Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer
Abstract
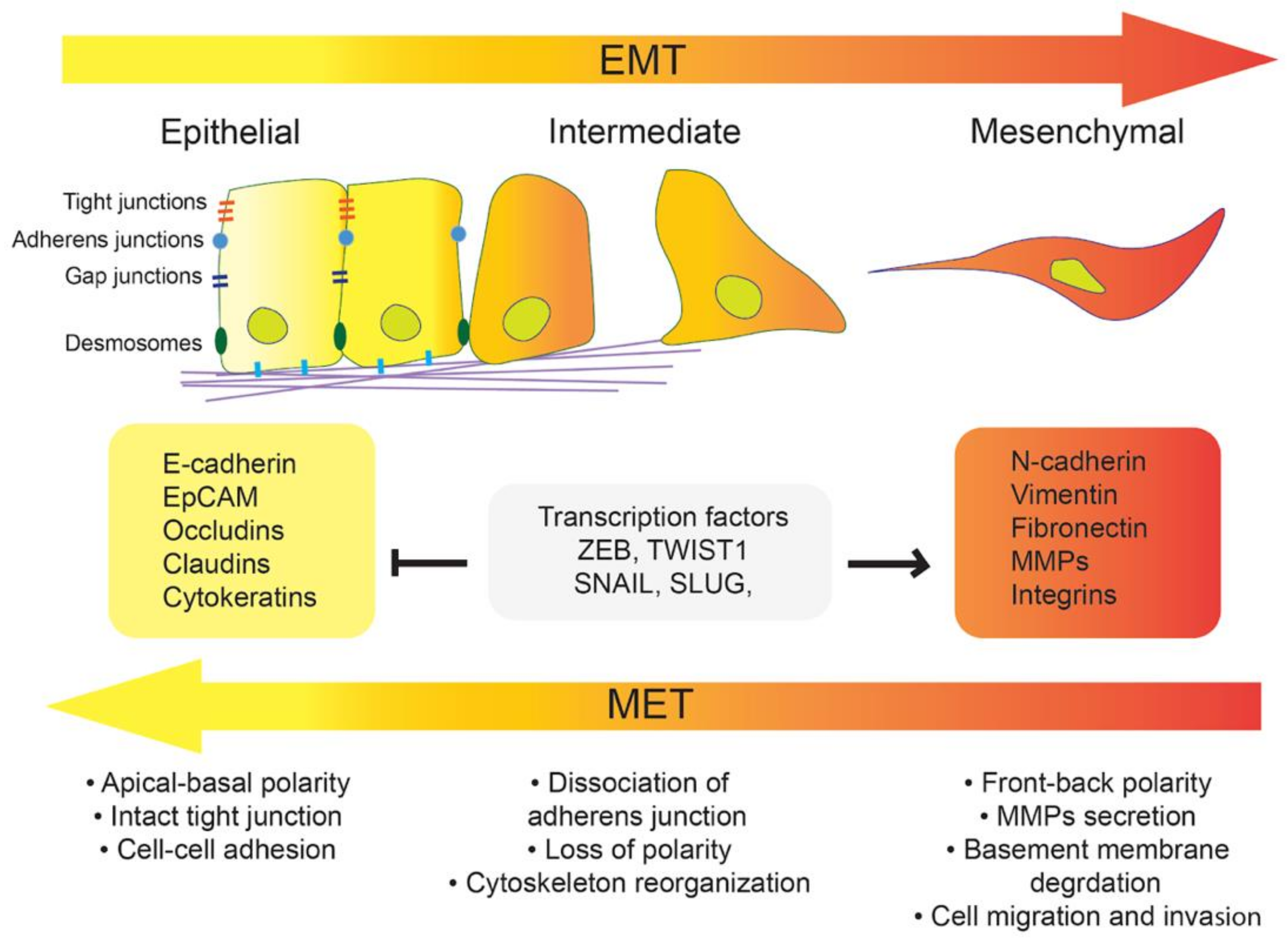
1. Introduction
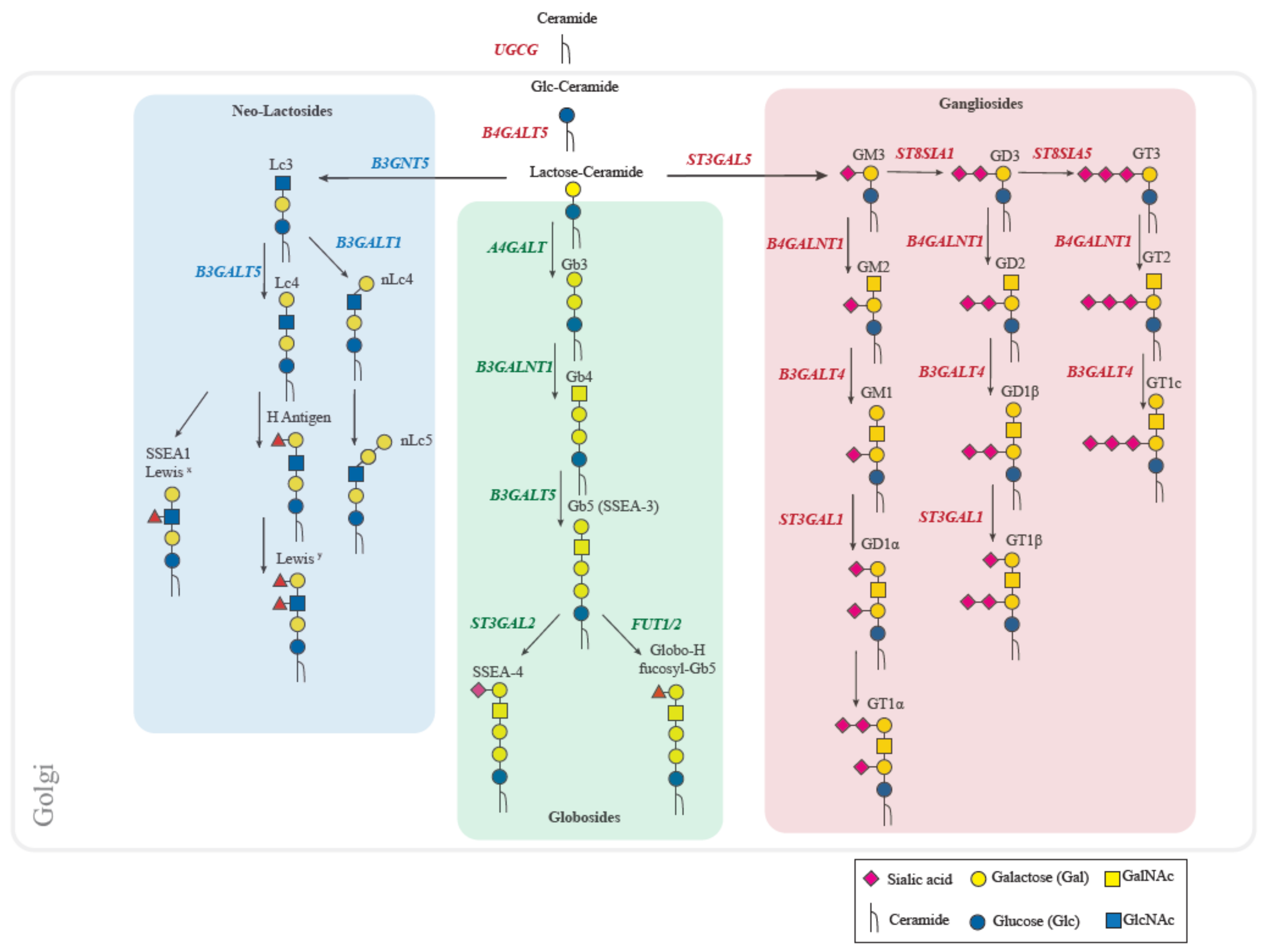
2. Human Glycosphingolipids Biosynthesis
3. Globo-Series GSLs
3.1. Gb3 and Gb4
3.2. SSEA3, SSEA4, and GloboH
4. Ganglio-Series GSLs
4.1. Monosialylated Gangliosides
4.2. Disialylated Gangliosides
5. An Overview of Current Methodologies to Characterize GSLs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trelstad, R.L.; Hay, E.D.; Revel, J.D. Cell contact during early morphogenesis in the chick embryo. Dev. Biol. 1967, 16, 78–106. [Google Scholar] [CrossRef]
- Nakaya, Y.; Sheng, G. Epithelial to mesenchymal transition during gastrulation: An embryological view. Dev. Growth Differ 2008, 50, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Greenburg, G.; Hay, E.D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol. 1982, 95, 333–339. [Google Scholar] [CrossRef]
- Jordan, N.V.; Johnson, G.L.; Abell, A.N. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle 2011, 10, 2865–2873. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Matsuda, M.; Furuse, M.; Tsukita, S. Regulation of tight junctions during the epithelium-mesenchyme transition: Direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003, 116, 1959–1967. [Google Scholar] [CrossRef]
- Martinez-Estrada, O.M.; Culleres, A.; Soriano, F.X.; Peinado, H.; Bolos, V.; Martinez, F.O.; Reina, M.; Cano, A.; Fabre, M.; Vilaro, S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem. J. 2006, 394, 449–457. [Google Scholar] [CrossRef]
- Tobioka, H.; Isomura, H.; Kokai, Y.; Tokunaga, Y.; Yamaguchi, J.; Sawada, N. Occludin expression decreases with the progression of human endometrial carcinoma. Hum. Pathol. 2004, 35, 159–164. [Google Scholar] [CrossRef]
- Suh, Y.; Yoon, C.H.; Kim, R.K.; Lim, E.J.; Oh, Y.S.; Hwang, S.G.; An, S.; Yoon, G.; Gye, M.C.; Yi, J.M.; et al. Claudin-1 induces epithelial-mesenchymal transition through activation of the c-Abl-ERK signaling pathway in human liver cells. Oncogene 2013, 32, 4873–4882. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, A.; Berx, G. Cadherins and epithelial-to-mesenchymal transition. Prog. Mol. Biol. Transl. Sci. 2013, 116, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Bierie, B.; Kober, K.I.; Thiru, P.; Krall, J.A.; Zill, C.; Reinhardt, F.; Tam, W.L.; Weinberg, R.A. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 2016, 351, aad3680. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Ballestar, E.; Esteller, M.; Cano, A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell Biol. 2004, 24, 306–319. [Google Scholar] [CrossRef]
- Comijn, J.; Berx, G.; Vermassen, P.; Verschueren, K.; van Grunsven, L.; Bruyneel, E.; Mareel, M.; Huylebroeck, D.; van Roy, F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 2001, 7, 1267–1278. [Google Scholar] [CrossRef]
- Medici, D.; Hay, E.D.; Olsen, B.R. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol. Biol. Cell 2008, 19, 4875–4887. [Google Scholar] [CrossRef]
- Mendez, M.G.; Kojima, S.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lin, H.H.; Tang, M.J.; Wang, Y.K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Gravdal, K.; Halvorsen, O.J.; Haukaas, S.A.; Akslen, L.A. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin. Cancer Res. 2007, 13, 7003–7011. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; de Herreros, A.G. Epithelial-mesenchymal transition in cancer. Mol. Oncol. 2017, 11, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Shu, X.; Gassama-Diagne, A.; Thiery, J.P. Mesenchymal-epithelial transition in development and reprogramming. Nat. Cell Biol. 2019, 21, 44–53. [Google Scholar] [CrossRef]
- Regina Todeschini, A.; Hakomori, S.I. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Giussani, P.; Tringali, C.; Riboni, L.; Viani, P.; Venerando, B. Sphingolipids: Key regulators of apoptosis and pivotal players in cancer drug resistance. Int. J. Mol. Sci. 2014, 15, 4356–4392. [Google Scholar] [CrossRef]
- Zhang, T.; van Die, I.; Tefsen, B.; van Vliet, S.J.; Laan, L.C.; Zhang, J.; Ten Dijke, P.; Wuhrer, M.; Belo, A.I. Differential O- and Glycosphingolipid Glycosylation in Human Pancreatic Adenocarcinoma Cells with Opposite Morphology and Metastatic Behavior. Front. Oncol. 2020, 10, 732. [Google Scholar] [CrossRef]
- Jacob, F.; Alam, S.; Konantz, M.; Liang, C.Y.; Kohler, R.S.; Everest-Dass, A.V.; Huang, Y.L.; Rimmer, N.; Fedier, A.; Schotzau, A.; et al. Transition of Mesenchymal and Epithelial Cancer Cells Depends on alpha1-4 Galactosyltransferase-Mediated Glycosphingolipids. Cancer Res. 2018, 78, 2952–2965. [Google Scholar] [CrossRef]
- Liang, Y.J.; Ding, Y.; Levery, S.B.; Lobaton, M.; Handa, K.; Hakomori, S.I. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 4968–4973. [Google Scholar] [CrossRef]
- Russo, D.; Capolupo, L.; Loomba, J.S.; Sticco, L.; D’Angelo, G. Glycosphingolipid metabolism in cell fate specification. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Capasso, S.; Sticco, L.; Russo, D. Glycosphingolipids: Synthesis and functions. FEBS J. 2013, 280, 6338–6353. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, H.J.; Quiroga, R.; Ferrari, M.L. Cellular and molecular biology of glycosphingolipid glycosylation. J. Neurochem. 2011, 117, 589–602. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Polishchuk, E.; Di Tullio, G.; Santoro, M.; Di Campli, A.; Godi, A.; West, G.; Bielawski, J.; Chuang, C.C.; van der Spoel, A.C.; et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 2007, 449, 62–67. [Google Scholar] [CrossRef]
- Halter, D.; Neumann, S.; van Dijk, S.M.; Wolthoorn, J.; de Maziere, A.M.; Vieira, O.V.; Mattjus, P.; Klumperman, J.; van Meer, G.; Sprong, H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J. Cell Biol. 2007, 179, 101–115. [Google Scholar] [CrossRef]
- Brimble, S.N.; Sherrer, E.S.; Uhl, E.W.; Wang, E.; Kelly, S.; Merrill, A.H., Jr.; Robins, A.J.; Schulz, T.C. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells 2007, 25, 54–62. [Google Scholar] [CrossRef]
- Cheung, S.K.; Chuang, P.K.; Huang, H.W.; Hwang-Verslues, W.W.; Cho, C.H.; Yang, W.B.; Shen, C.N.; Hsiao, M.; Hsu, T.L.; Chang, C.F.; et al. Stage-specific embryonic antigen-3 (SSEA-3) and beta3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proc. Natl. Acad. Sci. USA 2016, 113, 960–965. [Google Scholar] [CrossRef]
- Chang, W.W.; Lee, C.H.; Lee, P.; Lin, J.; Hsu, C.W.; Hung, J.T.; Lin, J.J.; Yu, J.C.; Shao, L.E.; Yu, J.; et al. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 11667–11672. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Tsai, Y.T.; Ariga, T.; Yanagisawa, M. Structures, biosynthesis, and functions of gangliosides—An overview. J. Oleo Sci. 2011, 60, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Holst, S.; Stavenhagen, K.; Balog, C.I.; Koeleman, C.A.; McDonnell, L.M.; Mayboroda, O.A.; Verhoeven, A.; Mesker, W.E.; Tollenaar, R.A.; Deelder, A.M.; et al. Investigations on aberrant glycosylation of glycosphingolipids in colorectal cancer tissues using liquid chromatography and matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS). Mol. Cell Proteom. 2013, 12, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Falguieres, T.; Maak, M.; von Weyhern, C.; Sarr, M.; Sastre, X.; Poupon, M.F.; Robine, S.; Johannes, L.; Janssen, K.P. Human colorectal tumors and metastases express Gb3 and can be targeted by an intestinal pathogen-based delivery tool. Mol. Cancer Ther. 2008, 7, 2498–2508. [Google Scholar] [CrossRef]
- Kovbasnjuk, O.; Mourtazina, R.; Baibakov, B.; Wang, T.; Elowsky, C.; Choti, M.A.; Kane, A.; Donowitz, M. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 19087–19092. [Google Scholar] [CrossRef]
- Tan, T.Z.; Miow, Q.H.; Miki, Y.; Noda, T.; Mori, S.; Huang, R.Y.; Thiery, J.P. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol. Med. 2014, 6, 1279–1293. [Google Scholar] [CrossRef]
- Viel, T.; Dransart, E.; Nemati, F.; Henry, E.; Theze, B.; Decaudin, D.; Lewandowski, D.; Boisgard, R.; Johannes, L.; Tavitian, B. In vivo tumor targeting by the B-subunit of shiga toxin. Mol. Imaging 2008, 7, 239–247. [Google Scholar] [CrossRef]
- Melton-Celsa, A.R. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Engedal, N.; Skotland, T.; Torgersen, M.L.; Sandvig, K. Shiga toxin and its use in targeted cancer therapy and imaging. Microb. Biotechnol. 2011, 4, 32–46. [Google Scholar] [CrossRef]
- Stimmer, L.; Dehay, S.; Nemati, F.; Massonnet, G.; Richon, S.; Decaudin, D.; Klijanienko, J.; Johannes, L. Human breast cancer and lymph node metastases express Gb3 and can be targeted by STxB-vectorized chemotherapeutic compounds. BMC Cancer 2014, 14, 916. [Google Scholar] [CrossRef] [PubMed]
- Geyer, P.E.; Maak, M.; Nitsche, U.; Perl, M.; Novotny, A.; Slotta-Huspenina, J.; Dransart, E.; Holtorf, A.; Johannes, L.; Janssen, K.P. Gastric Adenocarcinomas Express the Glycosphingolipid Gb3/CD77: Targeting of Gastric Cancer Cells with Shiga Toxin B-Subunit. Mol. Cancer Ther. 2016, 15, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Desselle, A.; Chaumette, T.; Gaugler, M.H.; Cochonneau, D.; Fleurence, J.; Dubois, N.; Hulin, P.; Aubry, J.; Birkle, S.; Paris, F. Anti-Gb3 monoclonal antibody inhibits angiogenesis and tumor development. PLoS ONE 2012, 7, e45423. [Google Scholar] [CrossRef] [PubMed]
- Arab, S.; Russel, E.; Chapman, W.B.; Rosen, B.; Lingwood, C.A. Expression of the verotoxin receptor glycolipid, globotriaosylceramide, in ovarian hyperplasias. Oncol. Res. 1997, 9, 553–563. [Google Scholar] [PubMed]
- Alam, S.; Anugraham, M.; Huang, Y.L.; Kohler, R.S.; Hettich, T.; Winkelbach, K.; Grether, Y.; Lopez, M.N.; Khasbiullina, N.; Bovin, N.V.; et al. Altered (neo-) lacto series glycolipid biosynthesis impairs alpha2-6 sialylation on N-glycoproteins in ovarian cancer cells. Sci. Rep. 2017, 7, 45367. [Google Scholar] [CrossRef]
- Alam, S.; Fedier, A.; Kohler, R.S.; Jacob, F. Glucosylceramide synthase inhibitors differentially affect expression of glycosphingolipids. Glycobiology 2015, 25, 351–356. [Google Scholar] [CrossRef]
- Liang, Y.J.; Kuo, H.H.; Lin, C.H.; Chen, Y.Y.; Yang, B.C.; Cheng, Y.Y.; Yu, A.L.; Khoo, K.H.; Yu, J. Switching of the core structures of glycosphingolipids from globo- and lacto- to ganglio-series upon human embryonic stem cell differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 22564–22569. [Google Scholar] [CrossRef]
- Lin, R.J.; Kuo, M.W.; Yang, B.C.; Tsai, H.H.; Chen, K.; Huang, J.R.; Lee, Y.S.; Yu, A.L.; Yu, J. B3GALT5 knockout alters gycosphingolipid profile and facilitates transition to human naive pluripotency. Proc. Natl. Acad. Sci. USA 2020, 117, 27435–27444. [Google Scholar] [CrossRef]
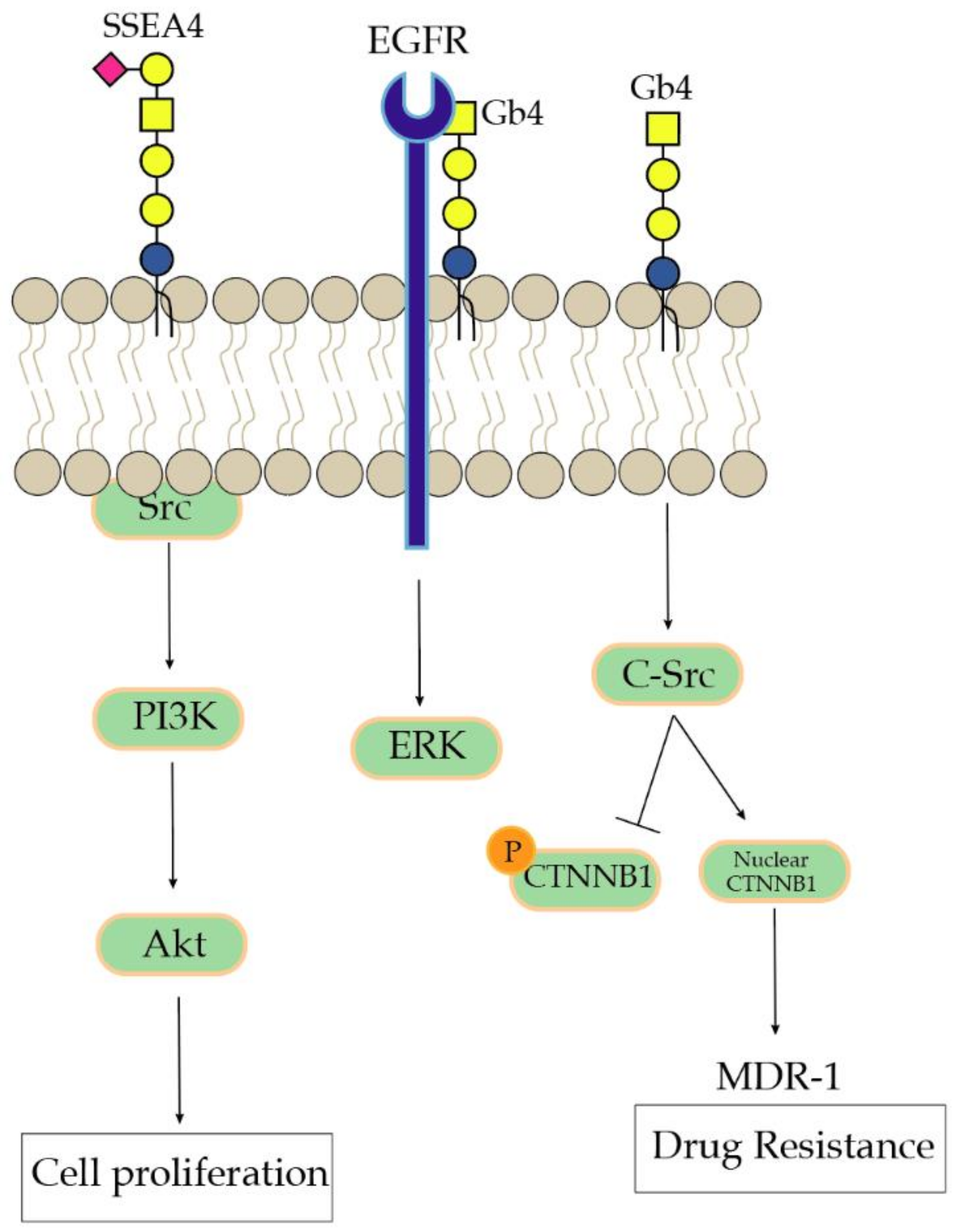
- Park, S.Y.; Kwak, C.Y.; Shayman, J.A.; Kim, J.H. Globoside promotes activation of ERK by interaction with the epidermal growth factor receptor. Biochim. Biophys. Acta 2012, 1820, 1141–1148. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Gupta, V.; Patwardhan, G.A.; Bhinge, K.; Zhao, Y.; Bao, J.; Mehendale, H.; Cabot, M.C.; Li, Y.T.; Jazwinski, S.M. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol. Cancer 2010, 9, 145. [Google Scholar] [CrossRef]
- Gooding, A.J.; Schiemann, W.P. Epithelial-Mesenchymal Transition Programs and Cancer Stem Cell Phenotypes: Mediators of Breast Cancer Therapy Resistance. Mol. Cancer Res. 2020, 18, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramaniyan, K.; Harichandan, A.; Schilbach, K.; Mack, A.F.; Bedke, J.; Stenzl, A.; Kanz, L.; Niederfellner, G.; Buhring, H.J. Expression of stage-specific embryonic antigen-4 (SSEA-4) defines spontaneous loss of epithelial phenotype in human solid tumor cells. Glycobiology 2015, 25, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.W.; Wang, P.Y.; Yeh, S.C.; Chuang, P.K.; Li, S.T.; Wu, C.Y.; Khoo, K.H.; Hsiao, M.; Hsu, T.L.; Wong, C.H. Stage-specific embryonic antigen-4 as a potential therapeutic target in glioblastoma multiforme and other cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chu, T.Y.; Ding, D.C. Human fallopian tube epithelial cells exhibit stemness features, self-renewal capacity, and Wnt-related organoid formation. J. Biomed. Sci. 2020, 27, 32. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, Y.; Hu, Y.; Zhou, C.; Hu, Y.; Chen, H. Stage-specific embryonic antigen 4 expression in epithelial ovarian carcinoma. Int. J. Gynecol Cancer 2010, 20, 958–964. [Google Scholar] [CrossRef]
- Steelant, W.F.; Kawakami, Y.; Ito, A.; Handa, K.; Bruyneel, E.A.; Mareel, M.; Hakomori, S. Monosialyl-Gb5 organized with cSrc and FAK in GEM of human breast carcinoma MCF-7 cells defines their invasive properties. FEBS Lett. 2002, 531, 93–98. [Google Scholar] [CrossRef]
- Van Slambrouck, S.; Steelant, W.F. Clustering of monosialyl-Gb5 initiates downstream signalling events leading to invasion of MCF-7 breast cancer cells. Biochem. J. 2007, 401, 689–699. [Google Scholar] [CrossRef]
- Chuang, P.K.; Hsiao, M.; Hsu, T.L.; Chang, C.F.; Wu, C.Y.; Chen, B.R.; Huang, H.W.; Liao, K.S.; Chen, C.C.; Chen, C.L.; et al. Signaling pathway of globo-series glycosphingolipids and beta1,3-galactosyltransferase V (beta3GalT5) in breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3518–3523. [Google Scholar] [CrossRef]
- Kuo, H.H.; Lin, R.J.; Hung, J.T.; Hsieh, C.B.; Hung, T.H.; Lo, F.Y.; Ho, M.Y.; Yeh, C.T.; Huang, Y.L.; Yu, J.; et al. High expression FUT1 and B3GALT5 is an independent predictor of postoperative recurrence and survival in hepatocellular carcinoma. Sci. Rep. 2017, 7, 10750. [Google Scholar] [CrossRef]
- Suzuki, Y.; Haraguchi, N.; Takahashi, H.; Uemura, M.; Nishimura, J.; Hata, T.; Takemasa, I.; Mizushima, T.; Ishii, H.; Doki, Y.; et al. SSEA-3 as a novel amplifying cancer cell surface marker in colorectal cancers. Int. J. Oncol. 2013, 42, 161–167. [Google Scholar] [CrossRef][Green Version]
- Nitulescu, G.M.; van de Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Gradinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules 2019, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Bobowski, M.; Steenackers, A.; Le Bourhis, X.; Delannoy, P. How Do Gangliosides Regulate RTKs Signaling? Cells 2013, 2, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.W.; Choi, H.J.; Kwak, C.H.; Song, K.H.; Suh, S.J.; Kwon, K.M.; Chang, Y.C.; Park, Y.G.; Chang, H.W.; et al. Ganglioside GM3 participates in the TGF-beta1-induced epithelial-mesenchymal transition of human lens epithelial cells. Biochem. J. 2013, 449, 241–251. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Mi, W.; Yang, J.; Han, F.; Lu, X.; Yu, W. Silencing of GM3 synthase suppresses lung metastasis of murine breast cancer cells. Breast Cancer Res. 2008, 10, R1. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef]
- Toledo, M.S.; Suzuki, E.; Handa, K.; Hakomori, S. Effect of ganglioside and tetraspanins in microdomains on interaction of integrins with fibroblast growth factor receptor. J. Biol. Chem. 2005, 280, 16227–16234. [Google Scholar] [CrossRef]
- Choi, H.J.; Chung, T.W.; Kang, S.K.; Lee, Y.C.; Ko, J.H.; Kim, J.G.; Kim, C.H. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression--transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology 2006, 16, 573–583. [Google Scholar] [CrossRef]
- Sarkar, T.R.; Battula, V.L.; Werden, S.J.; Vijay, G.V.; Ramirez-Pena, E.Q.; Taube, J.H.; Chang, J.T.; Miura, N.; Porter, W.; Sphyris, N.; et al. GD3 synthase regulates epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene 2015, 34, 2958–2967. [Google Scholar] [CrossRef]
- Fukumoto, S.; Mutoh, T.; Hasegawa, T.; Miyazaki, H.; Okada, M.; Goto, G.; Furukawa, K.; Urano, T. GD3 synthase gene expression in PC12 cells results in the continuous activation of TrkA and ERK1/2 and enhanced proliferation. J. Biol. Chem. 2000, 275, 5832–5838. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Yan, Y.; Yuan, B.; Dasgupta, A.; Sun, J.; Mu, H.; Do, K.A.; Ueno, N.T.; Andreeff, M.; Battula, V.L. ST8SIA1 Regulates Tumor Growth and Metastasis in TNBC by Activating the FAK-AKT-mTOR Signaling Pathway. Mol. Cancer Ther. 2018, 17, 2689–2701. [Google Scholar] [CrossRef] [PubMed]
- Bremer, E.G.; Schlessinger, J.; Hakomori, S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J. Biol. Chem. 1986, 261, 2434–2440. [Google Scholar] [PubMed]
- Mirkin, B.L.; Clark, S.H.; Zhang, C. Inhibition of human neuroblastoma cell proliferation and EGF receptor phosphorylation by gangliosides GM1, GM3, GD1A and GT1B. Cell Prolif. 2002, 35, 105–115. [Google Scholar] [CrossRef]
- Mitsuda, T.; Furukawa, K.; Fukumoto, S.; Miyazaki, H.; Urano, T.; Furukawa, K. Overexpression of ganglioside GM1 results in the dispersion of platelet-derived growth factor receptor from glycolipid-enriched microdomains and in the suppression of cell growth signals. J. Biol. Chem. 2002, 277, 11239–11246. [Google Scholar] [CrossRef]
- Guan, F.; Schaffer, L.; Handa, K.; Hakomori, S.I. Functional role of gangliotetraosylceramide in epithelial-to-mesenchymal transition process induced by hypoxia and by TGF-{beta}. FASEB J. 2010, 24, 4889–4903. [Google Scholar] [CrossRef]
- Guan, F.; Handa, K.; Hakomori, S.I. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc. Natl. Acad. Sci. USA 2009, 106, 7461–7466. [Google Scholar] [CrossRef]
- Mutoh, T.; Tokuda, A.; Miyadai, T.; Hamaguchi, M.; Fujiki, N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. USA 1995, 92, 5087–5091. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Izumoto, S.; Suzuki, T.; Kinoshita, M.; Kagawa, N.; Wada, K.; Hashimoto, N.; Maruno, M.; Nakatsuji, Y.; Yoshimine, T. Ganglioside GM3 inhibits proliferation and invasion of glioma. J. Neurooncol. 2005, 71, 99–106. [Google Scholar] [CrossRef]
- Hashiramoto, A.; Mizukami, H.; Yamashita, T. Ganglioside GM3 promotes cell migration by regulating MAPK and c-Fos/AP-1. Oncogene 2006, 25, 3948–3955. [Google Scholar] [CrossRef]
- Chung, T.W.; Kim, S.J.; Choi, H.J.; Kim, K.J.; Kim, M.J.; Kim, S.H.; Lee, H.J.; Ko, J.H.; Lee, Y.C.; Suzuki, A.; et al. Ganglioside GM3 inhibits VEGF/VEGFR-2-mediated angiogenesis: Direct interaction of GM3 with VEGFR-2. Glycobiology 2009, 19, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Mathow, D.; Chessa, F.; Rabionet, M.; Kaden, S.; Jennemann, R.; Sandhoff, R.; Grone, H.J.; Feuerborn, A. Zeb1 affects epithelial cell adhesion by diverting glycosphingolipid metabolism. EMBO Rep. 2015, 16, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Investig. 2012, 122, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Hamamura, K.; Hotta, H.; Matsumoto, Y.; Nishida, Y.; Hattori, H.; Furukawa, K.; Ueda, M.; Furukawa, K. Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Sci. 2012, 103, 1656–1664. [Google Scholar] [CrossRef]
- Vantaku, V.; Donepudi, S.R.; Ambati, C.R.; Jin, F.; Putluri, V.; Nguyen, K.; Rajapakshe, K.; Coarfa, C.; Battula, V.L.; Lotan, Y.; et al. Expression of ganglioside GD2, reprogram the lipid metabolism and EMT phenotype in bladder cancer. Oncotarget 2017, 8, 95620–95631. [Google Scholar] [CrossRef]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside G(D2) in small cell lung cancer cell lines: Enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar]
- Cazet, A.; Bobowski, M.; Rombouts, Y.; Lefebvre, J.; Steenackers, A.; Popa, I.; Guerardel, Y.; Le Bourhis, X.; Tulasne, D.; Delannoy, P. The ganglioside G(D2) induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the G(D3) synthase. Glycobiology 2012, 22, 806–816. [Google Scholar] [CrossRef]
- Cazet, A.; Lefebvre, J.; Adriaenssens, E.; Julien, S.; Bobowski, M.; Grigoriadis, A.; Tutt, A.; Tulasne, D.; Le Bourhis, X.; Delannoy, P. GD(3) synthase expression enhances proliferation and tumor growth of MDA-MB-231 breast cancer cells through c-Met activation. Mol. Cancer Res. 2010, 8, 1526–1535. [Google Scholar] [CrossRef]
- Aloia, A.; Petrova, E.; Tomiuk, S.; Bissels, U.; Deas, O.; Saini, M.; Zickgraf, F.M.; Wagner, S.; Spaich, S.; Sutterlin, M.; et al. The sialyl-glycolipid stage-specific embryonic antigen 4 marks a subpopulation of chemotherapy-resistant breast cancer cells with mesenchymal features. Breast Cancer Res. 2015, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Noto, Z.; Yoshida, T.; Okabe, M.; Koike, C.; Fathy, M.; Tsuno, H.; Tomihara, K.; Arai, N.; Noguchi, M.; Nikaido, T. CD44 and SSEA-4 positive cells in an oral cancer cell line HSC-4 possess cancer stem-like cell characteristics. Oral Oncol. 2013, 49, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Anugraham, M.; Pochechueva, T.; Tse, B.W.; Alam, S.; Guertler, R.; Bovin, N.V.; Fedier, A.; Hacker, N.F.; Huflejt, M.E.; et al. The glycosphingolipid P(1) is an ovarian cancer-associated carbohydrate antigen involved in migration. Br. J. Cancer 2014, 111, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wen, L.; Ma, X.; Chen, Z.; Yu, Y.; Zhu, J.; Wang, Y.; Liu, Z.; Liu, H.; Wu, D.; et al. High expression of lactotriaosylceramide, a differentiation-associated glycosphingolipid, in the bone marrow of acute myeloid leukemia patients. Glycobiology 2012, 22, 930–938. [Google Scholar] [CrossRef]
- Illuzzi, G.; Bernacchioni, C.; Aureli, M.; Prioni, S.; Frera, G.; Donati, C.; Valsecchi, M.; Chigorno, V.; Bruni, P.; Sonnino, S.; et al. Sphingosine kinase mediates resistance to the synthetic retinoid N-(4-hydroxyphenyl)retinamide in human ovarian cancer cells. J. Biol. Chem. 2010, 285, 18594–18602. [Google Scholar] [CrossRef]
- Noll, E.N.; Lin, J.; Nakatsuji, Y.; Miller, R.H.; Black, P.M. GM3 as a novel growth regulator for human gliomas. Exp. Neurol. 2001, 168, 300–309. [Google Scholar] [CrossRef]
- Chung, T.W.; Choi, H.J.; Kim, S.J.; Kwak, C.H.; Song, K.H.; Jin, U.H.; Chang, Y.C.; Chang, H.W.; Lee, Y.C.; Ha, K.T.; et al. The ganglioside GM3 is associated with cisplatin-induced apoptosis in human colon cancer cells. PLoS ONE 2014, 9, e92786. [Google Scholar] [CrossRef]
- Jin, U.H.; Chung, T.W.; Song, K.H.; Kwak, C.H.; Choi, H.J.; Ha, K.T.; Chang, Y.C.; Lee, Y.C.; Kim, C.H. Ganglioside GM3 is required for caffeic acid phenethyl ester-induced megakaryocytic differentiation of human chronic myelogenous leukemia K562 cells. Biochem. Cell Biol. 2014, 92, 243–249. [Google Scholar] [CrossRef]
- Wang, H.; Isaji, T.; Satoh, M.; Li, D.; Arai, Y.; Gu, J. Antitumor effects of exogenous ganglioside GM3 on bladder cancer in an orthotopic cancer model. Urology 2013, 81, 210.e11–210.e15. [Google Scholar] [CrossRef]
- Marijan, S.; Markotic, A.; Mastelic, A.; Rezic-Muzinic, N.; Pilkington, L.I.; Reynisson, J.; Culic, V.C. Glycosphingolipid expression at breast cancer stem cells after novel thieno[2,3-b]pyridine anticancer compound treatment. Sci. Rep. 2020, 10, 11876. [Google Scholar] [CrossRef]
- Yamada, T.; Bando, H.; Takeuchi, S.; Kita, K.; Li, Q.; Wang, W.; Akinaga, S.; Nishioka, Y.; Sone, S.; Yano, S. Genetically engineered humanized anti-ganglioside GM2 antibody against multiple organ metastasis produced by GM2-expressing small-cell lung cancer cells. Cancer Sci. 2011, 102, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.H.; Ryu, J.S.; Kim, C.H.; Ko, K.; Ma, J.Y.; Hwang, K.A.; Choo, Y.K. Relationship between ganglioside expression and anti-cancer effects of the monoclonal antibody against epithelial cell adhesion molecule in colon cancer. Exp. Mol. Med. 2011, 43, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Bos, P.D.; Zhang, X.H.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; van de Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Lee, J.M.; Kwon, K.M.; Kwak, C.H.; Abekura, F.; Park, J.Y.; Cho, S.H.; Lee, K.; Chang, Y.C.; Lee, Y.C.; et al. Exogenous and Endogeneous Disialosyl Ganglioside GD1b Induces Apoptosis of MCF-7 Human Breast Cancer Cells. Int. J. Mol. Sci. 2016, 17, 652. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Wang, C.Y.; Wang, I.A.; Chen, Y.W.; Li, L.T.; Lin, C.Y.; Ho, M.Y.; Chou, T.L.; Wang, Y.H.; Chiou, S.P.; et al. Interaction of glycosphingolipids GD3 and GD2 with growth factor receptors maintains breast cancer stem cell phenotype. Oncotarget 2017, 8, 47454–47473. [Google Scholar] [CrossRef]
- Dobrenkov, K.; Ostrovnaya, I.; Gu, J.; Cheung, I.Y.; Cheung, N.K. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatric Blood Cancer 2016, 63, 1780–1785. [Google Scholar] [CrossRef]
- Yeh, S.C.; Wang, P.Y.; Lou, Y.W.; Khoo, K.H.; Hsiao, M.; Hsu, T.L.; Wong, C.H. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef]
- Webb, T.J.; Li, X.; Giuntoli, R.L., 2nd; Lopez, P.H.; Heuser, C.; Schnaar, R.L.; Tsuji, M.; Kurts, C.; Oelke, M.; Schneck, J.P. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 2012, 72, 3744–3752. [Google Scholar] [CrossRef]
- Hakomori, S.I. Structure and function of glycosphingolipids and sphingolipids: Recollections and future trends. Biochim. Biophys. Acta 2008, 1780, 325–346. [Google Scholar] [CrossRef]
- Taki, T.; Kasama, T.; Handa, S.; Ishikawa, D. A simple and quantitative purification of glycosphingolipids and phospholipids by thin-layer chromatography blotting. Anal. Biochem. 1994, 223, 232–238. [Google Scholar] [CrossRef]
- Taki, T.; Ishikawa, D. TLC blotting: Application to microscale analysis of lipids and as a new approach to lipid-protein interaction. Anal. Biochem. 1997, 251, 135–143. [Google Scholar] [CrossRef]
- Fuchs, B. Analysis of phospolipids and glycolipids by thin-layer chromatography-matrix-assisted laser desorption and ionization mass spectrometry. J. Chromatogr. A 2012, 1259, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.N.; Silva, L.C.; Futerman, A.H.; Prieto, M. Effect of ceramide structure on membrane biophysical properties: The role of acyl chain length and unsaturation. Biochim. Biophys. Acta 2011, 1808, 2753–2760. [Google Scholar] [CrossRef]
- Farwanah, H.; Kolter, T. Lipidomics of glycosphingolipids. Metabolites 2012, 2, 134–164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, A.A.; Joseph, V.; Dan, V.M.; Jaleel, A.; Kumar, T.R.S.; Kartha, C.C. Untargeted metabolomics reveals alterations in metabolites of lipid metabolism and immune pathways in the serum of rats after long-term oral administration of Amalaki rasayana. Mol. Cell Biochem. 2020, 463, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, U.; Daniluk, J.; Kucharski, R.; Kowalczyk, T.; Pietrowska, K.; Samczuk, P.; Filimoniuk, A.; Kretowski, A.; Lebensztejn, D.; Ciborowski, M. Untargeted Metabolomics and Inflammatory Markers Profiling in Children With Crohn’s Disease and Ulcerative Colitis-A Preliminary Study. Inflamm. Bowel Dis. 2019, 25, 1120–1128. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Pryce, J.; Rochfort, S. Comprehensive Characterization of Bovine Milk Lipids: Phospholipids, Sphingolipids, Glycolipids, and Ceramides. J. Agric. Food Chem. 2020, 68, 6726–6738. [Google Scholar] [CrossRef]
- Balis, F.M.; Busch, C.M.; Desai, A.V.; Hibbitts, E.; Naranjo, A.; Bagatell, R.; Irwin, M.; Fox, E. The ganglioside GD2 as a circulating tumor biomarker for neuroblastoma. Pediatric Blood Cancer 2020, 67, e28031. [Google Scholar] [CrossRef]
- Teuber, K.; Schiller, J.; Jakop, U.; Lupold, S.; Orledge, J.M.; Blount, J.D.; Royle, N.J.; Hoodless, A.; Muller, K. MALDI-TOF mass spectrometry as a simple tool to determine the phospholipid/glycolipid composition of sperm: Pheasant spermatozoa as one selected example. Anim. Reprod. Sci. 2011, 123, 270–278. [Google Scholar] [CrossRef]
- Soltwisch, J.; Heijs, B.; Koch, A.; Vens-Cappell, S.; Hohndorf, J.; Dreisewerd, K. MALDI-2 on a Trapped Ion Mobility Quadrupole Time-of-Flight Instrument for Rapid Mass Spectrometry Imaging and Ion Mobility Separation of Complex Lipid Profiles. Anal. Chem. 2020, 92, 8697–8703. [Google Scholar] [CrossRef]
- Anugraham, M.; Everest-Dass, A.V.; Jacob, F.; Packer, N.H. A platform for the structural characterization of glycans enzymatically released from glycosphingolipids extracted from tissue and cells. Rapid Commun. Mass Spectrom. 2015, 29, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Vainauskas, S.; Stockmann, H.; McManus, C.; Taron, C.H.; Rudd, P.M. Comprehensive Profiling of Glycosphingolipid Glycans Using a Novel Broad Specificity Endoglycoceramidase in a High-Throughput Workflow. Anal. Chem. 2016, 88, 4795–4802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wongtrakul-Kish, K.; Walsh, I.; Sim, L.C.; Mak, A.; Liau, B.; Ding, V.; Hayati, N.; Wang, H.; Choo, A.; Rudd, P.M.; et al. Combining Glucose Units, m/z, and Collision Cross Section Values: Multiattribute Data for Increased Accuracy in Automated Glycosphingolipid Glycan Identifications and Its Application in Triple Negative Breast Cancer. Anal. Chem. 2019, 91, 9078–9085. [Google Scholar] [CrossRef]
- Bien, T.; Perl, M.; Machmuller, A.C.; Nitsche, U.; Conrad, A.; Johannes, L.; Muthing, J.; Soltwisch, J.; Janssen, K.P.; Dreisewerd, K. MALDI-2 Mass Spectrometry and Immunohistochemistry Imaging of Gb3Cer, Gb4Cer, and Further Glycosphingolipids in Human Colorectal Cancer Tissue. Anal. Chem. 2020, 92, 7096–7105. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yu, H.; Ma, T.; Yang, F.; Jia, L.; Zhang, C.; Zhang, J.; Niu, L.; Yang, J.; Zhang, Z.; et al. Analysis of Glycosphingolipid Glycans by Lectin Microarrays. Anal. Chem. 2019, 91, 10663–10671. [Google Scholar] [CrossRef] [PubMed]
- Rossdam, C.; Konze, S.A.; Oberbeck, A.; Rapp, E.; Gerardy-Schahn, R.; von Itzstein, M.; Buettner, F.F.R. Approach for Profiling of Glycosphingolipid Glycosylation by Multiplexed Capillary Gel Electrophoresis Coupled to Laser-Induced Fluorescence Detection To Identify Cell-Surface Markers of Human Pluripotent Stem Cells and Derived Cardiomyocytes. Anal. Chem. 2019, 91, 6413–6418. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, M.; Yu, M.; Fu, Q.; Jiang, H.; Yu, G.; Li, G. Gangliosides profiling in serum of breast cancer patient: GM3 as a potential diagnostic biomarker. Glycoconj. J. 2019, 36, 419–428. [Google Scholar] [CrossRef]
- Talabnin, K.; Talabnin, C.; Kumagai, T.; Sutatum, N.; Khiaowichit, J.; Dechsukhum, C.; Ishihara, M.; Azadi, P.; Sripa, B. Ganglioside GM2: A potential biomarker for cholangiocarcinoma. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Colsch, B.; Woods, A.S. Localization and imaging of sialylated glycosphingolipids in brain tissue sections by MALDI mass spectrometry. Glycobiology 2010, 20, 661–667. [Google Scholar] [CrossRef]

| GSL | KEGG | Tumor Type | Associated Cell Characteristics | References | |
|---|---|---|---|---|---|
| Globosides | Gb3 | A4GALT | Neuroblastoma | Anti-Gb3 antibody inhibits angiogenesis and tumor development | [53] |
| A4GALT | Ovarian | Promotes EMT, cell migration, chemoresistance and reduces cell proliferation | [30] | ||
| A4GALT | Gastric | Gb3 is expressed in gastric adenocarcinoma | [52] | ||
| A4GALT | Colon | Upregulated in metastatic colon cancer, promotes cell invasiveness, and tumor growth | [46] | ||
| Gb4 | B3GALNT1 | Colorectal | EtDO-P4 reduces cell proliferation. Exogenous Gb4 activates EGFR and induces the ERK pathway | [59] | |
| SSEA3 (Gb5) | B3GALT5 | Breast | Promotes cell proliferation, tumor growth, cancer stemness | [40,65,68] | |
| B3GALT5 | Colorectal | High tumorgenicity and promote cell proliferation in vivo | [70] | ||
| GloboH | FUT1 and FUT2 | Breast | Promotes cell invasion, reduced apoptosis | [68] | |
| SSEA4 | ST3GAL1, ST3GAL2 | Glioblastoma | Anti-SSEA4 antibody inhibits tumor growth in vivo | [63] | |
| ST3GAL1, ST3GAL2 | Ovarian | Loss of SSEA4 correlated with advanced tumor stage and poor cell differentiation | [65] | ||
| ST3GAL1, ST3GAL2 | Breast | SSEA4 promotes chemoresistance, tumorigenicity in vivo and promotes EMT | [101] | ||
| ST3GAL1, ST3GAL2 | Prostate | SSEA4-positive cells downregulate epithelial cell-associated markers, promotes EMT and cell–ECM adhesion | [62] | ||
| ST3GAL1, ST3GAL2 | Oral | Cancer stemness, promote tumorigenicity in vivo | [102] | ||
| Lacto-neolacto | P1 | A4GALT | Ovarian | Promotes cell migration | [103] |
| Lc3, nLc4 | B3GNT5 | Leukemia | Acute myeloid leukemia initiation and differentiation | [104] | |
| Gangliosides | GM3 | ST3GAL5 (SIAT9) | Ovarian | Retinoid-resistant ovarian cancer cells have higher level of GM3 | [105] |
| ST3GAL5 (SIAT9) | Breast | ST3GAL5 silencing inhibits cell migration | [77] | ||
| ST3GAL5 (SIAT9) | Glioblastoma | Exogenous GM3 inhibits proliferation/migration | [90,106] | ||
| ST3GAL5 (SIAT9) | Colorectal | Induces cisplatin-induced apoptosis | [107] | ||
| ST3GAL5 (SIAT9) | Leukemia | Promotes leukemia cell line differentiation | [108] | ||
| ST3GAL5 (SIAT9) | Bladder | Exogenous GM3 inhibits proliferation, cell adhesion | [109] | ||
| GM2 | B4GALNT1 | Breast | GM2 higher expression is associated with cancer cell stemness | [110] | |
| B4GALNT1 | Ovarian | Retinoid-resistant ovarian cancer cells have higher level of GM2 | [105] | ||
| B4GALNT1 | Lung cancer | Promotes metastasis and tumorigenicity in vivo | [111] | ||
| GM1 | B3GALT4 | Colorectal | Appears upon anti-EpCAM-based inhibition of cell proliferation | [112] | |
| GD1α * | ST6GALNAC3 ST6GALNAC4 ST6GALNAC5 ST6GALNAC6 | Breast | Promotes brain metastasis and cell adhesion (ST6GALNAC5 in breast cancer cells and enhances their adhesion to brain endothelial cells) | [113] | |
| GD1b | B3GALT4 | Breast | Exogenous and endogenous GD1b induces apoptosis in vitro | [114] | |
| GD2 | B4GALNT1 | Breast | Proliferation, cancer stemness | [95,115] | |
| B4GALNT1 | Lung | Promotes proliferation and invasion | [98] | ||
| B4GALNT1 | Sarcoma | Enhancement of malignant properties | [96,116] | ||
| GD3 | ST8SIA1 | Glioblastoma | Promotes proliferation and invasion | [117] | |
| ST8SIA1 | Breast | Promotes migration/invasion, metastasis in vivo and cell adhesion | [81,83,115] | ||
| ST8SIA1 | Ovarian | Inhibits the antitumor NKT cell response | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cumin, C.; Huang, Y.-L.; Everest-Dass, A.; Jacob, F. Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer. Biomolecules 2021, 11, 62. https://doi.org/10.3390/biom11010062
Cumin C, Huang Y-L, Everest-Dass A, Jacob F. Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer. Biomolecules. 2021; 11(1):62. https://doi.org/10.3390/biom11010062
Chicago/Turabian StyleCumin, Cécile, Yen-Lin Huang, Arun Everest-Dass, and Francis Jacob. 2021. "Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer" Biomolecules 11, no. 1: 62. https://doi.org/10.3390/biom11010062
APA StyleCumin, C., Huang, Y.-L., Everest-Dass, A., & Jacob, F. (2021). Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer. Biomolecules, 11(1), 62. https://doi.org/10.3390/biom11010062





