Visual System Impairment in a Mouse Model of Krabbe Disease: The Twitcher Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Visual Cortex Recording
2.3. Immunohistochemistry and Confocal Imaging
2.4. Western Blot
2.5. Psychosine and GALC Activity Quantification in TWI Tissues
2.6. Statistical Analysis
3. Results
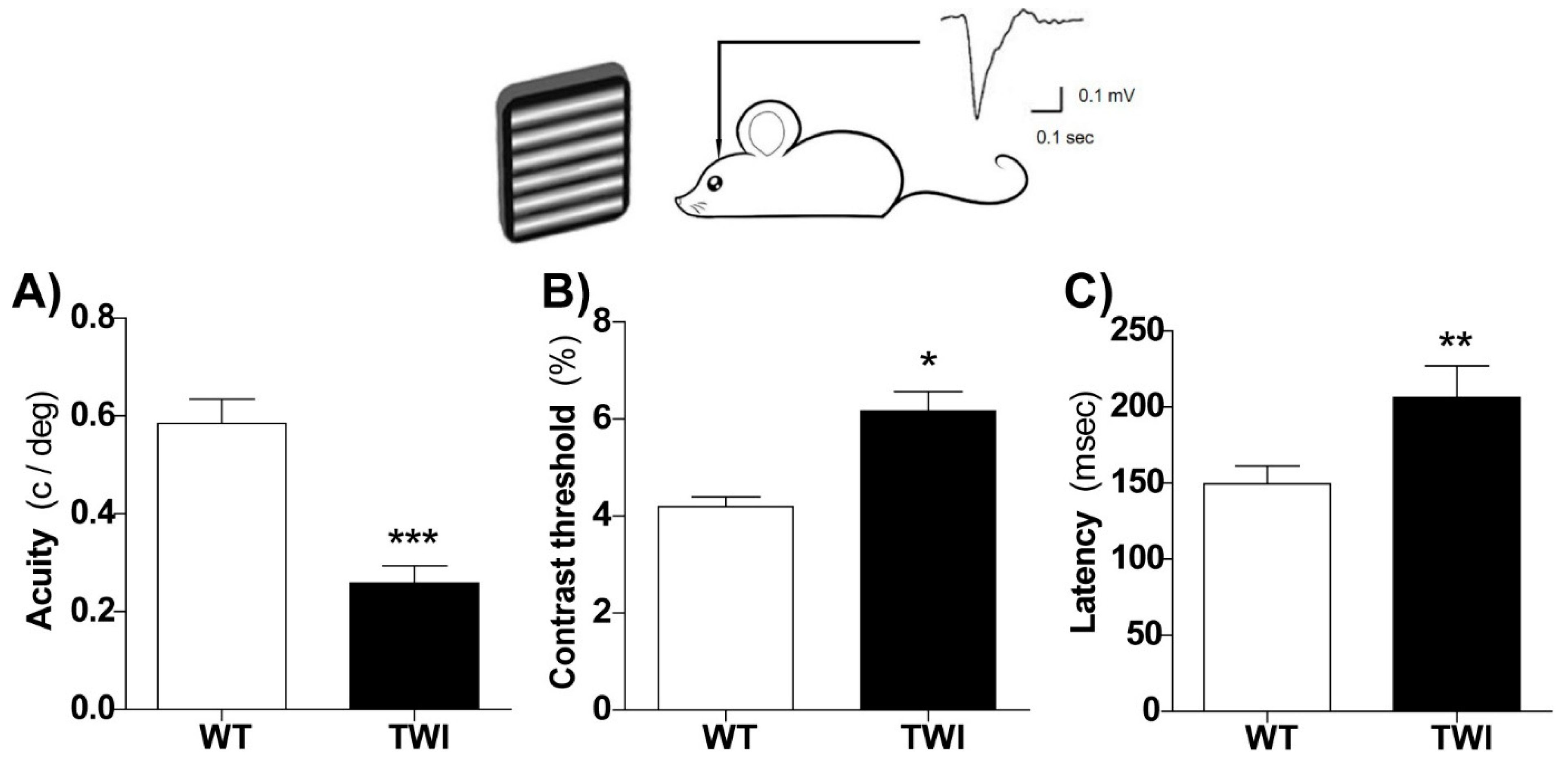
3.1. Functional Alterations in the Visual System of the Twitcher Mouse
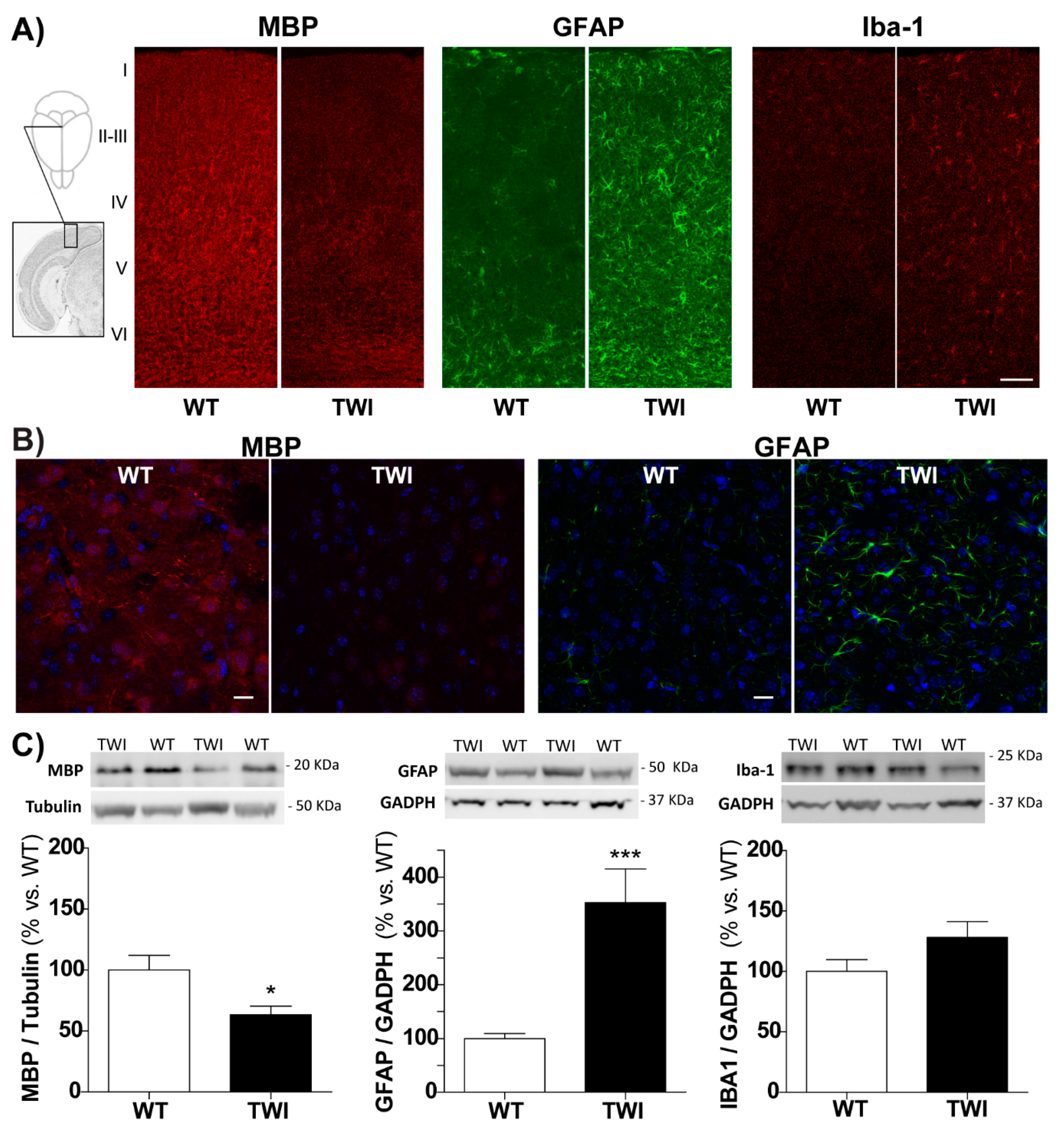
3.2. Demyelination and Gliosis in Twitcher Mice
3.3. Psychosine Accumulation in Twitcher Tissues
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Calderwood, L.; Wenger, D.A.; Matern, D.; Dahmoush, H.; Watiker, V.; Lee, C. Rare Saposin A deficiency: Novel variant and psychosine analysis. Mol. Genet. Metab. 2020, 129, 161–164. [Google Scholar] [CrossRef]
- Giussani, P.; Prinetti, A.; Tringali, C. The role of Sphingolipids in myelination and myelin stability and their involvement in childhood and adult demyelinating disorders. J. Neurochem. 2020, 1–12. [Google Scholar] [CrossRef]
- White, A.B.; Givogri, M.I.; Lopez-Rosas, A.; Cao, H.; Van Breemen, R.; Thinakaran, G. Psychosine accumulates in membrane microdomains in the brain of Krabbe patients, disrupting the raft architecture. J. Neurosci. 2009, 29, 6068–6077. [Google Scholar] [CrossRef] [PubMed]
- White, A.B.; Givogri, M.I.; Lopez-Rosas, A.; Cao, H.; van Breemen, R.; Thinakaran, G.; Bongarzone, E.R. Persistence of psychosine in brain lipid rafts is a limiting factor in the therapeutic recovery of a mouse model for Krabbe disease. J. Neurosci. Res. 2011, 89, 6068–6077. [Google Scholar] [CrossRef] [PubMed]
- Del Grosso, A.; Antonini, S.; Angella, L.; Tonazzini, I.; Signore, G.; Cecchini, M. Lithium improves cell viability in psychosine-treated MO3.13 human oligodendrocyte cell line via autophagy activation. J. Neurosci. Res. 2016, 94, 1246–1260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Benitez, B.A.; Nagree, M.S.; Dearborn, J.T.; Jiang, X.; Guzman, M.A.; Woloszynek, J.C.; Giaramita, A.; Yip, B.K.; et al. Genetic ablation of acid ceramidase in Krabbe disease confirms the psychosine hypothesis and identifies a new therapeutic target. Proc. Natl. Acad. Sci. USA 2019, 116, 20097–20103. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Bongarzone, E.R. Synaptic failure: The achilles tendon of sphingolipidoses. J. Neurosci. Res. 2016, 94, 1031–1036. [Google Scholar] [CrossRef]
- Voccoli, V.; Tonazzini, I.; Signore, G.; Caleo, M.; Cecchini, M. Role of extracellular calcium and mitochondrial oxygen species in psychosine-induced oligodendrocyte cell death. Cell. Death Dis. 2014, 5, e1529. [Google Scholar] [CrossRef]
- de Vito, G.; Cappello, V.; Tonazzini, I.; Cecchini, M.; Piazza, V. RP-CARS reveals molecular spatial order anomalies in myelin of an animal model of Krabbe disease. J. Biophotonics 2017, 10, 385–389. [Google Scholar] [CrossRef]
- Graziano, A.C.E.; Cardile, V. History, genetic, and recent advances on Krabbe disease. Gene 2015, 555, 2–13. [Google Scholar] [CrossRef]
- Parlanti, P.; Cappello, V.; Brun, F.; Tromba, G.; Rigolio, R.; Tonazzini, I.; Cecchini, M.; Piazza, V.; Gemmi, M. Size and specimen-dependent strategy for x-ray micro-ct and tem correlative analysis of nervous system samples. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Megha, J.; De Jesus, O. “Krabbe Disease.” StatPearls. 2020. Available online: https://pubmed.ncbi.nlm.nih.gov/32965986 (accessed on 23 December 2020).
- Wenger, D.A.; Rafi, M.A.; Luzi, P. Krabbe disease: One Hundred years from the bedside to the bench to the bedside. J. Neurosci. Res. 2016, 94, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, B.; Sourander, P.; Svennerholm, L. Diagnosis of Krabbe’s infantile leucodystrophy. J. Neurol. Neurosurg Psychiatry 1963, 26, 195–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beltran-Quintero, M.L.; Bascou, N.A.; Poe, M.D.; Wenger, D.A.; Saavedra-Matiz, C.A.; Nichols, M.J.; Escolar, M.L. Early progression of Krabbe disease in patients with symptom onset between 0 and 5 months. Orphanet J. Rare Dis. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Lyon, G.; Hagberg, B.; Evrard, P.; Allaire, C.; Pavone, L.; Vanier, M. Symptomatology of late onset krabbe’s leukodystrophy: The european experience. Dev Neurosci. 1991, 13, 240–244. [Google Scholar] [CrossRef]
- Bascou, N.; Derenzo, A.; Poe, M.D.; Escolar, M.L. A prospective natural history study of Krabbe disease in a patient cohort with onset between 6 months and 3 years of life. Orphanet J. Rare Dis. 2018, 13, 1–17. [Google Scholar] [CrossRef]
- Debs, R.; Froissart, R.; Aubourg, P.; Papeix, C.; Douillard, C.; Degos, B.; Fontaine, B.; Audoin, B.; Lacour, A.; Said, G.; et al. Krabbe disease in adults: Phenotypic and genotypic update from a series of 11 cases and a review. J. Inherit. Metab. Dis. 2013, 36, 859–868. [Google Scholar] [CrossRef]
- Emery, J.M.; Green, W.R.; Huff, D.S. Krabbe’s disease. Histopathology and ultrastructure of the eye. Am. J. Ophthalmol. 1972, 74, 400–406. [Google Scholar] [CrossRef]
- Milton Lima Garcia, A.; Martins Menezes Morais, N.; Ohlweiler, L.; Isabel Bragatti Winckler, M.; Ranzan, J.; Alfonso Pinto Artigalás, O.; Pinto, L.L.; Netto, C.B.; Ashton-Prolla, P.; Vedolin, L.; et al. Optic nerve enlargement and leukodystrophy An unusual finding of the infantile form of Krabbe disease EspEssamEnto dE vias óptiCas E LEuCodistrofia: Um aChado pouCo frEqüEntE da forma infantiL. Arq. Neuropsiquiatr. 2010, 68, 816–818. [Google Scholar] [CrossRef][Green Version]
- Brodsky, M.C.; Hunter, J.S. Positional ocular flutter and thickened optic nerves as sentinel signs of Krabbe disease. J. AAPOS 2011, 15, 595–597. [Google Scholar] [CrossRef]
- Aldosari, M.; Altuwaijri, M.; Husain, A.M. Brain-stem auditory and visual evoked potentials in children with Krabbe disease. Clin. Neurophysiol. 2004, 115, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, K. The Twitcher Mouse: A Model for Krabbe Disease and for Experimental Therapies. Brain Pathol. 1995, 5, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, C.E. Neurological and neurobehavioral development of the mutant “twitcher” mouse. Behav. Brain Res. 1987, 25, 143–153. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, K. Myelin Pathology in the Twitcher Mouse. Ann. N. Y. Acad. Sci. 1990, 605, 313–324. [Google Scholar] [CrossRef]
- Grosso, A.; Galliani, M.; Angella, L.; Santi, M.; Tonazzini, I.; Parlanti, G.; Signore, G.; Cecchini, M. Brain-targeted enzyme-loaded nanoparticles: A breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease. Sci. Adv. 2019, 5, eaax7462. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.S.; Issa, Y.; Jakubauskas, B.; Stoskute, M.; Elackattu, V.; Marshall, J.N.; Bogue, W.; Nguyen, D.; Hauck, Z.; Rue, E.; et al. Long-Term Improvement of Neurological Signs and Metabolic Dysfunction in a Mouse Model of Krabbe’s Disease after Global Gene Therapy. Mol. Ther. 2018, 26, 874–889. [Google Scholar] [CrossRef]
- Kalatzis, V. The ocular anomalies in a cystinosis animal model mimic disease pathogenesis (Pediatric Research 62, (156–162)). Pediatr. Res. 2007, 62, 558. [Google Scholar] [CrossRef]
- Simpson, J.; Nien, C.J.; Flynn, K.; Jester, B.; Cherqui, S.; Jester, J. Quantitative in vivo and ex vivo confocal microscopy analysis of corneal cystine crystals in the Ctns -/- knockout mouse. Mol. Vis. 2011, 17, 2212–2220. [Google Scholar]
- Denny, C.A.; Alroy, J.; Pawlyk, B.S.; Sandberg, M.A.; D’Azzo, A.; Seyfried, T.N. Neurochemical, morphological, and neurophysiological abnormalities in retinas of Sandhoff and GM1 gangliosidosis mice. J. Neurochem. 2007, 101, 1294–1302. [Google Scholar] [CrossRef]
- Intartaglia, D.; Giamundo, G.; Marrocco, E.; Maffia, V.; Salierno, F.G.; Nusco, E. Retinal Degeneration in MPS-IIIA Mouse Model. Front. Cell Dev. Biol. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Sakai, N.; Inui, K.; Tatsumi, N.; Fukushima, H.; Nishigaki, T.; Taniike, M.; Nishimoto, J.; Tsukamoto, H.; Yanagihara, I.; Ozono, K.; et al. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe’s disease. J. Neurochem. 1996, 66, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Drechsel, D.; Schmid, M.T.; Ninkovic, J.; Irmler, M.; Brill, M.S.; Restani, L.; Gianfranceschi, L.; Cerri, C.; Weber, S.N.; et al. AP2γ regulates basal progenitor fate in a region- and layer-specific manner in the developing cortex. Nat. Neurosci. 2009, 12, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; Genovesi, S.; Maggia, M.; Cenni, M.C.; Zunino, G.; Sgadò, P.; Caleo, M.; Bozzi, Y. Altered GABAergic markers, increased binocularity and reduced plasticity in the visual cortex of engrailed-2 knockout mice. Front. Cell Neurosci. 2014, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Del Grosso, A.; Angella, L.; Tonazzini, I.; Moscardini, A.; Giordano, N.; Caleo, M.; Rocchiccioli, S.; Cecchini, M. Dysregulated autophagy as a new aspect of the molecular pathogenesis of Krabbe disease. Neurobiol. Dis. 2019, 129, 195–207. [Google Scholar] [CrossRef]
- Tonazzini, I.; Van Woerden, G.M.; Masciullo, C.; Mientjes, E.J.; Elgersma, Y.; Cecchini, M. The role of ubiquitin ligase E3A in polarized contact guidance and rescue strategies in UBE3A-deficient hippocampal neurons. Mol. Autism. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Leat, S.J.; Yadav, N.K.; Irving, E.L. Development of visual acuity and contrast sensitivity in children. J. Optom. 2009, 2, 19–26. [Google Scholar] [CrossRef]
- Gianfranceschi, L.; Fiorentini, A.; Maffei, L. Behavioural visual acuity of wild type and bcl2 transgenic mouse. Vis. Res. 2000, 39, 569–574. [Google Scholar] [CrossRef]
- Porciatti, V.; Pizzorusso, T.; Maffei, L. The visual physiology of the wild type mouse determined with pattern VEPs. Vis. Res. 1999, 39, 3071–3081. [Google Scholar] [CrossRef]
- Al-Essa, M.A.; Bakheet, S.M.; Patay, Z.J.; Powe, J.E.; Ozand, P.T. Clinical and cerebral FDG PET scan in a patient with Krabbe’s disease. Pediatr. Neurol. 2000, 22, 44–47. [Google Scholar] [CrossRef]
- LeBlanc, J.J.; DeGregorio, G.; Centofante, E.; Vogel-Farley, V.K.; Barnes, K.; Kaufmann, W.E.; Fagiolini, M.; Nelson, C.A. Visual evoked potentials detect cortical processing deficits in Rett syndrome. Ann. Neurol. 2015, 78, 775–786. [Google Scholar] [CrossRef]
- Brownstein, S. Optic Nerve in Globoid Leukodystrophy (Krabbe’s Disease). Arch. Ophthalmol. 1978, 96, 864. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, N.I.; Kreher, C.; Favret, J.; Nguyen, D.; Bongarzone, E.R.; Wrabetz, L.; Feltri, M.L.; Shin, D. Brainstem development requires galactosylceramidase and is critical for pathogenesis in a model of Krabbe disease. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.M.; Bagel, J.H.; Nguyen, D.; Lykken, E.A.; Salvador, J.P.; Jiang, X.; Swain, G.P.; Assenmacher, C.A.; Hendricks, I.J.; Miyadera, K.; et al. Krabbe disease successfully treated via monotherapy of intrathecal gene therapy. J. Clin. Investig. 2020, 130, 4906–4920. [Google Scholar] [CrossRef] [PubMed]
- Hennig, A.K.; Levy, B.; Ogilvie, J.M.; Vogler, C.A.; Galvin, N.; Bassnett, S.; Sands, M.S. Intravitreal gene therapy reduces lysosomal storage in specific areas of the CNS in mucopolysaccharidosis VII mice. J. Neurosci. 2003, 23, 3302–3307. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonazzini, I.; Cerri, C.; Del Grosso, A.; Antonini, S.; Allegra, M.; Caleo, M.; Cecchini, M. Visual System Impairment in a Mouse Model of Krabbe Disease: The Twitcher Mouse. Biomolecules 2021, 11, 7. https://doi.org/10.3390/biom11010007
Tonazzini I, Cerri C, Del Grosso A, Antonini S, Allegra M, Caleo M, Cecchini M. Visual System Impairment in a Mouse Model of Krabbe Disease: The Twitcher Mouse. Biomolecules. 2021; 11(1):7. https://doi.org/10.3390/biom11010007
Chicago/Turabian StyleTonazzini, Ilaria, Chiara Cerri, Ambra Del Grosso, Sara Antonini, Manuela Allegra, Matteo Caleo, and Marco Cecchini. 2021. "Visual System Impairment in a Mouse Model of Krabbe Disease: The Twitcher Mouse" Biomolecules 11, no. 1: 7. https://doi.org/10.3390/biom11010007
APA StyleTonazzini, I., Cerri, C., Del Grosso, A., Antonini, S., Allegra, M., Caleo, M., & Cecchini, M. (2021). Visual System Impairment in a Mouse Model of Krabbe Disease: The Twitcher Mouse. Biomolecules, 11(1), 7. https://doi.org/10.3390/biom11010007








