Abstract
The microbiota-harboring human gut is an exquisitely active ecosystem that has evolved in a constant symbiosis with the human host. It produces numerous compounds depending on its metabolic capacity and substrates availability. Diet is the major source of the substrates that are metabolized to end-products, further serving as signal molecules in the microbiota-host cross-talk. Among these signal molecules, branched-chain amino acids (BCAAs) has gained significant scientific attention. BCAAs are abundant in animal-based dietary sources; they are both produced and degraded by gut microbiota and the host circulating levels are associated with the risk of type 2 diabetes. This review aims to summarize the current knowledge on the complex relationship between gut microbiota and its functional capacity to handle BCAAs as well as the host BCAA metabolism in insulin resistance development. Targeting gut microbiota BCAA metabolism with a dietary modulation could represent a promising approach in the prevention and treatment of insulin resistance related states, such as obesity and diabetes.
1. Introduction
Intestinal microbiota composition and function are hypothesized to play a pivotal role in the development of major non-communicable diseases including type 2 diabetes mellitus (T2D), chronic kidney disease, neurodegenerative diseases, and arterial hypertension [1]. The outbreak of the interest in microbiome and its relationship to human health was enabled by the rapid development of the next genome sequencing (NGS) technology in the late 1990s and the term “microbiota” was conceptualized in 2001 [2]. The first studies focused mainly on the microbiota composition but soon it became evident that the products of the microbial metabolism are at least as important as the bacteria inhabiting specific niches of the human body. The way that the intestinal microbiota processes diet components impacts the availability of biologically active end-products. Many of these compounds convey signaling functions as they mediate host–microbiota crosstalk and substantially affect the host physiology. For example, dietary fiber polysaccharides that cannot be cleaved by human glycosidases are fermented by gut microbiota to form short-chain fatty acids (SCFA) that exert pleiotropic effects on the host. They serve as the main energy source for colonocytes [3]; contribute to the maintenance of intestinal barrier [4]; stimulate the maturation of gut-associated immune cells with anti-inflammatory potential [5]; or regulate energy expenditure in the liver, adipose tissue, or skeletal muscle [6]. Among other well-studied end-products are choline, an essential nutrient which is utilized by gut microbes and its deficiency leads to the NASH-like syndrome [7], or acetaldehyde derived from ethanol by intestinal microbiota, which has been shown to disrupt tight junctions between intestinal mucosal cells [8].
Among other emerging microbiota-derived compounds, branched-chain amino acids (BCAAs) have recently gained a significant scientific attention. Indeed, there has been a long-lasting strand of evidence that elevated circulating BCAAs are associated with insulin resistance states such as obesity and diabetes [9] and may even predict cancer development [10,11]. Compared to mammals that are dependent on external supply of BCAAs, numerous gut bacteria possess metabolic pathways allowing for the BCAA biosynthesis. Therefore, it is reasonable to hypothesize that the composition and metabolic performance of intestinal microbiota contribute to the BCAA availability to the host and are, at least partially, responsible for their effect on the host metabolism.
The purpose of the narrative review is to summarize the current knowledge on BCAA body metabolism with respect to the relationship between the BCAA and insulin resistance (IR) and to provide evidence that intestinal microbiota metabolic activity may represent the potential mechanistic link between the diet composition, BCAA availability, and IR development.
2. Branched-Chain Amino Acids Are Both Nutrients and Signaling Molecules
BCAAs circulate in plasma as free amino acids (AAs) and are being resorbed virtually to all tissues using specific carriers. BCAAs are unique among other AA as they undergo neither intestinal nor liver catabolism as none of these tissues contain the first key deamination enzyme (BCAT, reviewed below). Therefore, their systemic circulating concentrations reflect the administered or resorbed dose, respectively [12,13]. Moreover, leucine is among the most abundant AA in bodily proteins [14], which both predispose BCAAs to be the nutrient-sensing signals for many target tissues [15]. At the cellular level, BCAAs serve as (i) direct building blocks or nitrogen donors for proteosynthesis; (ii) energy/anaplerosis substrate while degraded eventually down to the final glucogenic (propionyl and succinyl-CoA) and ketogenic (acetyl-coA and acetoacetate) products and oxidized; or (iii) nutritional signals via mTOR activation. The intracellular pathways of BCAAs are summarized in Figure 1.
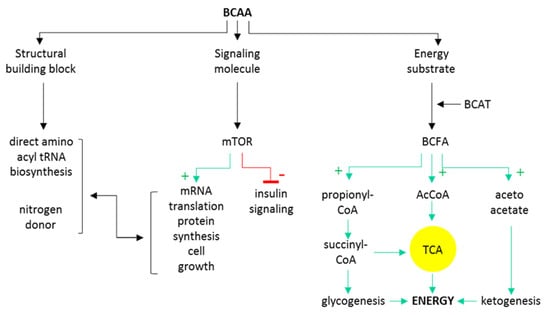
Figure 1.
Schematic overview of major intracellular BCAA metabolic and signaling pathways. BCAT, branched-chain amino acid aminotransferase; BCFA, branched-chain fatty acids; AcCoA, acetyl coenzyme A; TCA, tricarboxylic acid cycle.
BCAAs share the first two katabolic enzymatic steps. The first is transamination catalyzed by branched-chain amino acid aminotransferase (BCAT) where deamination occurs and amino-residue is transferred to α-ketoglutarate to eventually form glutamate/glutamine. The product of the reaction is a keto-analogue of respective BCAAs, i.e., branched-chain fatty acid (BCFA), α-keto-isocaproate, α-keto-β-metylvalerate, or keto-isovalerate. A BCAT-catalyzed reaction is reversible and runs near equilibrium which means that the direction of the reaction depends on the availability of BCFA and nitrogen donors. Moreover, there exists an interorgan flux of branched carbon skeleton to prevent the loss of these essential compounds [16]. A further step towards eventual oxidation is an oxidative decarboxylation catalyzed by a branched-chain α-keto-dehydrogenase complex (BCKDH). The oxidative decarboxylation is an irreversible reaction that is highly regulated and coupled with NADH reduction. Products of decarboxylation are isovaleryl-CoA, isobutyryl-CoA and α-methyl-butyryl-CoA. These substrates consequently undergo a series of dehydrogenase mitochondrial reactions to eventually form degradation end-products acetoacetate, acetyl-CoA a propionyl-CoA. Importantly, at each catabolic step downstream from BCKDH, the oxidation intermediates may be used for anaplerosis, synthesis of FA or cholesterol [17].
BCAAs, namely leucine, signal to mTOR complex (mammalian/mechanistic target of rapamycin). This is an evolutionary-conserved nutrient-sensing serine–threonine kinase with two major complexes: mTORC1, which is responsible for cells’ anabolism to catabolism switch via control of metabolic state; and mTORC2, which downstream targets control cell survival, proliferation and cytoskeleton dynamics. The complex is an integrating system where many metabolic signals, both nutritional and hormonal, converge. Major phosphorylation targets of mTORC are p70S6 serine kinase 1 (S6K1) and 4EBP1 (binding protein 1 for eukaryotic initiation factor 4E) [15] that serve as regulators of transcriptional activity leading to many anabolic processes including protein synthesis, autophagy inhibition, and cell growth. Of note, phosphorylated S6K1 inactivates the insulin receptor substrate (IRS) and downregulates the insulin cascade activity, decreasing insulin-dependent substrate uptake in the time of the BCAA abundance [18].
3. Circulating BCAAs Associated with Insulin Resistance
Individual BCAAs share common structural resemblance and their circulating levels are synchronized in many clinical states, physical activity, fasting [19], and diabetes [17]. The association between the elevated circulating BCAAs and insulin resistance was described more than fifty years ago but it has just recently received a significant scientific acknowledgement [20,21]. Peripheral BCAA levels predict a risk of incident diabetes up to twelve years before its manifestation [22] and lifestyle intervention [20]. In line with this observation, bariatric surgery [23] that reverts metabolic syndrome decreases circulating BCAAs too. Whether there is any causal role in BCAA triggering IR remains to be confirmed. Nevertheless, a few plausible mechanisms of the increased BCAA availability interfering with insulin functions were described (Figure 2).
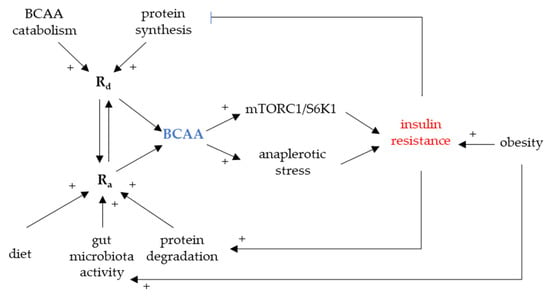
Figure 2.
BCAA and insulin resistance development. mTORC1, mammalian/mechanistic target of rapamycin complex 1; Ra, rate of appearance; Rd, rate of disappearance; S6K1, p70S6 serine kinase 1.
Firstly, the activation of downstream mediator of mTOR SK61 leads to the downregulation of insulin receptor substrate (IRS1 and IRS2) [9,18]. It was shown in vitro that BCAAs induce mTOR signaling in skeletal muscle reflected by a decreased glucose uptake [24]. Persistent mTOR activation due to a chronic BCAA overload may therefore contribute to insulin resistance [25].
Secondly, BCAA catabolic intermediates feed into other metabolic pathways, namely TCA, potentially disturbing fine-tuned equilibria dependent on substrate availability. This effect is also referred to as anaplerotic stress [26]. Several BCFA could cause mitochondrial dysfunction and inactivation of pyruvate dehydrogenase in the liver and heart. Once bound to CoA, BCFA can be only transported out of the mitochondria as acylcarnitines. Acylcarnitines are abundant in various organic acidurias and their levels correlate with functional impairment, namely mitochondrial dysfunction. As several BCAA-derived acylcarnitines are elevated in circulation of T2DM patients, it was suggested that this effect may link elevated BCAAs with mitochondrial dysfunction in metabolically active organs [27].
The association is far from being straightforward. It has been repeatedly shown that BCAAs exert insulinotropic effects via multiple mechanisms on beta cells: insulin synthesis activated by mTOR [28], acting on glutamate dehydrogenase [28], and stimulating incretin secretion [12]. Having such properties, it has been suggested that BCAA and/or diets rich in BCAAs could improve metabolic status in T2DM [29,30].
Moreover, BCAAs stimulate muscle protein synthesis and inhibit protein degradation [31]. It was shown that namely leucine and its derivates, when supplemented, can not only revert sarcopenia in patients with T2DM but also ameliorate their insulin sensitivity [32,33]. BCAAs were, hence, suggested as nutriceuticals to prevent lean mass loss [34,35].
Circulating levels of BCAAs depend on several factors modulating their appearance/disappearance. Rate of disappearance (Rd) is determined by protein synthesis, urinary excretion, and catabolism/oxidation in tissues whereas the rate of appearance (Ra) is determined by dietary intake, intestinal resorption, and tissue protein degradation. Ra/Rd mismatch leading to elevated circulating BCAAs could be eventually caused by a shift in any of these.
Factors decreasing Rd. Decreased protein synthesis, decreased oxidation, and increased excretion were all suggested as players in the elevated BCAA levels [25,36,37]. BCAAs constitute a significant portion of acyl-CoAs feeding lipogenesis in adipose tissue [38,39]. As it was shown, gene expression of BCKDH is down-regulated in adipose tissue of IR subjects; once the storage capacity is exceeded, there is a BCAA spill-over to the systemic circulation [40]. BCAAs stimulate protein synthesis [41] and are major building blocks and nitrogen donors for skeletal muscle. There is a postprandial flux of BCAAs into the skeletal muscle [42] increasing their Rd. It is conceivable that decreased protein synthesis may be also linked to elevated BCAAs.
Factors increasing Ra. As revised earlier, BCAAs stimulate protein synthesis whereas insulin blocks protein degradation. Loss of insulin action could lead to disinhibition of protein breakdown in the skeletal muscle and, therefore, to increased Ra of BCAAs [43]. As per increased dietary intake, BCAA intake does not contribute to fasting but to postprandial BCAA levels [12,25]. Anyhow, it may modulate other dependent factors as it was shown that dietary protein composition has the major impact on BCAA concentrations [44,45]. An important link between diet and bioavailability of BCAAs, i.e., the amount of digested and resorbed BCAAs, has emerged: gut microbiota composition and metabolic capacity [46,47,48].
4. Gut Microbiota as the Source of Amino Acids for the Host
Diet has been considered the main source of essential nutrients but recent research has unraveled the great importance of a “hidden metabolic organ”—the gut microbiota—in modulation of the availability of many necessary compounds to the host. Bacteria are capable of numerous biochemical processes, including digestion of proteins or peptides that escaped absorption in the upper part of the digestive tract [49,50] or AA synthesis from nonspecific nitrogen sources [51]. Therefore, the metabolic requirement of AAs may not be covered only by the diet but also by AAs synthesized de novo by the gastrointestinal microbiota [52]. This assumption was confirmed by experiments in which nitrogen-containing compounds (ammonium chloride, urea) labeled with 15N were provided in the diet and the label was later found in the AA pool of the host [53,54,55]. The final availability of the microbiota-derived AA to the host depends both on the macronutrient composition of the diet (i.e., diet rich in carbohydrates blocks protein fermentation) and the gut microbiota functional capacity. Consequently, the alterations in the gut microbiota composition, called dysbiosis, may substantially contribute to the metabolic deregulation of the host.
While identification of the bacterial species responsible for proteolytic fermentation was originally based on correlative methods and the culture on AAs containing media, the newer approach capitalize on Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of annotated human gut bacterial genomes [56]. Unfortunately, all approaches face considerable limits.
Correlation studies comparing gut microbiota composition and feces/serum metabolome do not establish causality. Furthermore, as the small intestine seems to be responsible for a large part of the uptake of microbiota-derived AAs [57], composition of ileal microbiota is much more relevant, especially concerning microbial source of AAs. Nevertheless, most of the available studies describe composition of fecal microbiota that reflects rather the situation in the colon than in the small intestine. The fermentation studies are usually performed in simplified conditions while, in the real intestine, the availability of other diet-derived substrates can affect the metabolic activity of the intestinal microflora and thus also the products potentially available to the host. In the KEGG database, approx. 21% of the reactions remain unclassified, allowing only for a crude estimation of the gut microbiota functional capacity [58].
In spite of these limitations, several protein fermenters and amino acid producers have already been identified. In vitro experiments showed major protein fermenters: Clostridium, Fusobacterium, Bacteroides, Actinomyces, Propionibacterium, and Peptostreptococci [59]. The most abundant AA-fermenting bacteria in the human small intestine are bacteria belonging to the Clostridium clusters, the Bacillus-Lactobacillus-Streptococcus groups, and Proteobacteria [60]. In the large intestine of healthy humans, bacteria belonging to the Clostridia and Peptostreptococci appear to be the most prevalent species involved in AA fermentation [60,61,62]. BCFA abundance, i.e., the marker of BCAA fermentation, has also been correlated with decreased Firmicutes and increased unknown Bacteroidetes, as well as Prevotella spp., Bacteroides ovatus, Bacteroides thetaiotamicron, and Clostridium spp. in an artificial colon model of high-protein diets [63].
Gut bacterial proteins encompass a higher ratio of BCAAs to other amino acids compared with the mammalian organism [60]. They represent important nutrients in bacterial physiology and ensure multiple functions, including protein synthesis, nutrient signaling, adaptation to amino acid starvation, or regulation of virulence gene expression [64]. Bacteria synthesize BCAAs through conserved pathways that are present in fungi and plants but absent in mammals (Figure 3); these biosynthetic pathways also provide intermediates for the synthesis of BCFA [64]. SCFA and BCFA synthesis share the same multienzyme pathway; however, the substrates that initiate the pathway differ. Acetyl-CoA serves as the substrate for SCFA synthesis, whereas branched-chain acyl-CoA serves as the substrate for BCFA synthesis [65] (Zhang 2008). Depending on the metabolic requirements, BCFAs may be converted to BCAAs [66,67] (Sun 2020).
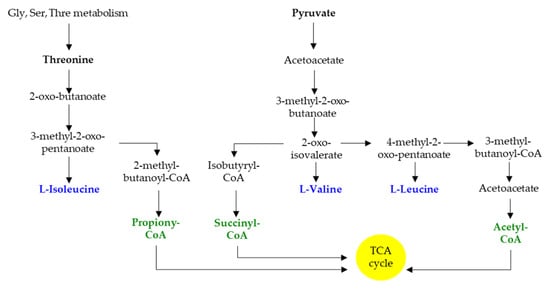
Figure 3.
Schematic representation of BCAA and BCFA biosynthesis. Gly, glycine; Ser, serine; Thre, threonine.
The actual metabolic program of bacteria significantly depends on available substrates. A landmark study by David et al. showed that short switch to an animal- or vegetable-based diet was associated with prompt alteration in fecal metabolome composition. A plant-based diet was associated with an increase in fecal SCFA while an animal-based diet led to the higher production of BCFAs (Figure 4) [68] (David). While literature dealing with the effect of dietary carbohydrates or fiber on bacterial fermentation program is quite rich, there is much less information available regarding microbial breakdown and fermentation of proteins. Based on the rather scarce evidence, it can be concluded that high protein intake is associated with an increase in Bacteroides, Fusobacterium, Proteobacteria, Desulfovibrio, Bilophila wadsworthia, Clostridium, Ruminociccus, Eubacterium, A. putredinis and Bacteroides sp., the last two genera positively correlating with fecal BCFAs. On the other hand, a decrease in Firmicutes, Archaea, Megasphera, Selenomonas, Acidaminococcus, Bifidobacterium, and Prevotella was observed. High-protein diets tend to lead to a decrease in the SCFA production, while BCAA and BCFA stool content is elevated [69].
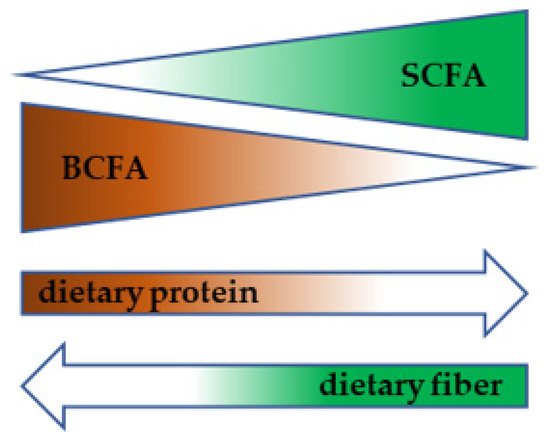
Figure 4.
Diet-dependent metabolic switch in gut microbiota.
The above mentioned findings support the hypothesis that microbiota-related components may be an important contributor to the diet-derived end-products available to the host.
5. Findings Derived from Observational Studies
5.1. Human Studies
Obesity, insulin resistance, and T2D are associated with elevated levels of circulating BCAA. In the landmark study involving 277 insulin-resistant, non-diabetic Danish subjects, Pedersen et al. demonstrated that the abundance of genes encoding enzymes involved in BCAA synthesis positively correlated with insulin resistance while the opposite relationship was observed in the case of genes encoding bacterial inward transporters for these AA [46]. Furthermore, they identified Prevotella copri and Bacteroides vulgatus as the main species responsible for the elevated BCAA biosynthesis and Butyrivibrio crosstus and Eubacterium siraeum for the decreased inward BCAA transport. Several other studies have reported altered microbiota composition and functional capacity for BCAA biosynthesis/degradation in both human and animal models of obesity or T2D since then. A metagenome-wide association study combined with serum metabolomic profiling performed in 118 obese and 105 lean healthy young Chinese individuals unraveled obesity-associated gut microbial signature linked to changes in circulating metabolites. Among other findings, the authors showed that microbiota of obese individuals have higher potential to produce aromatic and branched-chain AA whereas the BCAA degradation pathways were depleted [47]. In line with these findings, serum concentrations of Phe, Tyr, Leu, Ile, and Val were higher in obese than in lean subjects. These AA were inversely correlated with species from Bacteroides genus, including B. thetaiotaomicron, B. intestinalis, B. ovatus, and B. uniformis. The Malmö Offspring Study, encompassing 92 Swedish adults, revealed strong positive association between the concentration of serum BCAA and related metabolites such as BCFAs (3-methyl-2-oxovalerate, α-ketoisovalerate and α-ketoisocaproate) or short-chain acylcarnitines (isovalerylcarnitine) and BMI [70]. Furthermore, the strongest predictor of a high BMI in this study, glutamate, is a byproduct of the first step of BCAA oxidation where the amino group is transferred to α-ketoglutarate. Metabolites predictive of a higher BMI were correlated with four gut bacterial genera. In case of Dorea, Blautia and Ruminococcus (all belonging to the Lachnoclostridiaceae family) there was a positive correlation while in the case of the order SHA-98 the relationship was inverse. The associations between the gut microbiota composition and circulating metabolites that may have an impact on cardiovascular or metabolic traits were addressed in METSIM study involving 531 Finnish males [71]. In this study, 40 significant associations between 17 traits and 23 unique bacterial operational taxonomic units (OTUs) were identified. In line with the study by Ottosson et al. [70], BCAAs were positively associated with the abundance of Blautia, a high BMI, and high homeostatic model assessment of insulin resistance (HOMA-IR) values. In addition, BCAA negatively correlated with Christensenellaceae. Wang et al. compared the gut microbiota and serum metabolome of relatively small groups of subjects following different dietary patterns, i.e., vegans (VG, n = 12), lacto-ovo-vegetarians (VEG, n = 12), and omnivores (OMNI, n = 12) [72]. Twenty-six and twenty-seven metabolites, including BCAAs, significantly contributed to the separation of OMNI from VEG and VG, respectively, with BCAA being higher in the OMNI group. The microbiome composition defined by the whole-genome sequencing of VG and VEG was significantly different from the OMNI group at the level of family, genus, and species. Interestingly, P. copri was identified by Pedersen et al. as the microorganism most positively associated with elevated serum BCAAs and was more abundant in the gut microbiota of the VG and VEG groups. On the other hand, metatranscriptomic analysis identified the BCAA degradation pathway as significantly enriched in VG and VEG compared with the OMNI group.
While the above-mentioned cross-sectional studies brought at least partly convergent outcomes, quite opposite results were obtained in a study of Mesnage describing metabolome and microbiome changes during a very different physiological situation, i.e., fasting and refeeding [73]. The study participants were examined at baseline, immediately after 10 days of fasting, after 4 days of refeeding and after 3 months of a habitual diet. The BCAA content dramatically increased (p = 0.0005) during fasting, returned to baseline after refeeding and declined significantly after 3 months. Regarding microbiome composition, fasting resulted in a decrease in Lachnospiraceae and Ruminococcaceae and a concomitant increase in the Bacteroides and Proteobacteria abundance. While Lachnospiraceae negatively correlated with the BCAA levels, Bacteroidetes correlated with them positively. This discrepancy points out the pitfalls of correlation studies and underlies the importance of the mechanistic approach. The outcomes from the above mentioned studies are summarized in Table 1.

Table 1.
Conditions associated with altered circulating BCAA concentration: human studies.
5.2. Animal Studies
While it is difficult to adjust to a different dietary intake of individual AA in large human trials, animal studies allow for such a standardization and, therefore, for a more precise identification of microbiota-derived BCAA in the circulation. Several studies addressing the relationship between gut microbiota, circulating metabolites, and metabolic health were performed in animal models of obesity (mice fed a high-fat diet) [48,74] or diabetes (rats fed a high-fat diet and treated with streptozotocin) [75] (Table 2). In all models, serum BCAAs were significantly elevated and could be regarded as serum biomarkers of obesity-related insulin resistance. HFD induced significant changes in the gut microbiota composition characterized by the increased Firmicutes to Bacteroidetes ratio but, at the genus level, the outcomes were quite heterogeneous. Pathway analysis based on the metabolite abundances [48,75] or PICRUSt analysis based on the gut microbiota composition [74] identified an increased BCAA biosynthesis pathway in the HFD groups. The BCAA degradation pathway was decreased in HFD-fed mice only in the study of Zeng et al. [48] but not in the others.

Table 2.
Conditions associated with altered circulating BCAA concentration: animal studies.
6. Mechanistic Studies
6.1. The Effect of Bacterial Taxa Manipulation on the BCAA Metabolism
Numerous animal experiments demonstrated that manipulation of gut microbiota influences the systemic BCAA pool (Table 3). One type of experimental setting involved direct colonization of experimental animals with selected bacteria or the whole fecal microbiome. The first study in this field [76] demonstrated that ex-germ free mice humanized with fecal bacteria from obese individuals reproduced the obese phenotype associated with the elevated serum concentration of BCAAs. The PICRUSt analysis revealed the enrichment of AA-related pathways in the gut microbiome including valine, leucine, and isoleucine biosynthesis. As described above, P. copri was identified as the strongest driver species for the positive association between microbial BCAA synthesis in the gut and the traits of insulin resistance in a Danish cohort of non-diabetic males [46]. Aiming to confirm a causal relationship, Pedersen et al. performed an experiment in which conventionally raised HFD-fed mice, which were low in BCAAs, were repeatedly gavaged with P. copri for three weeks. Three weeks of P. copri challenge aggravated the glucose intolerance, increased the serum BCAA concentration, and reduced insulin sensitivity compared with sham-gavaged animals. Interestingly, P. copri represented only a minor component of the gut microbiome and it was also the only bacteria affected by the gavage during the whole experiment; this suggests that even low-abundant taxa may significantly affect the host metabolism. In another study, Zeng et al. [48] described the effect of gavaging conventionally raised HFD-fed mice with Bacteroides ovatus, i.e., bacteria that negatively correlated with circulating BCAA in human obesity [47]. Gavage with live, but not heat-killed B. ovatus, ameliorated diet-induced obesity, improved serum lipid profile, and significantly reduced the serum and fecal BCAA concentration compared with the sham-gavaged animals. The same effect was observed using B. thetaiotaomicron [47].

Table 3.
The effect of specific bacterial taxa on BCAAs concentration in serum.
6.2. The Effect of Microbiota Modulation with Phytochemicals or Symbiotics on the BCAA Metabolism
A different strategy is based on the administration of compounds affecting the gut microbiome (Table 4). This manipulation is subsequently translated into serum AA, resp. BCAA composition as well as to the glucose metabolism-related parameters. To our knowledge, this effect was described after a treatment with citrus polymethoxyflavones (PMFE) [48], Luffa cylindrical (luffa) [74], berberine [77], glucomannans [75], and inulin [78]. The chemical nature of these compounds is quite heterogeneous. Inulin and glucomannan are indigestible polysaccharides (dietary fiber); berberine is a quaternary salt of benzylisoquinoline alkaloid; PMFE belongs to flavonoids; and luffa is a crude mixture of components including polyphenols, flavonoids, saponin, triterpenoids, and oleanolic acid. Their effect was tested in animal models of HFD-induced obesity and insulin resistance [48,74,77], diabetes [75] or in growing pigs [78]. All treatments alleviated HFD-induced obesity and ameliorated the glucose and/or lipid profiles. This effect was microbiota-dependent as it was partly or completely abolished by antibiotic treatment [74,77] or transferable by fecal transplantation from treated mice to HFD-fed ones [77]. The common effect of all interventions at the metabolome level was a decrease in serum BCAA concentrations. Regarding the gut microbiome composition, the effect of individual treatments is extremely variable but, in all studies, the authors reported altered functionality of the gut microbiota manifesting as either the downregulation of BCAA biosynthetic pathways [74,75], the upregulation of BCAA degradation pathways [48,78], or both [77]. The functional capacity of the gut microbiota was derived from its composition using PICRUSt [74,77], predicted by pathway analysis based on metabolome composition [48] or using both approaches [75,78]. Currently, there is only one available human study describing the effect of symbiotic (SG) administration (Bifidobacterium lactis UBBLa-70 + fructooligosaccharide) in combination with low-energy intervention in obese women [79]. Both interventions led to a comparable weight loss but the serum BCAA concentration decreased only in the SG-supplemented group. Furthermore, a positive correlation for changes in Verrucomicrobia and isoleucine, as well as for Firmicutes and isoleucine, and total BCAAs were observed in the SG group. Sun et al. described the effect of the gentamicin intervention in mice infected with the Influenza virus. Besides the reduced survival of the mice, gentamicin treatment also resulted in an increased abundance of Bacteroidetes, decreased abundance of Proteobacteria, and elevated serum BCAA concentration [79].

Table 4.
The effect of microbiota modulation with phytochemicals or symbiotics on BCAAs concentration in serum.
7. Conclusions
Their unique biochemical and physiological characteristics predispose BCAAs to be the nutrient-sensing signals for many target tissues. BCAAs, namely leucine, signal to mTOR complex, an evolutionary conserved nutrient-sensing system ensuring optimal metabolic adaptation to nutrient availability and energy status. BCAAs play an essential role in physiologically switching off the insulin signaling; however, when present in excess, they are associated with insulin resistance and T2DM development. Being essential AAs, BCAAs cannot be synthesized and mammalian organism depends on their external sources. Besides the diet, the gut microbiota seem to be a substantial source of these nutrients. Here, we summarize the evidence for the gut microbiota being the player influencing the host circulating BCAA pool, thus potentially contributing to obesity and metabolic disorders. As per the mechanistic links, altered microbiome functional capacity manifested either by an increased abundance of BCAA biosynthesis and/or an decreased abundance of BCAA degradation pathways in the gut bacterial metagenome have been suggested. The data from microbiota-targeted intervention studies suggest that modulating the BCAA availability by affecting the gut microbiota may represent a promising therapeutic approach. Unfortunately, functional redundancy of microbial genomes is probably what currently precludes the identification of particular bacterial taxa responsible for BCAA modulating effects. Further research in this field is warranted in order to find the most effective ways to the prevention and treatment of insulin-resistant states.
Author Contributions
Conceptualization, J.G. and M.C.; writing—original draft preparation, review and editing, J.G. and M.C. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant MH CR no. NV18-01-00040, by the institutional support PROGRES Q36, by MH CR—DRO (Institute for Clinical and Experimental medicine—IKEM, IN 00023001”) and by EFSD mentorship program supported by AstraZeneca.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabello-Olmo, M.; Arana, M.; Urtasun, R.; Encio, I.J.; Barajas, M. Role of Postbiotics in Diabetes Mellitus: Current Knowledge and Future Perspectives. Foods 2021, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cresci, G.A.; Glueck, B.; McMullen, M.R.; Xin, W.; Allende, D.; Nagy, L.E. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J. Gastroenterol. Hepatol. 2017, 32, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; de Roos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mao, Z.; Ye, X.; Zuo, T. Human Gut Microbiome and Liver Diseases: From Correlation to Causation. Microorganisms 2021, 9, 1017. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Galle, P.R. Animal models of non-alcoholic steatohepatitis: Of mice and man. Dig. Dis. 2010, 28, 247–254. [Google Scholar] [CrossRef]
- Rao, R.K. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol. Biol. 2008, 447, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katagiri, R.; Goto, A.; Nakagawa, T.; Nishiumi, S.; Kobayashi, T.; Hidaka, A.; Budhathoki, S.; Yamaji, T.; Sawada, N.; Shimazu, T.; et al. Increased Levels of Branched-Chain Amino Acid Associated With Increased Risk of Pancreatic Cancer in a Prospective Case-Control Study of a Large Cohort. Gastroenterology 2018, 155, 1474–1482.e1. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hazra, A.; Lawler, P.R.; Chandler, P.D.; Chasman, D.I.; Buring, J.E.; Lee, I.M.; Cheng, S.; Manson, J.E.; Mora, S. Circulating branched-chain amino acids and long-term risk of obesity-related cancers in women. Sci. Rep. 2020, 10, 16534. [Google Scholar] [CrossRef] [PubMed]
- Gojda, J.; Strakova, R.; Plihalova, A.; Tuma, P.; Potockova, J.; Polak, J.; Andel, M. Increased Incretin But Not Insulin Response after Oral versus Intravenous Branched Chain Amino Acids. Ann. Nutr. Metab. 2017, 70, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Felig, P.; Hagenfeldt, L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J. Clin. Investig. 1976, 57, 987–999. [Google Scholar] [CrossRef]
- Vellai, T. How the amino acid leucine activates the key cell-growth regulator mTOR. Nature 2021, 596, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.; Zampieri, T.T.; Donato, J., Jr. Reviewing the Effects of L-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis. Nutrients 2015, 7, 3914–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.T. Metabolism of BCAAs. In Branched Chain Amino Acids in Clinical Nutrition; Rajendram, R., Preedy, V.R., Patel, V.B., Eds.; Humana Press: Totowa, NJ, USA, 2015; Volume 1, pp. 13–24. [Google Scholar]
- Tremblay, F.; Krebs, M.; Dombrowski, L.; Brehm, A.; Bernroider, E.; Roth, E.; Nowotny, P.; Waldhausl, W.; Marette, A.; Roden, M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005, 54, 2674–2684. [Google Scholar] [CrossRef] [Green Version]
- Nair, K.S.; Woolf, P.D.; Welle, S.L.; Matthews, D.E. Leucine, glucose, and energy metabolism after 3 days of fasting in healthy human subjects. Am. J. Clin. Nutr. 1987, 46, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Kamaura, M.; Nishijima, K.; Takahashi, M.; Ando, T.; Mizushima, S.; Tochikubo, O. Lifestyle modification in metabolic syndrome and associated changes in plasma amino acid profiles. Circ. J. 2010, 74, 2434–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurtz, P.; Makinen, V.P.; Soininen, P.; Kangas, A.J.; Tukiainen, T.; Kettunen, J.; Savolainen, M.J.; Tammelin, T.; Viikari, J.S.; Ronnemaa, T.; et al. Metabolic signatures of insulin resistance in 7098 young adults. Diabetes 2012, 61, 1372–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Lips, M.A.; Van Klinken, J.B.; van Harmelen, V.; Dharuri, H.K.; AC’t Hoen, P.A.; Laros, J.F.; van Ommen, G.J.; Janssen, I.M.; Van Ramshorst, B.; Van Wagensveld, B.A.; et al. Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care 2014, 37, 3150–3156. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Brameld, J.M.; Parr, T.; Murton, A.J. Leucine and mTORc1 act independently to regulate 2-deoxyglucose uptake in L6 myotubes. Amino Acids 2020, 52, 477–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Biswas, D.; Duffley, L.; Pulinilkunnil, T. Role of branched-chain amino acid-catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. FASEB J. 2019, 33, 8711–8731. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Yao, W.; He, Q.; Shao, Y.; Zheng, R.; Huang, F. L-leucine stimulates glutamate dehydrogenase activity and glutamate synthesis by regulating mTORC1/SIRT4 pathway in pig liver. Anim. Nutr. 2018, 4, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Bermudez-Silva, F.J.; Andre, C.; Elie, M.; Romero-Zerbo, S.Y.; Leste-Lasserre, T.; Belluomo, I.; Duchampt, A.; Clark, S.; Aubert, A.; et al. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS ONE 2013, 8, e74705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, K.; Yu, Y.H.; Hou, J.; Zhang, Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr. Metab. 2010, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hey, P.; Gow, P.; Testro, A.G.; Apostolov, R.; Chapman, B.; Sinclair, M. Nutraceuticals for the treatment of sarcopenia in chronic liver disease. Clin. Nutr. ESPEN 2021, 41, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; van Loon, L.J. Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr. Rev. 2011, 69, 675–689. [Google Scholar] [CrossRef]
- Maykish, A.; Sikalidis, A.K. Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia-Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. J. Pers. Med. 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, J.D.P.; Howell, S.L.; Teixeira, F.J.; Pimentel, G.D. Dietary Amino Acids and Immunonutrition Supplementation in Cancer-Induced Skeletal Muscle Mass Depletion: A Mini-Review. Curr. Pharm. Des. 2020, 26, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Boulet, M.M.; Chevrier, G.; Grenier-Larouche, T.; Pelletier, M.; Nadeau, M.; Scarpa, J.; Prehn, C.; Marette, A.; Adamski, J.; Tchernof, A. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E736–E746. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Xie, G.; Jia, W.; Jia, W. Insulin resistance and the metabolism of branched-chain amino acids. Front. Med. 2013, 7, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crown, S.B.; Marze, N.; Antoniewicz, M.R. Catabolism of Branched Chain Amino Acids Contributes Significantly to Synthesis of Odd-Chain and Even-Chain Fatty Acids in 3T3-L1 Adipocytes. PLoS ONE 2015, 10, e0145850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.E. Observations of branched-chain amino acid administration in humans. J. Nutr. 2005, 135, 1580S–1584S. [Google Scholar] [CrossRef] [Green Version]
- Gojda, J.; Waldauf, P.; Hruskova, N.; Blahutova, B.; Krajcova, A.; Urban, T.; Tuma, P.; Rasova, K.; Duska, F. Lactate production without hypoxia in skeletal muscle during electrical cycling: Crossover study of femoral venous-arterial differences in healthy volunteers. PLoS ONE 2019, 14, e0200228. [Google Scholar] [CrossRef] [Green Version]
- Everman, S.; Meyer, C.; Tran, L.; Hoffman, N.; Carroll, C.C.; Dedmon, W.L.; Katsanos, C.S. Insulin does not stimulate muscle protein synthesis during increased plasma branched-chain amino acids alone but still decreases whole body proteolysis in humans. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E671–E677. [Google Scholar] [CrossRef] [Green Version]
- Calbet, J.A.; MacLean, D.A. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J. Nutr. 2002, 132, 2174–2182. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, M.; Stenberg, M.; Frid, A.H.; Holst, J.J.; Bjorck, I.M. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: The role of plasma amino acids and incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.L.; Li, S.Z.; Xiao, P.T.; Cai, Y.Y.; Chu, C.; Chen, B.Z.; Li, P.; Li, J.; Liu, E.H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tome, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tome, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef]
- Reitzer, L. Biosynthesis of Glutamate, Aspartate, Asparagine, L-Alanine, and D-Alanine. EcoSal Plus 2004, 1. [Google Scholar] [CrossRef] [PubMed]
- Torrallardona, D.; Harris, C.I.; Coates, M.E.; Fuller, M.F. Microbial amino acid synthesis and utilization in rats: Incorporation of 15N from 15NH4Cl into lysine in the tissues of germ-free and conventional rats. Br. J. Nutr. 1996, 76, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metges, C.C.; El-Khoury, A.E.; Henneman, L.; Petzke, K.J.; Grant, I.; Bedri, S.; Pereira, P.P.; Ajami, A.M.; Fuller, M.F.; Young, V.R. Availability of intestinal microbial lysine for whole body lysine homeostasis in human subjects. Am. J. Physiol. 1999, 277, E597–E607. [Google Scholar] [CrossRef] [PubMed]
- Metges, C.C.; Petzke, K.J.; Hennig, U. Gas chromatography/combustion/isotope ratio mass spectrometric comparison of N-acetyl- and N-pivaloyl amino acid esters to measure 15N isotopic abundances in physiological samples: A pilot study on amino acid synthesis in the upper gastro-intestinal tract of minipigs. J. Mass Spectrom. 1996, 31, 367–376. [Google Scholar] [CrossRef]
- Millward, D.J.; Forrester, T.; Ah-Sing, E.; Yeboah, N.; Gibson, N.; Badaloo, A.; Boyne, M.; Reade, M.; Persaud, C.; Jackson, A. The transfer of 15N from urea to lysine in the human infant. Br. J. Nutr. 2000, 83, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef]
- Sridharan, G.V.; Choi, K.; Klemashevich, C.; Wu, C.; Prabakaran, D.; Pan, L.B.; Steinmeyer, S.; Mueller, C.; Yousofshahi, M.; Alaniz, R.C.; et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat. Commun. 2014, 5, 5492. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.A.; Macfarlane, G.T. Enumeration of human colonic bacteria producing phenolic and indolic compounds: Effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J. Appl. Bacteriol. 1996, 81, 288–302. [Google Scholar] [CrossRef]
- Dai, Z.L.; Wu, G.; Zhu, W.Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, C.; Macfarlane, G.T. Influence of pH, nutrient availability, and growth rate on amine production by Bacteroides fragilis and Clostridium perfringens. Appl. Environ. Microbiol. 1989, 55, 2894–2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tome, D. Re-print of “Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host”. Pharmacol. Res. 2013, 69, 114–126. [Google Scholar] [CrossRef]
- Aguirre, M.; Eck, A.; Koenen, M.E.; Savelkoul, P.H.; Budding, A.E.; Venema, K. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res. Microbiol. 2016, 167, 114–125. [Google Scholar] [CrossRef]
- Kaiser, J.C.; Heinrichs, D.E. Branching Out: Alterations in Bacterial Physiology and Virulence Due to Branched-Chain Amino Acid Deprivation. mBio 2018, 9, e01188-18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Branched-chain amino acid catabolism of Thermoanaerobacter pseudoethanolicus reveals potential route to branched-chain alcohol formation. Extremophiles 2020, 24, 121–133. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, Q.; Zhang, Y.; Gao, H. Derepression of bkd by the FadR loss dictates elevated production of BCFAs and isoleucine starvation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158577. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Fernandez, E.N.; Putz, P.; Rowland, I.; Swann, J.; Turk, J.; Vermeiren, J.; et al. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur. J. Nutr. 2018, 57, 25–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottosson, F.; Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Almgren, P.; Fernandez, C.; Melander, O.; Orho-Melander, M. Connection Between BMI-Related Plasma Metabolite Profile and Gut Microbiota. J. Clin. Endocrinol. Metab. 2018, 103, 1491–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Org, E.; Blum, Y.; Kasela, S.; Mehrabian, M.; Kuusisto, J.; Kangas, A.J.; Soininen, P.; Wang, Z.; Ala-Korpela, M.; Hazen, S.L.; et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 2017, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wan, Y.; Yin, K.; Wei, Y.; Wang, B.; Yu, X.; Ni, Y.; Zheng, J.; Huang, T.; Song, M.; et al. Lower Circulating Branched-Chain Amino Acid Concentrations Among Vegetarians are Associated with Changes in Gut Microbial Composition and Function. Mol. Nutr. Food Res. 2019, 63, e1900612. [Google Scholar] [CrossRef]
- Mesnage, R.; Grundler, F.; Schwiertz, A.; Le Maho, Y.; Wilhelmi de Toledo, F. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J. Nutr. Sci. 2019, 8, e36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yue, Y.; Shi, M.; Tian, M.; Ji, J.; Liao, X.; Hu, X.; Chen, F. Dietary Luffa cylindrica (L.) Roem promotes branched-chain amino acid catabolism in the circulation system via gut microbiota in diet-induced obese mice. Food Chem. 2020, 320, 126648. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Yin, J.; Nie, S. Multiomics Approach to Explore the Amelioration Mechanisms of Glucomannans on the Metabolic Disorder of Type 2 Diabetic Rats. J. Agric. Food Chem. 2021, 69, 2632–2645. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, S.J.; Liu, J.; Wang, A.T.; Meng, X.T.; Yang, Z.R.; Peng, C.; Guan, H.S.; Wang, C.Y.; Yan, D. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E73–E85. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Xia, B.; Tang, S.; Liu, L.; Xie, J.; Zhang, H. Bioregional Alterations in Gut Microbiome Contribute to the Plasma Metabolomic Changes in Pigs Fed with Inulin. Microorganisms 2020, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; He, Z.; Li, J.; Gong, S.; Yuan, S.; Li, T.; Ning, N.; Xing, L.; Zhang, L.; Chen, F.; et al. Gentamicin Induced Microbiome Adaptations Associate With Increased BCAA Levels and Enhance Severity of Influenza Infection. Front. Immunol. 2020, 11, 608895. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).