Prophylactic Treatment with Vitamins C and B2 for Methotrexate-Induced Gastrointestinal Mucositis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culturing Conditions
2.2. Bacterial Growth Experiments
2.3. Materials
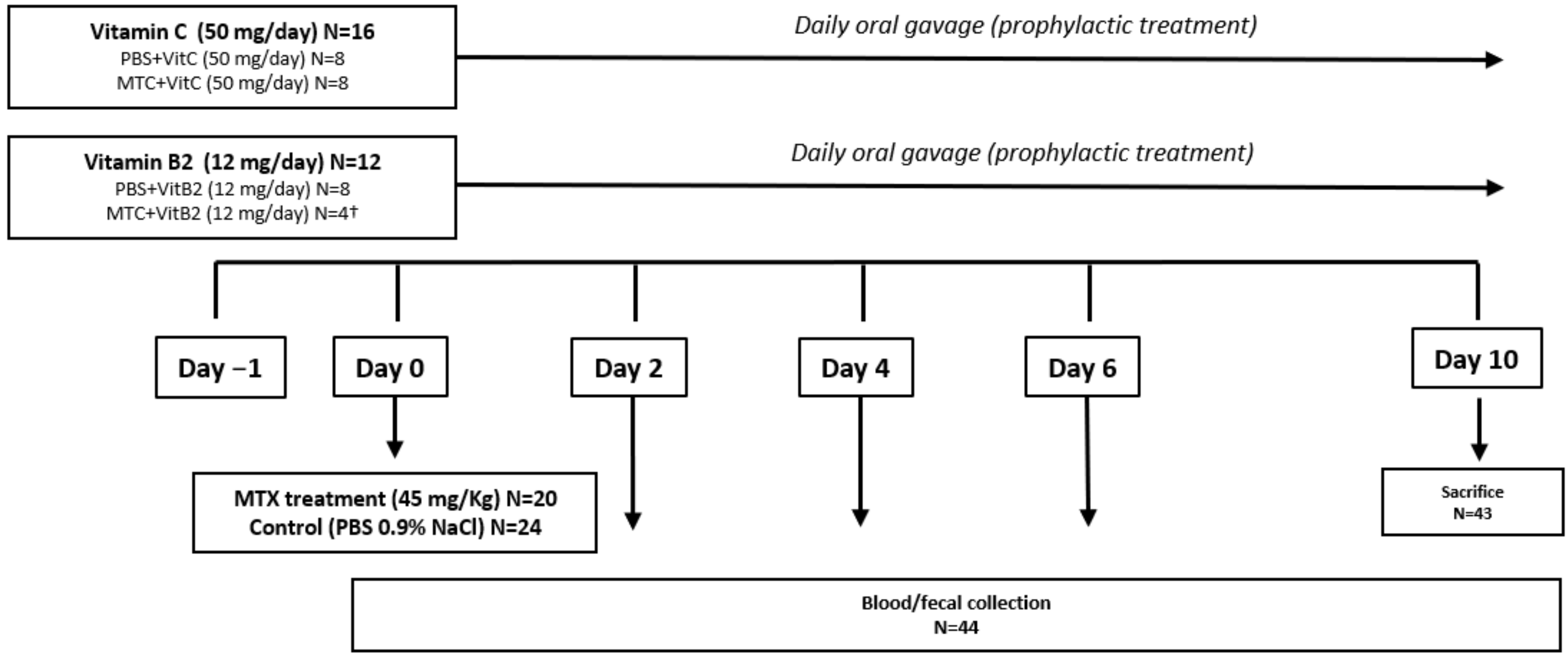
2.4. Animals and Ethics
2.5. Ethical Statement and Husbandry Specifications
2.6. Experimental Procedures
2.7. Plasma Citrulline Levels Determination
2.8. Microbiome Analysis
2.8.1. DNA Extraction
2.8.2. PCR Amplification of V3 and V4 Region of the 16S rRNA Gene
2.8.3. PCR Purification, Library Preparation and Illumina Sequencing
2.8.4. Statistical Analysis and Bioinformatics
3. Results
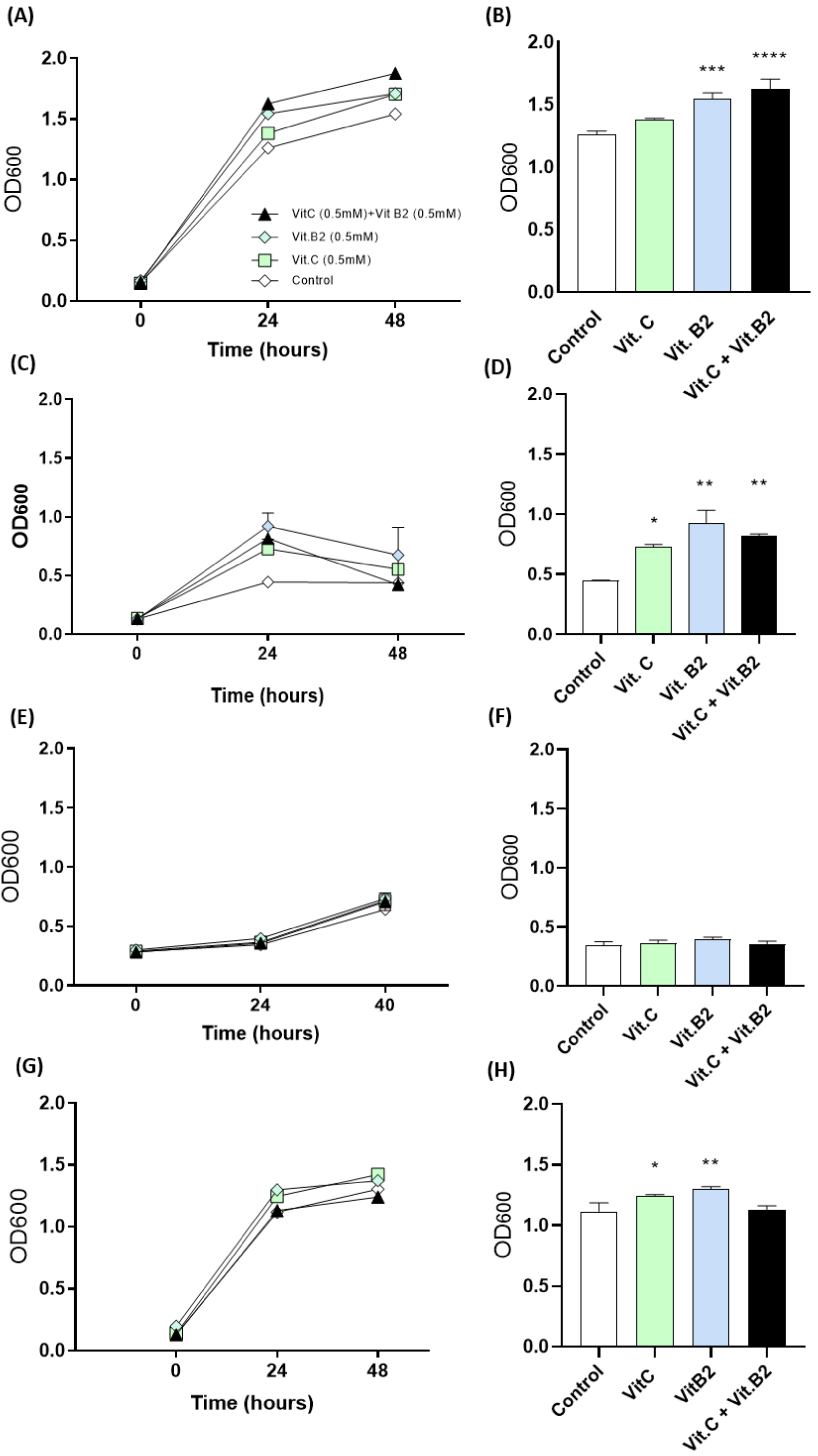
3.1. In Vitro Findings
Vitamins C and B2 Enhance Growth of Anaerobic Bacteria under Oxidative Stress
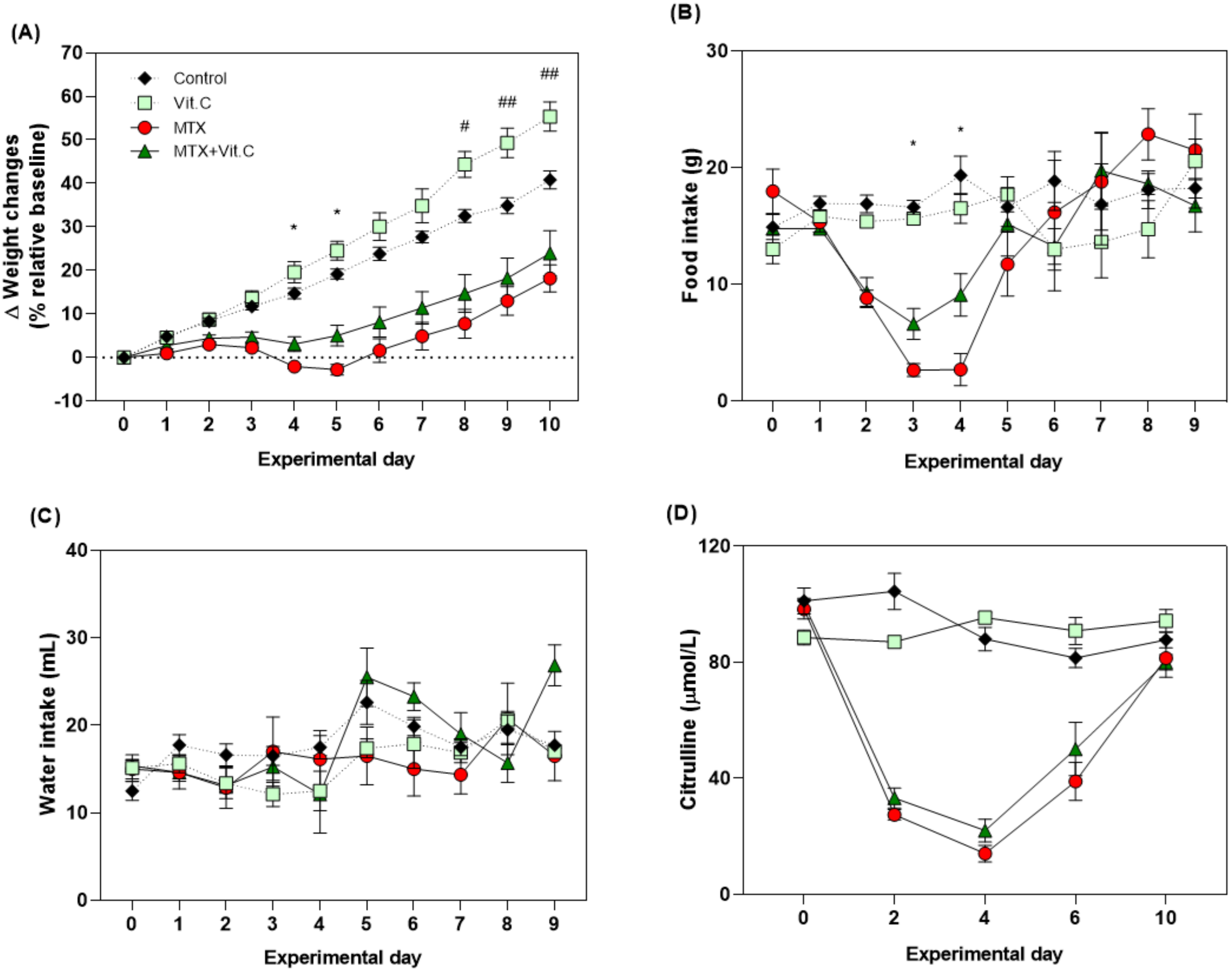
3.2. In Vivo Findings
3.2.1. Baseline Data
3.2.2. Vitamin C but Not Vitamin B2 Promotes Body Weight and Food Intake during MTX-Induced Mucositis
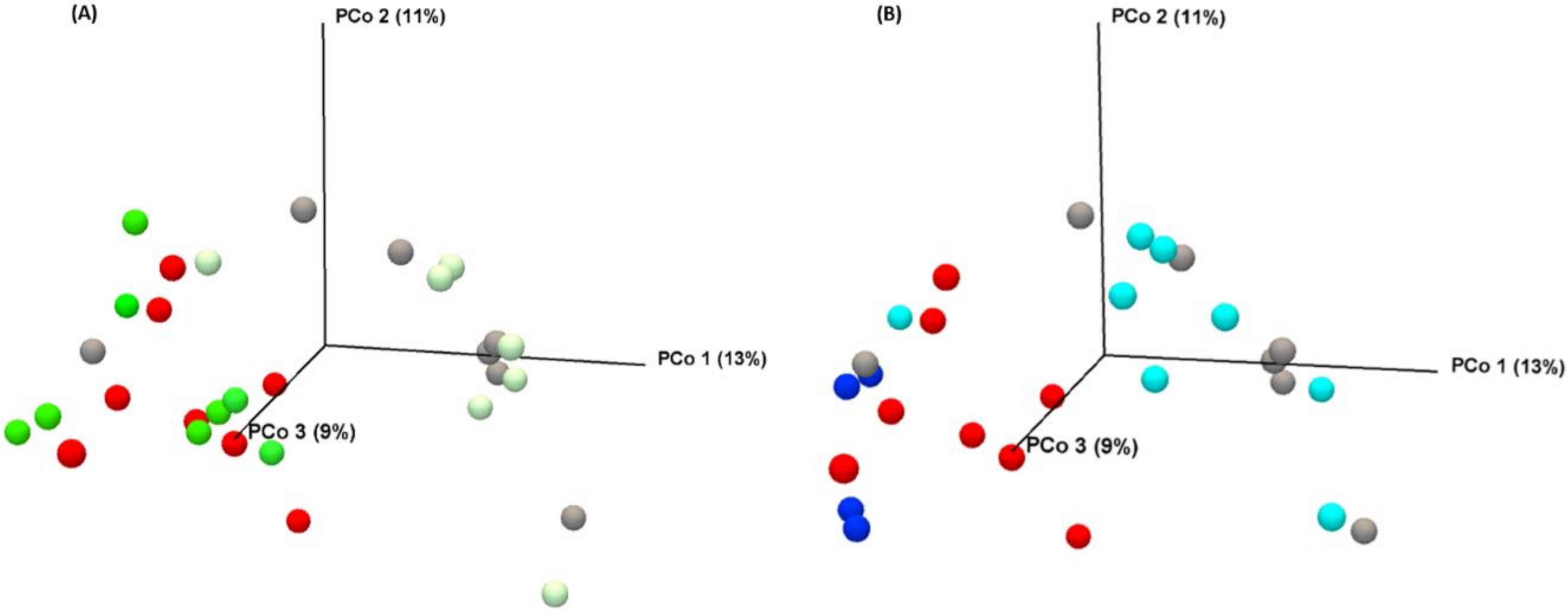
3.2.3. Vitamins Fail to Modulate MTX-Induced Microbial Disruption
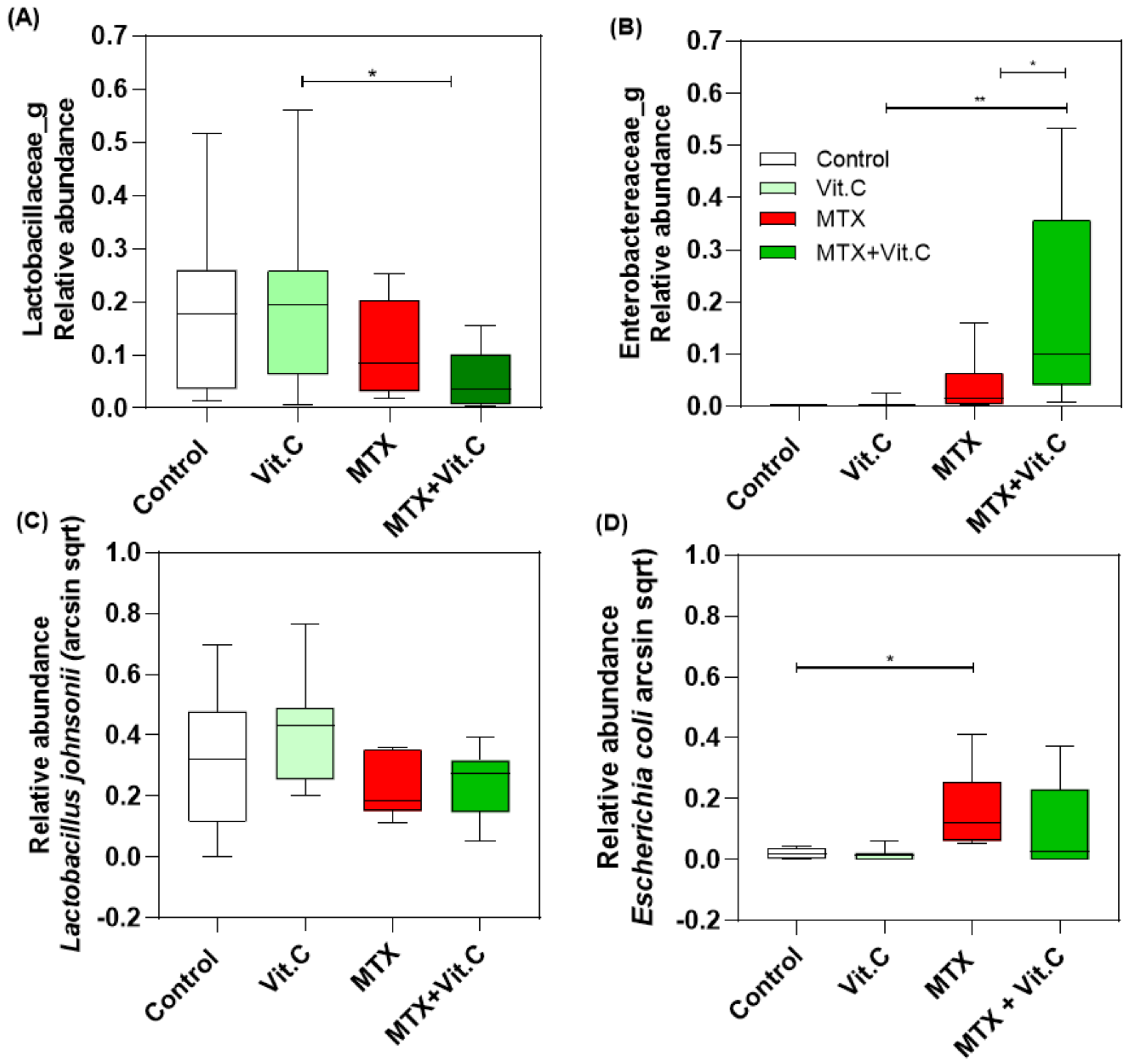
3.2.4. Vitamin C Supplementation in MTX-Treated Rats Increases Enterobacteriaceae Family at Day 4
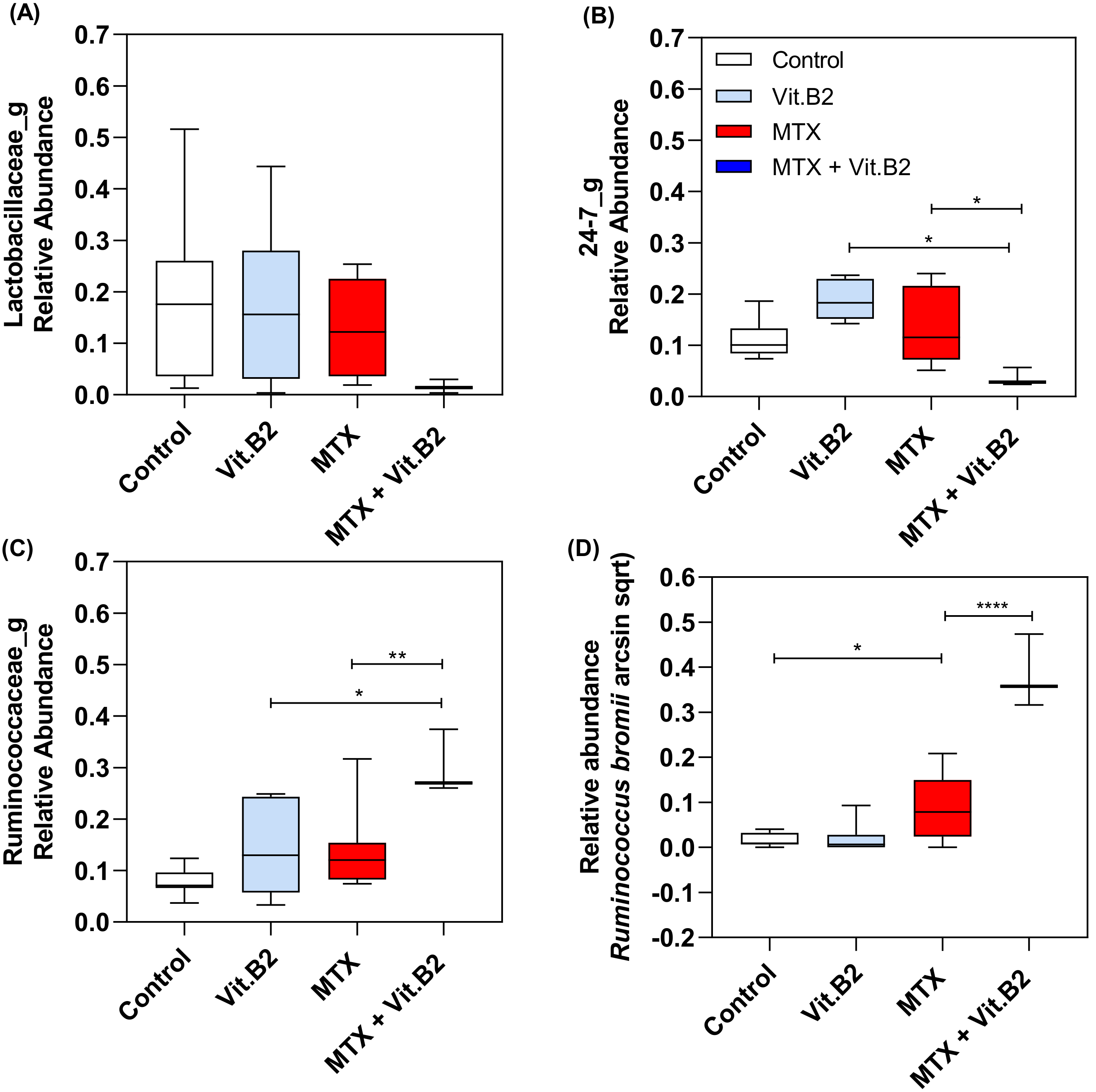
3.2.5. Vitamin B2 Induced Changes in the Composition of the Gut Microbiota during Recovery of MTX-Induced Mucositis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on Cancer Therapy-Induced Mucosal Injury: Pathogenesis, Measurement, Epidemiology, and Consequences for Patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Sonis, S.T. Pathobiology of mucositis. Semin. Oncol. Nurs. 2004, 20, 11–15. [Google Scholar] [CrossRef]
- Sonis, S.T. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009, 45, 1015–1020. [Google Scholar] [CrossRef]
- Gubatan, J.; Moss, A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018. [Google Scholar] [CrossRef]
- Wadleigh, R.G.; Redman, R.S.; Graham, M.L.; Krasnow, S.H.; Anderson, A.; Cohen, M.H. Vitamin E in the treatment of chemotherapy-induced mucositis. Am. J. Med. 1992. [Google Scholar] [CrossRef]
- Nejatinamini, S.; Debenham, B.J.; Clugston, R.D.; Mawani, A.; Parliament, M.; Wismer, W.V.; Mazurak, V.C. Poor vitamin status is associated with skeletal muscle loss and mucositis in head and neck cancer patients. Nutrients 2018, 10, 1236. [Google Scholar] [CrossRef]
- Masri, O.A.; Chalhoub, J.M.; Sharara, A.I. Role of vitamins in gastrointestinal diseases. World J. Gastroenterol. 2015. [Google Scholar] [CrossRef]
- Al-asmari, A.K.; Khan, A.Q.; Al-qasim, A.M.; Al-yousef, Y. Ascorbic acid attenuates antineoplastic drug 5-fluorouracil induced gastrointestinal toxicity in rats by modulating the expression of inflammatory mediators. Toxicol. Rep. 2015, 2, 908–916. [Google Scholar] [CrossRef]
- Khan, M.T.; Browne, W.R.; van Dijl, J.M.; Harmsen, H.J.M. How Can Faecalibacterium prausnitzii Employ Riboflavin for Extracellular Electron Transfer? Antioxid. Redox Signal. 2012, 17, 1433–1440. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Subramenium, G.A.; Marchant, J.S.; Said, H.M. Tumor necrosis factor alpha reduces intestinal vitamin C uptake: A role for NF-κB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003. [Google Scholar] [CrossRef]
- Kondo, K.; Hiramoto, K.; Yamate, Y.; Goto, K.; Sekijima, H.; Ooi, K. Ameliorative effect of high-dose Vitamin C administration on dextran sulfate sodium-induced colitis mouse model. Biol. Pharm. Bull. 2019. [Google Scholar] [CrossRef]
- De Moreno de LeBlanc, A.; Levit, R.; de Giori, G.S.; LeBlanc, J.G. Vitamin Producing Lactic Acid Bacteria as Complementary Treatments for Intestinal Inflammation. Antiinflamm. Antiallergy. Agents Med. Chem. 2018. [Google Scholar] [CrossRef][Green Version]
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Evaluation of the effect of soymilk fermented by a riboflavin-producing Lactobacillus plantarum strain in a murine model of colitis. Benef. Microbes 2017. [Google Scholar] [CrossRef]
- Robien, K.; Schubert, M.M.; Bruemmer, B.; Lloid, M.E.; Potter, J.D.; Ulrich, C.M. Predictors of oral mucositis in patients receiving hematopoietic cell transplants for chronic myelogenous leukemia. J. Clin. Oncol. 2004. [Google Scholar] [CrossRef]
- Bartram, A.K.; Lynch, M.D.J.; Stearns, J.C.; Moreno-Hagelsieb, G.; Neufeld, J.D. Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Appl. Environ. Microbiol. 2011. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004. [Google Scholar] [CrossRef]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef]
- Kuiken, N.S.S.; Rings, E.H.H.M.; Alffenaar, J.W.C.; Havinga, R.; Jurdzinski, A.; Groen, A.K.; Tissing, W.J.E. Tumor Necrosis Factor-Alpha Inhibitor Etanercept Does Not Alter Methotrexate-Induced Gastrointestinal Mucositis in Rats. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e28–e34. [Google Scholar] [CrossRef]
- Fijlstra, M.; Tissing, W.J.E.; Stellaard, F.; Verkade, H.J.; Rings, E.H.H.M. Reduced absorption of long-chain fatty acids during methotrexate-induced gastrointestinal mucositis in the rat. Clin. Nutr. 2013, 32, 452–459. [Google Scholar] [CrossRef]
- Van Vliet, M.J.; Harmsen, H.J.M.; de Bont, E.S.J.M.; Tissing, W.J.E. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010, 6, 1–7. [Google Scholar] [CrossRef]
- Van Vliet, M.J.; Tissing, W.J.E.; Rings, E.H.H.M.; Koetse, H.A.; Stellaard, F.; Kamps, W.A.; De Bont, E.S.J.M. Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr. Blood Cancer 2009, 53, 1188–1194. [Google Scholar] [CrossRef]
- Fijlstra, M.; Rings, E.H.H.M.; Verkade, H.J.; van Dijk, T.H.; Kamps, W.A.; Tissing, W.J.E. Lactose maldigestion during methotrexate-induced gastrointestinal mucositis in a rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G283-91. [Google Scholar] [CrossRef][Green Version]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019. [Google Scholar]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015. [Google Scholar] [CrossRef]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van De Wiele, T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley Des Varannes, S.; Le Vacon, F.; De La Cochetière, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef]
- Bowen, J.; Al-Dasooqi, N.; Bossi, P.; Wardill, H.; Van Sebille, Y.; Al-Azri, A.; Bateman, E.; Correa, M.E.; Raber-Durlacher, J.; Kandwal, A.; et al. The pathogenesis of mucositis: Updated perspectives and emerging targets. Support. Care Cancer 2019, 4023–4033. [Google Scholar] [CrossRef]
- Louise Pouncey, A.; James Scott, A.; Leslie Alexander, J.; Marchesi, J.; Kinross, J. Gut microbiota, chemotherapy and the host: The influence of the gut microbiota on cancer treatment. Ecancermedicalscience 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Wardill, H.R.; Tissing, W.J.E. Determining risk of severe gastrointestinal toxicity based on pretreatment gut microbial community in patients receiving cancer treatment. Curr. Opin. Support. Palliat. Care 2017, 11, 125–132. [Google Scholar] [CrossRef]
- Cereda, E.; Caraccia, M.; Caccialanza, R. Probiotics and mucositis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 399–404. [Google Scholar] [CrossRef]
- Prisciandaro, L.D.; Geier, M.S.; Butler, R.N.; Cummins, A.G.; Howarth, G.S. Evidence supporting the use of probiotics for the prevention and treatment of chemotherapy-induced intestinal mucositis. Crit. Rev. Food Sci. Nutr. 2011, 51, 239–247. [Google Scholar] [CrossRef]
- Meeker, S.; Seamons, A.; Maggio-Price, L.; Paik, J. Protective links between Vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 2016, 22, 933–948. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Das, A.B. Potential mechanisms of action for vitamin C in cancer: Reviewing the evidence. Front. Physiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Van Der Reest, J.; Gottlieb, E. Anti-cancer effects of Vitamin C revisited. Cell Res. 2016, 26, 269–270. [Google Scholar] [CrossRef]
- Xuan, Z.; An, Y.; Yang, D.; Wang, S.; Xu, Q.; Yuan, S. Exploration of the protection of riboflavin laurate on oral mucositis induced by chemotherapy or radiotherapy at the cellular level: What is the leading contributor? Int. J. Mol. Sci. 2013, 14, 4722–4733. [Google Scholar] [CrossRef]
- Li, H.-L.; Lu, L.; Wang, X.-S.; Qin, L.-Y.; Wang, P.; Qiu, S.-P.; Wu, H.; Huang, F.; Zhang, B.-B.; Shi, H.-L.; et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front. Cell. Infect. Microbiol. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Verghese, R.; Mathew, S.; David, A. Antimicrobial activity of Vitamin C demonstrated on uropathogenic Escherichia coli and Klebsiella pneumoniae. J. Curr. Res. Sci. Med. 2017. [Google Scholar] [CrossRef]
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Protective effect of the riboflavin-overproducing strain Lactobacillus plantarum CRL2130 on intestinal mucositis in mice. Nutrition 2018, 54, 165–172. [Google Scholar] [CrossRef]
- De Moreno de LeBlanc, A.; Perdigón, G. The application of probiotic fermented milks in cancer and intestinal inflammation. Proc. Nutr. Soc. 2010, 69, 421–428. [Google Scholar] [CrossRef]


| Baseline Body Weight in Each Experiments Group | ||
|---|---|---|
| Body Weight (g) | Plasma Citrulline (µmol/L) | |
| Control (N = 8) | 95.88 ± 0.22 | 101.16 ± 4.51 |
| Vitamin C (N = 8) | 96.34 ± 0.27 | 88.52 ± 2.53 |
| Vitamin B2 (N = 8) | 95.88 ± 0.22 | 90.29 ± 9.34 |
| MTX (N = 8) | 96.39 ± 0.34 | 98.33 ± 9.59 |
| MTX+VitC (N = 8) | 97.40 ± 0.38 | 100.05 ± 1.99 |
| MTX+VitB2 (N = 4) | 97.82 ± 0.88 | 104.70 ± 13.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Ferreira, A.R.; Wardill, H.R.; Havinga, R.; Tissing, W.J.E.; Harmsen, H.J.M. Prophylactic Treatment with Vitamins C and B2 for Methotrexate-Induced Gastrointestinal Mucositis. Biomolecules 2021, 11, 34. https://doi.org/10.3390/biom11010034
da Silva Ferreira AR, Wardill HR, Havinga R, Tissing WJE, Harmsen HJM. Prophylactic Treatment with Vitamins C and B2 for Methotrexate-Induced Gastrointestinal Mucositis. Biomolecules. 2021; 11(1):34. https://doi.org/10.3390/biom11010034
Chicago/Turabian Styleda Silva Ferreira, Ana Rita, Hannah R. Wardill, Rick Havinga, Wim J. E. Tissing, and Hermie J. M. Harmsen. 2021. "Prophylactic Treatment with Vitamins C and B2 for Methotrexate-Induced Gastrointestinal Mucositis" Biomolecules 11, no. 1: 34. https://doi.org/10.3390/biom11010034
APA Styleda Silva Ferreira, A. R., Wardill, H. R., Havinga, R., Tissing, W. J. E., & Harmsen, H. J. M. (2021). Prophylactic Treatment with Vitamins C and B2 for Methotrexate-Induced Gastrointestinal Mucositis. Biomolecules, 11(1), 34. https://doi.org/10.3390/biom11010034





