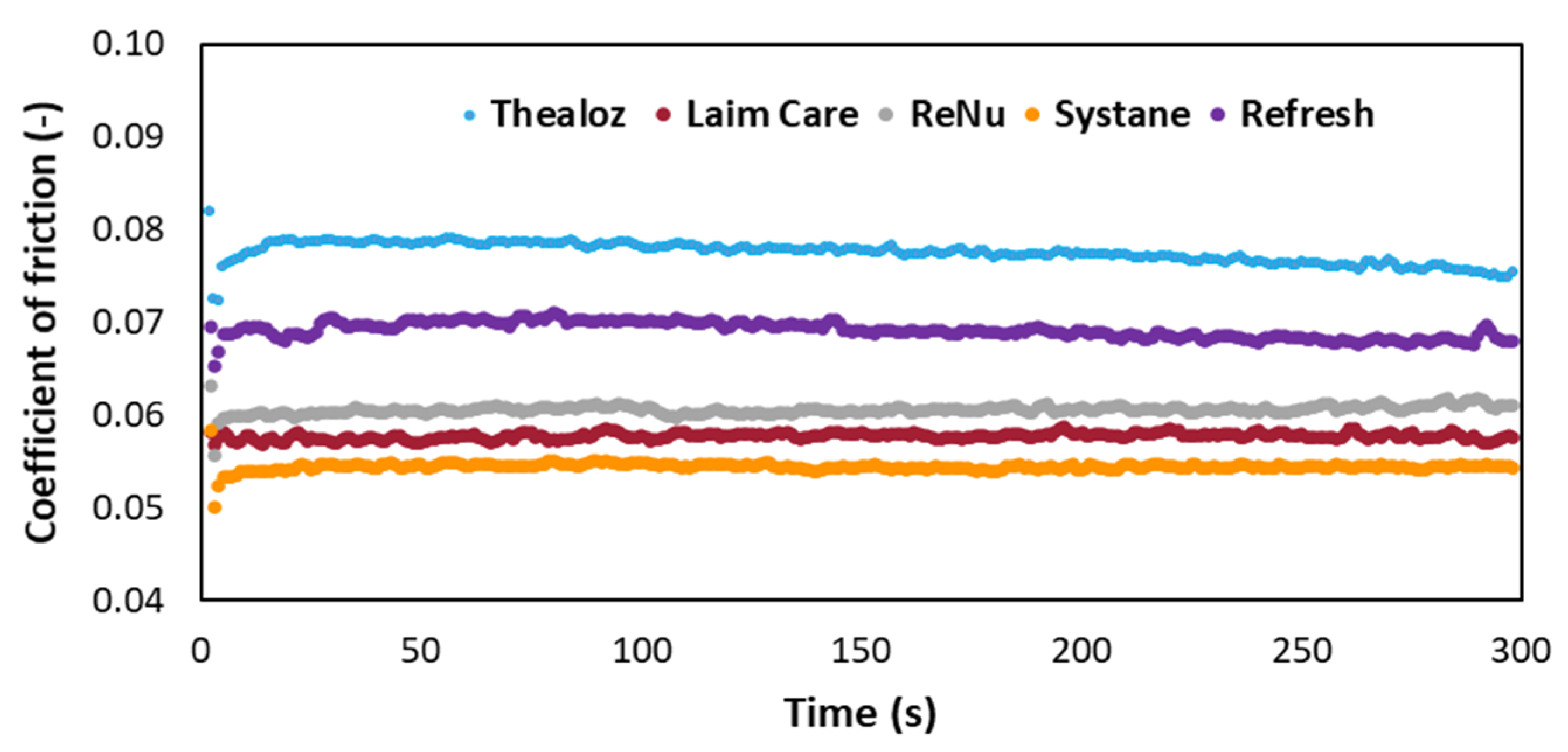
3.3. Determination of the Coefficient of Friction for Commercially Available Eye Drops
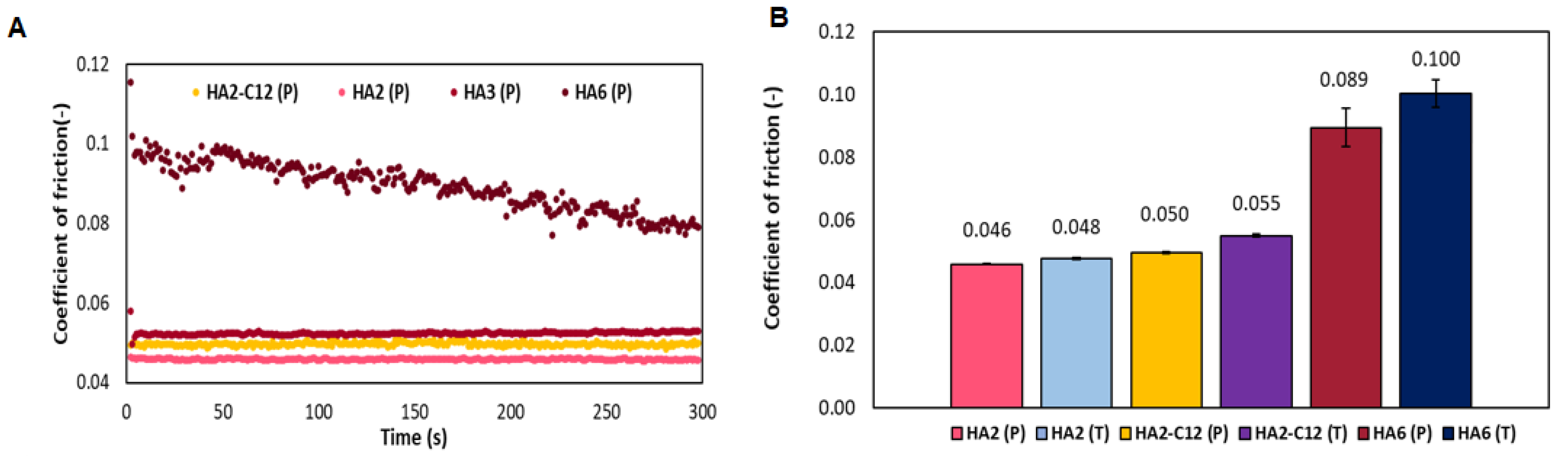
3.4. Determination of the Coefficient of Friction for Native HA
3.5. MucoadhesionProperties of HA Determined by Turbidimetric Titration
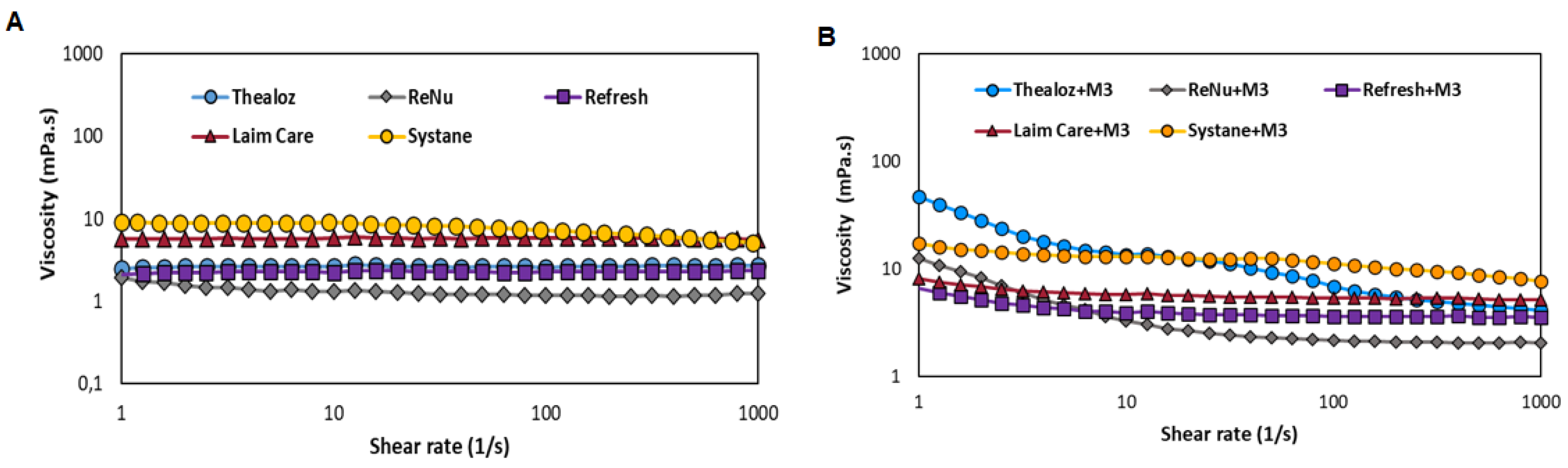
3.6. Mucoadhesive Properties Determined by Rheology
3.7. Sterilisation by Filtration
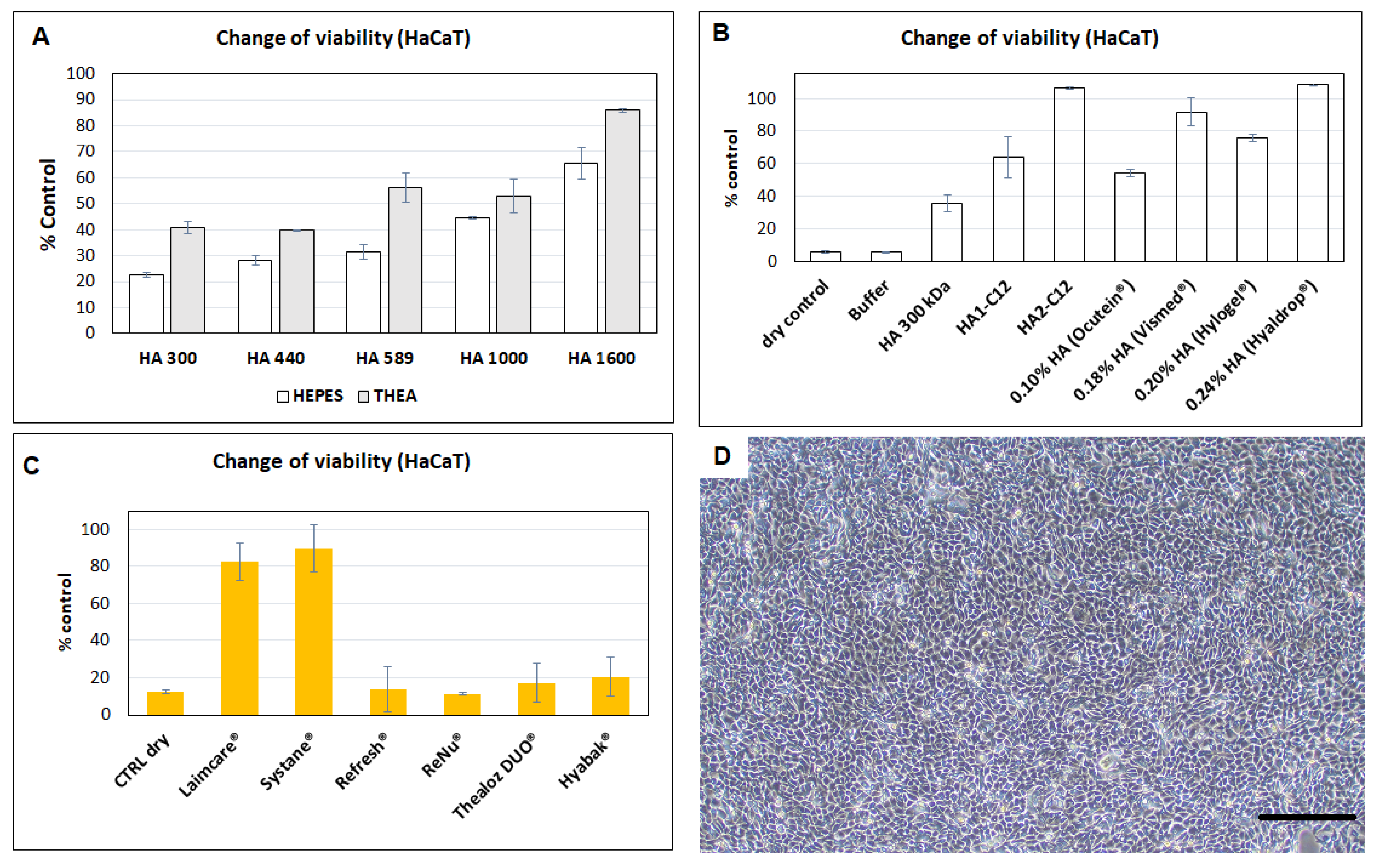
3.8. Evaluation of Protective Efficacies of HA and Derivatives Tested on HaCaT Cells against Dehydration
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Kolanu, S. Contact lens wear and dry eyes: Challenges and solutions. Clin. Optom. 2017, 9, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, A.; Shamloo, K.; Jhanji, V.; Sharma, A. Categorization of Marketed Artificial Tear Formulations Based on Their Ingredients: A Rational Approach for Their Use. J. Clin. Med. 2021, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lierheim, W.G.K. Why Chain Length of Hyaluronan in Eye Drops Matters. Diagnostics 2020, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Ángeles, G.; Nešporová, K. Hyaluronan and its derivatives for ophthalmology: Recent advances and future perspectives. Carbohydr. Polym. 2021, 259, 117697. [Google Scholar] [CrossRef]
- Aragona, P.; Simmons, P.A.; Wang, H.; Wang, T. Physicochemical Properties of Hyaluronic Acid-Based Lubricant Eye Drops. Transl. Vis. Sci. Technol. 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, S.; Sullivan, D.A.; Sullivan, B.D.; Sheardown, H.; Schmidt, T.A. Dose-Dependent and Synergistic Effects of Proteoglycan 4 on Boundary Lubrication at a Human Cornea–Polydimethylsiloxane Biointerface. Eye Contact Lens Sci. Clin. Pract. 2012, 38, 27–35. [Google Scholar] [CrossRef]
- Samsom, M.; Iwabuchi, Y.; Sheardown, H.; Schmidt, T.A. Proteoglycan 4 and hyaluronan as boundary lubricants for model contact lens hydrogels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1329–1338. [Google Scholar] [CrossRef]
- Sterner, O.; Karageorgaki, C.; Zürcher, M.; Zürcher, S.; Scales, C.W.; Fadli, Z.; Spencer, N.D.; Tosatti, S.G.P. Reducing Friction in the Eye: A Comparative Study of Lubrication by Surface-Anchored Synthetic and Natural Ocular Mucin Analogues. ACS Appl. Mater. Interfaces 2017, 9, 20150–20160. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, K.; Drolle, E.; Nakagawa, H.; Hisamura, R.; Ngo, W.; Jones, L. Impact of a low molecular weight hyaluronic acid derivative on contact lens wettability. Contact Lens Anterior Eye 2021, 44, 101334. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, W.; Fan, Y.; Kampf, N.; Wang, Y.; Klein, J. Effects of hyaluronan molecular weight on the lubrication of cartilage-emulating boundary-layers. Biomacromolecules 2020, 21, 4345–4354. [Google Scholar] [CrossRef]
- Marian, M.; Shah, R.; Gashi, B.; Zhang, S.; Bhavnani, K.; Wartzack, S.; Rosenkranz, A. Exploring the Lubrication Mechanisms of Synovial Fluids for Joint Longevity—A Perspective. Colloids Surf. B Biointerfaces 2021, 206, 111926. [Google Scholar] [CrossRef]
- Sforza, C.; Rango, M.; Galante, D.; Bresolin, N.; Ferrario, V. Spontaneous blinking in healthy persons: An optoelectronic study of eyelid motion. Ophthalmic Physiol. Opt. 2008, 28, 345–353. [Google Scholar] [CrossRef]
- Winkeljann, B.; Boettcher, K.; Balzer, B.N.; Lieleg, O. Mucin Coatings Prevent Tissue Damage at the Cornea-Contact Lens Interface. Adv. Mater. Interfaces 2017, 4, 1700186. [Google Scholar] [CrossRef]
- Kutálková, E.; Hrnčiřík, J.; Witasek, R.; Ingr, M.; Huerta-Ángeles, G.; Hermannová, M.; Velebný, V. The rate and evenness of the substitutions on hyaluronan grafted by dodecanoic acid influenced by the mixed-solvent composition. Int. J. Biol. Macromol. 2021, 189, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Ángeles, G.; Ondreáš, F.; Brandejsová, M.; Kopecká, K.; Vagnerová, H.; Kulhánek, J.; Drmota, T. Formulation of hyaluronan grafted with dodecanoic acid as a potential ophthalmic treatment. Carbohydr. Polym. 2020, 246, 116578. [Google Scholar] [CrossRef] [PubMed]
- Podzimek, S.; Hermannova, M.; Bilerova, H.; Bezakova, Z.; Velebny, V. Solution properties of hyaluronic acid and comparison of SEC-MALS-VIS data with off-line capillary viscometry. J. Appl. Polym. Sci. 2010, 116, 3013–3020. [Google Scholar] [CrossRef]
- Čožíková, D.; Šílová, T.; Moravcová, V.; Šmejkalová, D.; Pepeliaev, S.; Velebný, V.; Hermannová, M. Preparation and extensive characterization of hyaluronan with narrow molecular weight distribution. Carbohydr. Polym. 2017, 160, 134–142. [Google Scholar] [CrossRef]
- Shih, P.-J.; Huang, C.-J.; Huang, T.-H.; Lin, H.-C.; Yen, J.-Y.; Wang, I.-J.; Cao, H.-J.; Shih, W.-P.; Dai, C.-A. Estimation of the Corneal Young’s ModulusIn VivoBased on a Fluid-Filled Spherical-Shell Model with Scheimpflug Imaging. J. Ophthalmol. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pult, H.; Tosatti, S.G.; Spencer, N.D.; Asfour, J.-M.; Ebenhoch, M.; Murphy, P. Spontaneous Blinking from a Tribological Viewpoint. Ocul. Surf. 2015, 13, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Vilhena, L.; Ramalho, A. Study of the frictional behavior of soft contact lenses by an innovative method. Tribol. Int. 2021, 153, 106633. [Google Scholar] [CrossRef]
- Hermans, K.; Plas, D.V.D.; Schreurs, E.; Weyenberg, W.; Ludwig, A. Cytotoxicity and anti-inflammatory activity of cyclosporine A loaded PLGA nanoparticles for ocular use. Die Pharm. 2014, 69, 32–37. [Google Scholar]
- Tømmeraas, K.; Mellergaard, M.; Malle, B.M.; Skagerlind, P. New amphiphilic hyaluronan derivatives based on modification with alkenyl and aryl succinic anhydrides. Carbohydr. Polym. 2011, 85, 173–179. [Google Scholar] [CrossRef]
- Mun, J.; Mok, J.W.; Jeong, S.; Cho, S.; Joo, C.-K.; Hahn, S.K. Drug-eluting contact lens containing cyclosporine-loaded cholesterol-hyaluronate micelles for dry eye syndrome. RSC Adv. 2019, 9, 16578–16585. [Google Scholar] [CrossRef] [Green Version]
- Tram, N.; Swindle-Reilly, K.E. Rheological Properties and Age-Related Changes of the Human Vitreous Humor. Front. Bioeng. Biotechnol. 2018, 6, 199. [Google Scholar] [CrossRef] [Green Version]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact Lens Technology: From Fundamentals to Applications. Adv. Health Mater. 2019, 8, e1900368. [Google Scholar] [CrossRef]
- Nichols, J.J.; Willcox, M.D.P.; Bron, A.J.; Belmonte, C.; Ciolino, J.B.; Craig, J.P.; Dogru, M.; Foulks, G.N.; Jones, L.; Nelson, J.D.; et al. The TFOS International Workshop on Contact Lens Discomfort: Executive Summary. Investig. Opthalmol. Vis. Sci. 2013, 54, TFOS7–TFOS13. [Google Scholar] [CrossRef] [Green Version]
- Ubels, J.; Clousing, D.; Van Haitsma, T.; Hong, B.-S.; Stauffer, P.; Asgharian, B.; Meadows, D. Pre-clinical investigation of the efficacy of an artificial tear solution containing hydroxypropyl-guar as a gelling agent. Curr. Eye Res. 2004, 28, 437–444. [Google Scholar] [CrossRef]
- Doan, S.; Bremond-Gignac, D.; Chiambaretta, F. Comparison of the effect of a hyaluronate–trehalose solution to hyaluronate alone on Ocular Surface Disease Index in patients with moderate to severe dry eye disease. Curr. Med. Res. Opin. 2018, 34, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Chiambaretta, F.; Doan, S.; Labetoulle, M.; Rocher, N.; El Fekih, L.; Messaoud, R.; Khairallah, M.; Baudouin, C.; for the HA-trehalose Study Group. A Randomized, Controlled Study of the Efficacy and Safety of a New Eyedrop Formulation for Moderate to Severe Dry Eye Syndrome. Eur. J. Ophthalmol. 2017, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kern, J.R.; Perry, S.S.; Rex, J.; Rudy, A. Surface friction modification of lotrafilcon B contact lenses by commercial drops. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1464. [Google Scholar]
- Cohen, S.; Martin, A.; Sall, K. Evaluation of clinical outcomes in patients with dry eye disease using lubricant eye drops containing polyethylene glycol or carboxymethylcellulose. Clin. Ophthalmol. 2013, 8, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Albarkah, Y.A.; Green, R.J.; Khutoryanskiy, V.V. Probing the Mucoadhesive Interactions Between Porcine Gastric Mucin and Some Water-Soluble Polymers. Macromol. Biosci. 2015, 15, 1546–1553. [Google Scholar] [CrossRef]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Caramella, C. Characterization of chitosan hydrochloride–mucin interaction by means of viscosimetric and turbidimetric measurements. Eur. J. Pharm. Sci. 2000, 10, 251–257. [Google Scholar] [CrossRef]
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; D’Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of hyaluronan-based eye drop formulations. Carbohydr. Polym. 2016, 153, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef] [PubMed]
- Schömig, V.J.; Käsdorf, B.T.; Scholz, C.; Bidmon, K.; Lieleg, O.; Berensmeier, S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. RSC Adv. 2016, 6, 44932–44943. [Google Scholar] [CrossRef] [Green Version]
- Caretti, L.; Valerio, A.L.G.; Piermarocchi, R.; Badin, G.; Verzola, G.; Masarà, F.; Scalora, T.; Monterosso, C. Efficacy of carbomer sodium hyaluronate trehalose vs hyaluronic acid to improve tear film instability and ocular surface discomfort after cataract surgery. Clin. Ophthalmol. 2019, 13, 1157–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Goto, T.; Shiraishi, A.; Ohashi, Y. In Vitro Efficacy of Ocular Surface Lubricants Against Dehydration. Cornea 2013, 32, 1260–1264. [Google Scholar] [CrossRef]
- Huerta-Ángeles, G.; Brandejsová, M.; Kopecká, K.; Ondreáš, F.; Medek, T.; Židek, O.; Kulhánek, J.; Vagnerová, H.; Velebný, V. Synthesis and Physicochemical Characterization of Undecylenic Acid Grafted to Hyaluronan for Encapsulation of Antioxidants and Chemical Crosslinking. Polymers 2019, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.-L.; Dinu-Pîrvu, C.-E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Entry a | Sample b | DSGC (%) c | Mw (kDa) d (PDI) | Dry Matter (w/w) e % | ash (w/w) % | T% f | τ g |
|---|---|---|---|---|---|---|---|
| 1 | HA0-C12 | 9.33 ± 032 | 199.4 ± 5.9 (1.6) | 86.83 ± 0.66 | 6.6 ± 0.09 | 99.2 ± 0.21 | 2.1 ± 0.4 |
| 2 | HA1-C12 | 8.73 ± 0.12 | 239.3 ± 2.9 (1.4) | 88.10 ± 0.16 | 7.4 ± 0.33 | 98.8 ± 0.30 | 2.8 ± 0.5 |
| 3 | HA2-C12 | 9.03 ± 0.10 | 311.2 ± 5.2 (1.4) | 88.66 ± 1.5 | 7.8 ± 0.66 | 98.9 ± 0.44 | 3.8 ± 0.2 |
| 4 | HA3-C12 | 7.73 ± 0.04 | 434.5 ± 1.9 (1.5) | 90.03 ± 1.3 | 4.9 ± 0.12 | 94.7 ± 1.4 | 5.8 ± 1.6 |
| Entry | [%] | Sample | Medium | Viscosity (mPa·s) | Viscosity (mPa·s) HA + Mucin III | Mucoadhesive Index (%) |
|---|---|---|---|---|---|---|
| 1 | - | Mucin III | HEPES | 4.97 | -- | -- |
| 2 | 0.3 | HA2 | HEPES | 14.68 ± 0.01 | 25.12 ± 3.18 | 27.83 |
| 3 | 0.3 | HA5 | HEPES | 17.88 ± 0.002 | 10.07 ± 0.003 | −49.8 |
| 4 | 0.3 | HA6 | HEPES | 119.93 ± 0.001 | 21.30 ± 0.001 | −72.6 |
| 5 | 0.3 | HA2 | trehalose | 8.15 ± 0.001 | 12.73 ± 0.002 | −2.95 |
| 6 | 0.3 | HA5 | trehalose | 17.64 ± 0.001 | 28.48 ± 0.004 | 25.78 |
| 7 | 0.3 | HA6 | trehalose | 49.33 ± 0.003 | 69.34 ± 0.004 | 26.69 |
| 8 | 0.10 | HA2-C12 | HEPES | 3.8 ± 0.04 | 7.69 ± 0.11 | −12.31 |
| 9 | 0.20 | HEPES | 7.38 ± 0.02 | 21.65 ± 0.11 | 75.30 | |
| 10 | 0.30 | HEPES | 12.91 ± 0.19 | 331.4 ± 37.09 | 1753.5 | |
| 11 | 0.30 | HA2-C12 | trehalose | 16.77 ± 0.003 | 494.63 ± 0.010 | 2175.2 |
| 12 | 0.10 | HA3-C12 | HEPES | 6.57 ± 0.75 | 11.67 ± 0.12 | 1.12 |
| 13 | 0.20 | HEPES | 13.53 ± 0.37 | 58.07 ± 1.06 | 213.89 | |
| 14 | 0.30 | HEPES | 26.53 ± 0.96 | 156.83 ± 3.94 | 397.87 | |
| 15 | 0.30 | HA3-C12 | trehalose | 68.87 ± 0.001 | 226.30 ± 0.002 | 254.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černohlávek, M.; Brandejsová, M.; Štěpán, P.; Vagnerová, H.; Hermannová, M.; Kopecká, K.; Kulhánek, J.; Nečas, D.; Vrbka, M.; Velebný, V.; et al. Insight into the Lubrication and Adhesion Properties of Hyaluronan for Ocular Drug Delivery. Biomolecules 2021, 11, 1431. https://doi.org/10.3390/biom11101431
Černohlávek M, Brandejsová M, Štěpán P, Vagnerová H, Hermannová M, Kopecká K, Kulhánek J, Nečas D, Vrbka M, Velebný V, et al. Insight into the Lubrication and Adhesion Properties of Hyaluronan for Ocular Drug Delivery. Biomolecules. 2021; 11(10):1431. https://doi.org/10.3390/biom11101431
Chicago/Turabian StyleČernohlávek, Mikuláš, Martina Brandejsová, Petr Štěpán, Hana Vagnerová, Martina Hermannová, Kateřina Kopecká, Jaromír Kulhánek, David Nečas, Martin Vrbka, Vladimir Velebný, and et al. 2021. "Insight into the Lubrication and Adhesion Properties of Hyaluronan for Ocular Drug Delivery" Biomolecules 11, no. 10: 1431. https://doi.org/10.3390/biom11101431
APA StyleČernohlávek, M., Brandejsová, M., Štěpán, P., Vagnerová, H., Hermannová, M., Kopecká, K., Kulhánek, J., Nečas, D., Vrbka, M., Velebný, V., & Huerta-Angeles, G. (2021). Insight into the Lubrication and Adhesion Properties of Hyaluronan for Ocular Drug Delivery. Biomolecules, 11(10), 1431. https://doi.org/10.3390/biom11101431







