Effect of Pirfenidone on TGF-β1-Induced Myofibroblast Differentiation and Extracellular Matrix Homeostasis of Human Orbital Fibroblasts in Graves’ Ophthalmopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Culture of Orbital Fibroblasts
2.2. Chemicals and Antibodies
2.3. Determination of Cell Viability
2.4. Western Blot Analysis
2.5. MMP-2/-9 Enzyme Activity Assay
2.6. Statistical Analysis
3. Results
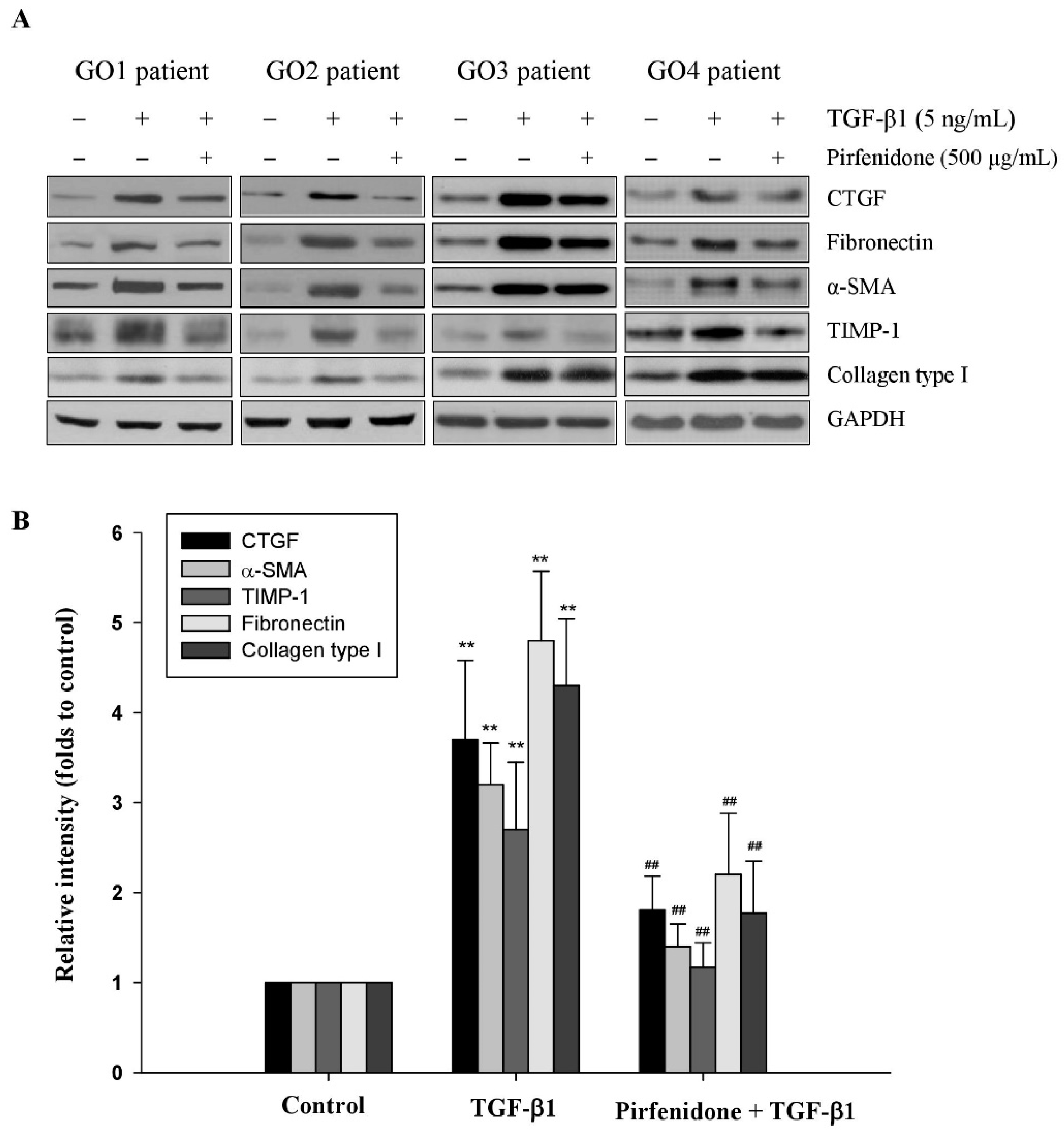
3.1. Pirfenidone Inhibited TGF-β1-Induced Fibrotic Protein Expression in GO Orbital Fibroblasts
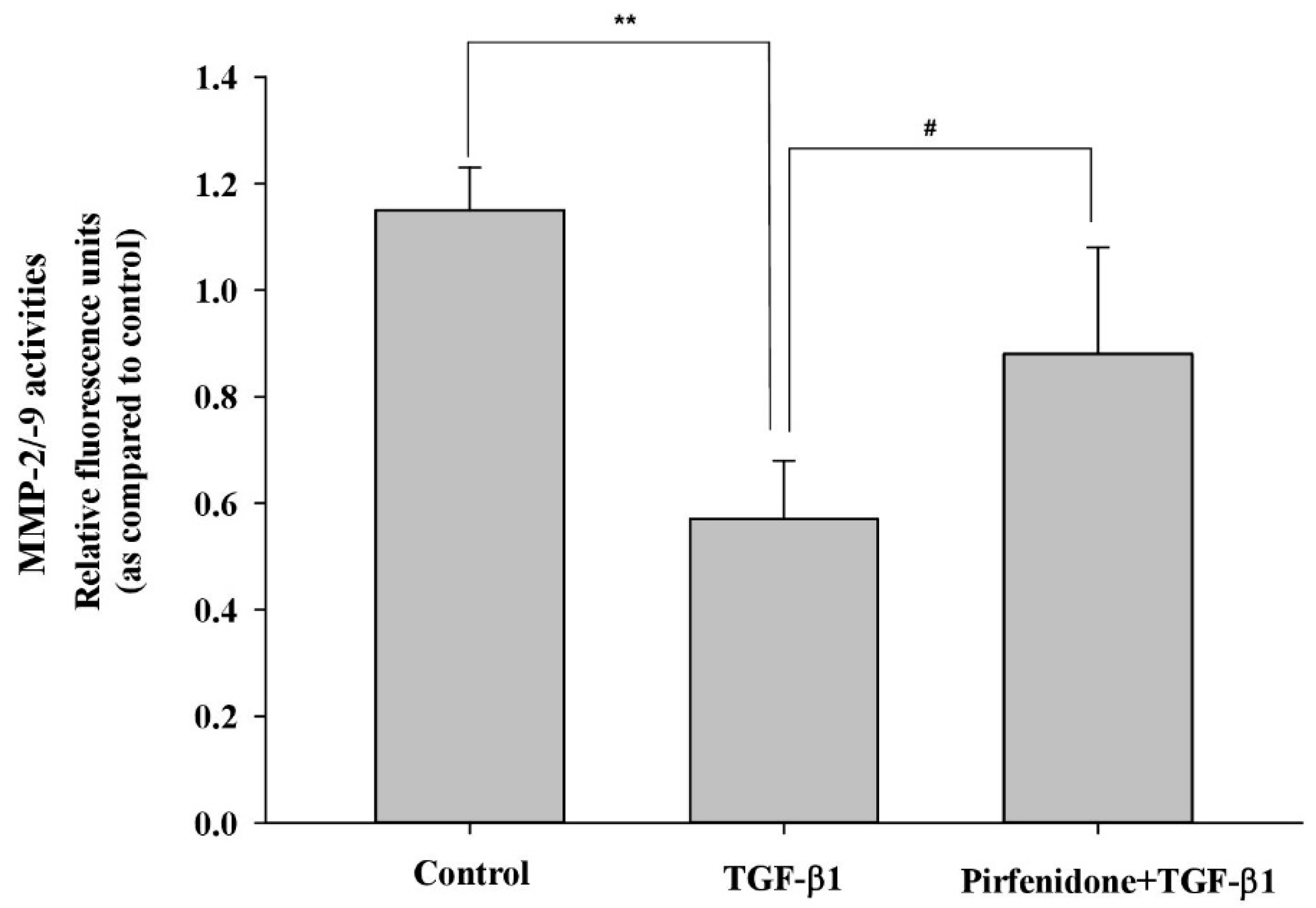
3.2. Pirfenidone Diminished TGF-β1-Mediated ECM Metabolism in GO Orbital Fibroblasts
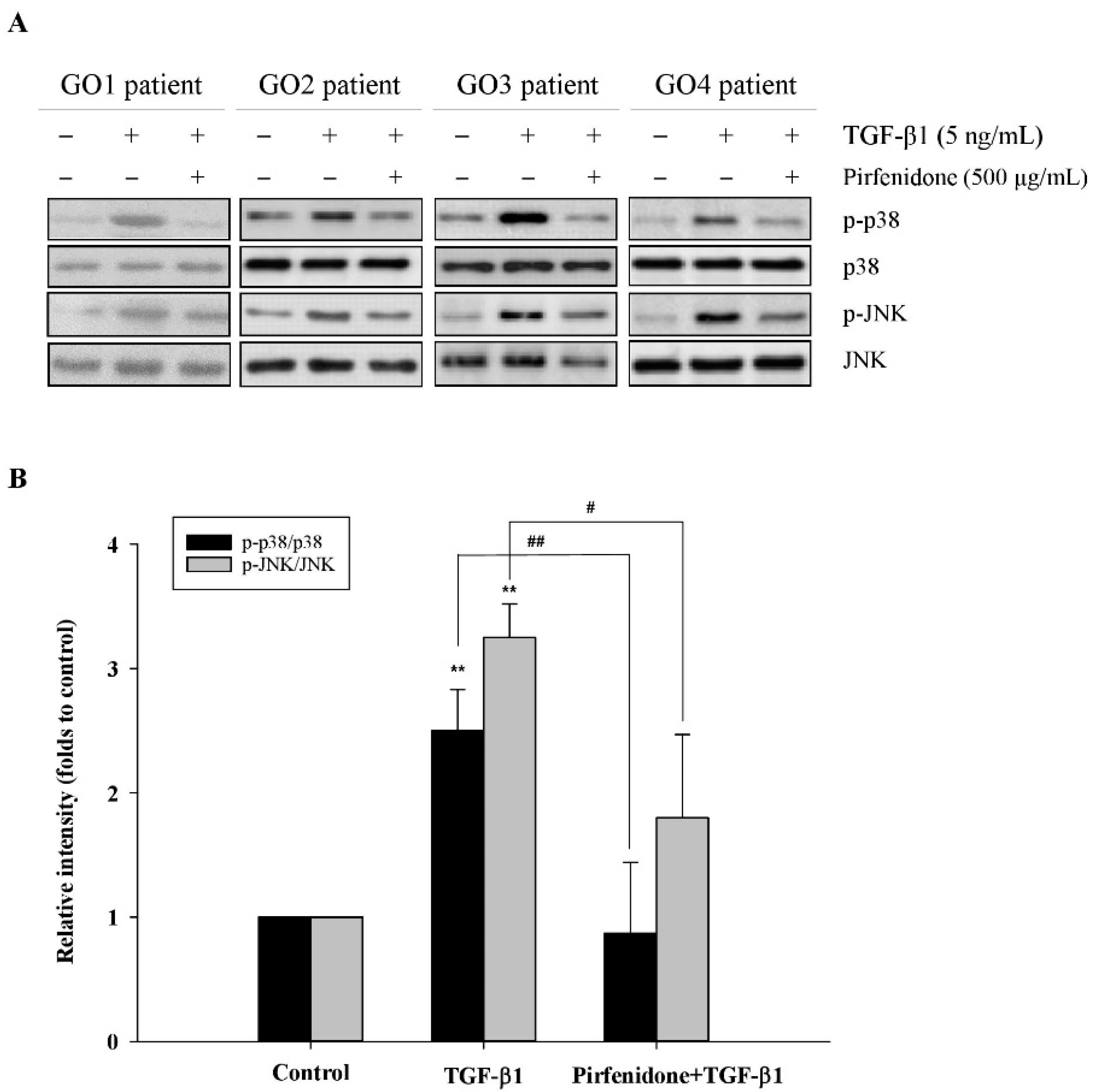
3.3. Pirfenidone Abolished TGF-β1-Induced p38 and JNK Phosphorylation in GO Orbital Fibroblasts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiromatsu, Y.; Eguchi, H.; Tani, J.; Kasaoka, M.; Teshima, Y. Graves’ ophthalmopathy: Epidemiology and natural history. Intern. Med. 2014, 53, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Khong, J.J.; McNab, A.A.; Ebeling, P.R.; Craig, J.E.; Selva, D. Pathogenesis of thyroid eye disease: Review and update on molecular mechanisms. Br. J. Ophthalmol. 2016, 100, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Dik, W.A.; Virakul, S.; van Steensel, L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp. Eye Res. 2016, 142, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Smith, T.J. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1735–1748. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic–Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, P.; Reszec, J.; Eckstein, A.; Johnson, K.; Grzybowski, A.; Chyczewski, L.; Mysliwiec, J. Markers of inflammation and fibrosis in the orbital fat/connective tissue of patients with Graves’ orbitopathy: Clinical implications. Mediat. Inflamm. 2014, 2014, 412158. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Wu, S.B.; Kao, S.C.; Kau, H.C.; Lee, F.; Wei, Y.H. The protective effect of antioxidants on orbital fibroblasts from patients with Graves’ ophthalmopathy in response to oxidative stress. Mol. Vis. 2013, 19, 927–934. [Google Scholar] [PubMed]
- Tsai, C.C.; Wu, S.B.; Kau, H.C.; Wei, Y.H. Essential role of connective tissue growth factor (CTGF) in transforming growth factor-β1 (TGF-β1)-induced myofibroblast transdifferentiation from Graves’ orbital fibroblasts. Sci. Rep. 2018, 8, 7276. [Google Scholar] [CrossRef] [PubMed]
- Koumas, L.; Smith, T.J.; Feldon, S.; Blumberg, N.; Phipps, R.P. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am. J. Pathol. 2003, 163, 1291–1300. [Google Scholar] [CrossRef]
- Hou, T.Y.; Wu, S.B.; Kau, H.C.; Tsai, C.C. JNK and p38 inhibitors prevent transforming growth factor-β1-induced myofibroblast transdifferentiation in human Graves’ orbital fibroblasts. Int. J. Mol. Sci. 2021, 22, 2952. [Google Scholar] [CrossRef]
- Richeldi, L.; Yasothan, U.; Kirkpatrick, P. Pirfenidone. Nat. Rev. Drug Discov. 2011, 10, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Bradford, W.Z.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Glasscock, K.F.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.H.; Nathan, S.D.; et al. Sensitivity analyses of the change in FVC in a Phase 3 trial of pirfenidone for idiopathic pulmonary fibrosis. Chest 2015, 148, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Gili, E.; Fagone, E.; Fruciano, M.; Iemmolo, M.; Vancheri, C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur. J. Pharm. Sci. 2014, 58, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Westra, I.M.; Oosterhuis, D.; Groothuis, G.M.; Olinga, P. The effect of antifibrotic drugs in rat precision-cut fibrotic liver slices. PLoS ONE 2014, 9, e95462. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, K.; Oka, T.; Wang, Q.; Ishizu, T.; Lee, J.K.; Miwa, K.; Akazawa, H.; Naito, A.T.; Sakata, Y.; Komuro, I. Pirfenidone exhibits cardioprotective effects by regulating myocardial fibrosis and vascular permeability in pressure-overloaded hearts. Am. J. Physiol Heart Circ. Physiol. 2015, 309, H512–H522. [Google Scholar] [CrossRef]
- Tampe, D.; Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 226–237. [Google Scholar] [CrossRef]
- Schaefer, C.J.; Ruhrmund, D.W.; Pan, L.; Seiwert, S.D.; Kossen, K. Antifibrotic activities of pirfenidone in animal models. Eur. Respir. Rev. 2011, 20, 85–97. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, G.F.; Liao, X.P.; Li, X.J.; Zhang, J.; Lin, H.; Chen, Z.; Zhang, X. Anti-fibrotic effects of pirfenidone by interference with the hedgehog signalling pathway in patients with systemic sclerosis-associated interstitial lung disease. Int. J. Rheum. Dis. 2018, 21, 477–486. [Google Scholar] [CrossRef]
- Wells, A.R.; Leung, K.P. Pirfenidone attenuates the profibrotic contractile phenotype of differentiated human dermal myofibroblasts. Biochem. Biophys. Res. Commun. 2020, 521, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.A.; Jeon, B.K.; Choi, Y.H.; Back, K.O.; Lee, J.B.; Kook, K.H. Pirfenidone attenuates the IL-1β-induced hyaluronic acid increase in orbital fibroblasts from patients with thyroid-associated ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2276–2283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tao, Y.; Chen, Q.; Zhao, C.; Yang, X.; Cun, Q.; Yang, W.; Zhang, Y.; Zhu, Y.; Zhong, H. The in vitro anti-fibrotic effect of Pirfenidone on human pterygium fibroblasts is associated with down-regulation of autocrine TGF-beta and MMP-1. Int. J. Med. Sci. 2020, 17, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Lee, K.; Ryu, S.W.; Im, M.; Kook, K.H.; Choi, C. Pirfenidone inhibits transforming growth factor-β1-induced fibrogenesis by blocking nuclear translocation of Smads in human retinal pigment epithelial cell line ARPE-19. Mol. Vis. 2012, 18, 1010–1020. [Google Scholar] [PubMed]
- Roztocil, E.; Hammond, C.L.; Gonzalez, M.O.; Feldon, S.E.; Woeller, C.F. The aryl hydrocarbon receptor pathway controls matrix metalloproteinase-1 and collagen levels in human orbital fibroblasts. Sci. Rep. 2020, 10, 8477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017, 9, a022129. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Wang, J.; Xu, C.; Huang, X.; Ruan, Z.; Dai, Y. Pirfenidone alleviates pulmonary fibrosis in vitro and in vivo through regulating Wnt/GSK-3beta/beta-catenin and TGF-beta1/Smad2/3 signaling pathways. Mol. Med. 2020, 26, 49. [Google Scholar] [CrossRef]
- Li, G.; Ren, J.; Hu, Q.; Deng, Y.; Chen, G.; Guo, K.; Li, R.; Li, Y.; Wu, L.; Wang, G.; et al. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-β signaling in a murine colitis model. Biochem. Pharmacol. 2016, 117, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Wang, B.; Nie, Y.; Wen, J.; Wang, Q.; Gu, C. Pirfenidone suppresses MAPK signalling pathway to reverse epithelial-mesenchymal transition and renal fibrosis. Nephrology (Carlton) 2017, 22, 589–597. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Y.; Liu, L.; Liu, X.; Xu, J.; Wu, K.; Yu, M. Pirfenidone induces G1 arrest in human Tenon’s fibroblasts in vitro involving AKT and MAPK signaling pathways. J. Ocul. Pharmacol. Ther. 2017, 33, 366–374. [Google Scholar] [CrossRef]
- Hall, C.L.; Wells, A.R.; Leung, K.P. Pirfenidone reduces profibrotic responses in human dermal myofibroblasts, in vitro. Lab. Investig. 2018, 98, 640–655. [Google Scholar] [CrossRef]
- Shi, K.; Wang, F.; Xia, J.; Zuo, B.; Wang, Z.; Cao, X. Pirfenidone inhibits epidural scar fibroblast proliferation and differentiation by regulating TGF-β1-induced Smad-dependent and -independent pathways. Am. J. Transl. Res. 2019, 11, 1593–1604. [Google Scholar] [PubMed]
- Kim, H.; Choi, Y.H.; Park, S.J.; Lee, S.Y.; Kim, S.J.; Jou, I.; Kook, K.H. Antifibrotic effect of Pirfenidone on orbital fibroblasts of patients with thyroid-associated ophthalmopathy by decreasing TIMP-1 and collagen levels. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3061–3066. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.S.; Kahaly, G.J.; Patel, A.; Sile, S.; Thompson, E.H.Z.; Perdok, R.; Fleming, J.C.; Fowler, B.T.; Marcocci, C.; Marinò, M.; et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N. Engl. J. Med. 2020, 382, 341–352. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-B.; Hou, T.-Y.; Kau, H.-C.; Tsai, C.-C. Effect of Pirfenidone on TGF-β1-Induced Myofibroblast Differentiation and Extracellular Matrix Homeostasis of Human Orbital Fibroblasts in Graves’ Ophthalmopathy. Biomolecules 2021, 11, 1424. https://doi.org/10.3390/biom11101424
Wu S-B, Hou T-Y, Kau H-C, Tsai C-C. Effect of Pirfenidone on TGF-β1-Induced Myofibroblast Differentiation and Extracellular Matrix Homeostasis of Human Orbital Fibroblasts in Graves’ Ophthalmopathy. Biomolecules. 2021; 11(10):1424. https://doi.org/10.3390/biom11101424
Chicago/Turabian StyleWu, Shi-Bei, Tzu-Yu Hou, Hui-Chuan Kau, and Chieh-Chih Tsai. 2021. "Effect of Pirfenidone on TGF-β1-Induced Myofibroblast Differentiation and Extracellular Matrix Homeostasis of Human Orbital Fibroblasts in Graves’ Ophthalmopathy" Biomolecules 11, no. 10: 1424. https://doi.org/10.3390/biom11101424
APA StyleWu, S.-B., Hou, T.-Y., Kau, H.-C., & Tsai, C.-C. (2021). Effect of Pirfenidone on TGF-β1-Induced Myofibroblast Differentiation and Extracellular Matrix Homeostasis of Human Orbital Fibroblasts in Graves’ Ophthalmopathy. Biomolecules, 11(10), 1424. https://doi.org/10.3390/biom11101424







