Glycosaminoglycans in Tissue Engineering: A Review
Abstract
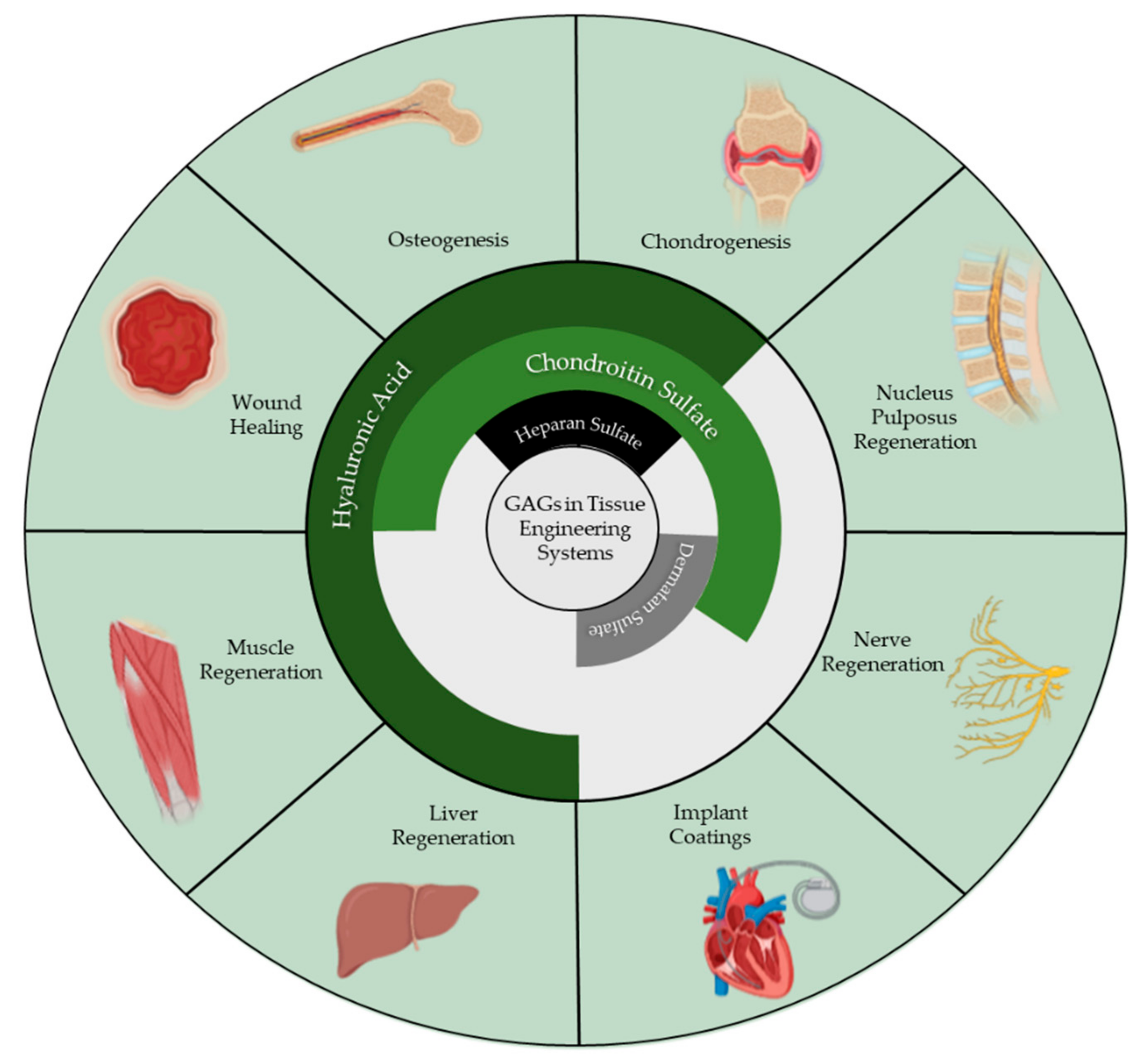
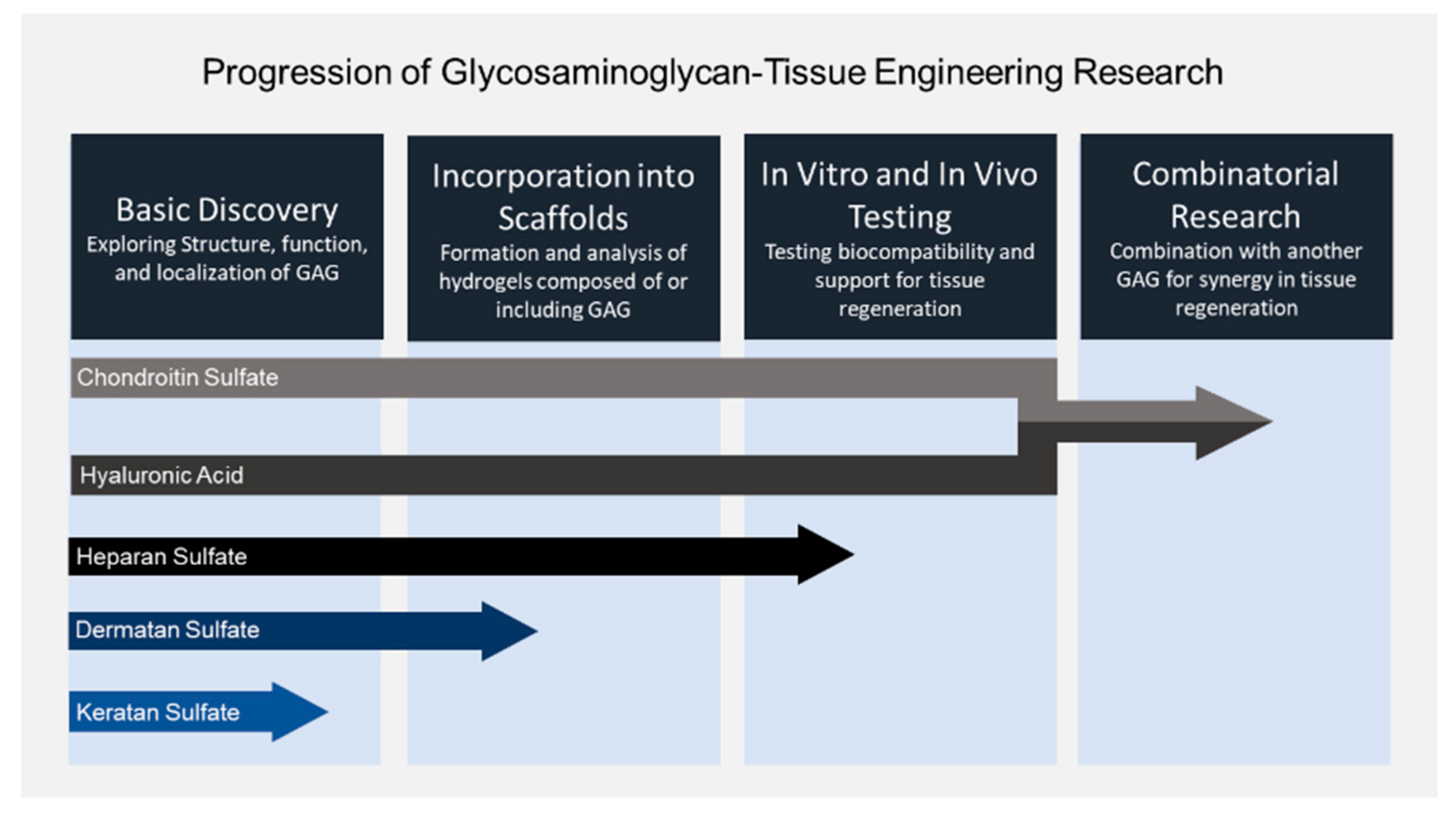
1. Introduction
2. Hyaluronic Acid
2.1. Hyaluronic Acid Supports Multiple Crosslinking Mechanisms
2.2. Hyaluronic Acid Blends
3. Chondroitin Sulfate
3.1. Sulfation and Sulfation Pattern Suport Biological Activity
3.2. Chondroitin Sulfate Blends
3.3. Processing Techniques and Manufacturing
4. Chondroitin Sulfate-Hyaluronic Acid Hybrid Tissue Engineering Systems
5. Dermatan Sulfate
6. Heparan Sulfate and Heparin
7. Keratan Sulfate
8. Summary and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blanco, A.; Blanco, G. (Eds.) Carbohydrates, in Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2017; Chapter 4; pp. 73–97. [Google Scholar]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.B.L.B.P.; Bartosikova, L.; Brauner, P.; Kolar, J.J.V.M. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Caterson, B.; Melrose, J. Keratan sulfate, a complex glycosaminoglycan with unique functional capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef] [PubMed]
- Crowder, K.M.; Gunther, J.M.; Jones, T.A.; Hale, B.D.; Zhang, H.Z.; Peterson, M.R.; Scheller, R.H.; Chavkin, C.; Bajjalieh, S.M. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc. Natl. Acad. Sci. USA 1999, 96, 15268–15273. [Google Scholar] [CrossRef]
- Meyer, K. The chemistry and biology of mucopolysaccharides and glycoproteins. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1938. [Google Scholar]
- Grossfeld, H.; Meyer, K.; Godman, G. Differentiation of Fibroblasts in Tissue Culture, as Determined by Mucopolysaccharide Production. Proc. Soc. Exp. Biol. Med. 1955, 88, 31–35. [Google Scholar] [CrossRef]
- Fessler, J.H. Water and Mucopolysaccharide as Structural Components of Connective Tissue. Nat. Cell Biol. 1957, 179, 426–427. [Google Scholar] [CrossRef]
- Anseth, A. Glycosaminoglycans in the developing corneal stroma. Exp. Eye Res. 1961, 1, 116–121. [Google Scholar] [CrossRef]
- Taylor, H.E. The role of mucopolysaccharides in the pathogenesis of intimal fibrosis and atherosclerosis of the human aorta. Am. J. Pathol. 1953, 29, 871–883. [Google Scholar]
- Altshuler, C.H.; Angevine, D.M. Acid mucopolysaccharide in degenerative disease of connective tissue, with special reference to serous inflammation. Am. J. Pathol. 1951, 27, 141–156. [Google Scholar]
- Stidworthy, G.; Masters, Y.F.; Shetlar, M. The effect of aging on mucopolysaccharide composition of human costal cartilage as measured by hexosamine and uronic acid content. J. Gerontol. 1958, 13, 10–13. [Google Scholar] [CrossRef]
- Bollet, A.J.; Goodwin, J.F.; Simpson, W.F.; Anderson, D.V. Mucopolysaccharide, protein and desoxyribosenucleic acid concentration of granulation tissue induced by polyvinyl sponges. Proc. Soc. Exp. Biol. Med. 1958, 99, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Driver, V.R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2012, 20, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Yamanlar, S.; Sant, S.; Boudou, T.; Picart, C.; Khademhosseini, A. Surface functionalization of hyaluronic acid hydrogels by polyelectrolyte multilayer films. Biomaterials 2011, 32, 5590–5599. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.-J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.; Chung, H.; et al. Tissue Reconstruction: Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy (Adv. Funct. Mater. 25/2015). Adv. Funct. Mater. 2015, 25, 3798. [Google Scholar] [CrossRef]
- Lou, J.; Stowers, R.; Nam, S.; Xia, Y.; Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 2018, 154, 213–222. [Google Scholar] [CrossRef]
- Yin, F.; Lin, L.; Zhan, S. Preparation and properties of cellulose nanocrystals, gelatin, hyaluronic acid composite hydrogel as wound dressing. J. Biomater. Sci. Polym. Ed. 2019, 30, 190–201. [Google Scholar] [CrossRef]
- Domingues, R.M.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.G.; Mano, J.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of Injectable Hyaluronic Acid/Cellulose Nanocrystals Bionanocomposite Hydrogels for Tissue Engineering Applications. Bioconjugate Chem. 2015, 26, 1571–1581. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Yoo, J.U.; Lundberg, M.; Dennis, J.E.; Huibregtse, B.A.; Goldberg, V.M.; I Caplan, A. Hyaluronan-based polymers in the treatment of osteochondral defects. J. Orthop. Res. 2000, 18, 773–780. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; De Ruijter, M.; Abbadessa, A.; Melchels, F.P.; Öner, F.C.; Dhert, W.J.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Schuurman, W.; Wijnberg, H.M.; Prins, H.-J.; Van Weeren, P.R.; Malda, J.; Alblas, J.; Dhert, W.J. Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined, Cell-Laden Hydrogel Scaffolds. Tissue Eng. Part C Methods 2012, 18, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Desando, G.; Ferrari, A.; Zini, N.; Mariani, E.; Grigolo, B. Hyaluronan scaffold supports osteogenic differentiation of bone marrow concentrate cells. J. Biol. Regul. Homeost. Agents 2016, 30, 409–420. [Google Scholar]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone Marrow Stromal Stem Cells: Nature, Biology, and Potential Applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Block, J.E. The role and effectiveness of bone marrow in osseous regeneration. Med. Hypotheses 2005, 65, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Sideris, E.; Griffin, D.R.; Ding, Y.; Li, S.; Weaver, W.M.; Di Carlo, D.; Hsiai, T.; Segura, T. Particle Hydrogels Based on Hyaluronic Acid Building Blocks. ACS Biomater. Sci. Eng. 2016, 2, 2034–2041. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Larson, J.C.; Isom, A.; Brey, E.M. Generation of Porous Poly(Ethylene Glycol) Hydrogels by Salt Leaching. Tissue Eng. Part C Methods 2009, 16, 905–912. [Google Scholar] [CrossRef]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J. Biomed. Mater. Res. 2000, 53, 1–7. [Google Scholar] [CrossRef]
- Hung, B.P.; Harvestine, J.N.; Saiz, A.M.; Gonzalez-Fernandez, T.; Sahar, D.E.; Weiss, M.L.; Leach, J.K. Defining hydrogel properties to instruct lineage- and cell-specific mesenchymal differentiation. Biomaterials 2019, 189, 1–10. [Google Scholar] [CrossRef]
- Poveda-Reyes, S.; Moulisova, V.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmeron-Sanchez, M.; Ferrer, G.G. Gelatin-Hyaluronic Acid Hydrogels with Tuned Stiffness to Counterbalance Cellular Forces and Promote Cell Differentiation. Macromol. Biosci. 2016, 16, 1311–1324. [Google Scholar] [CrossRef]
- Lin, H.; Beck, A.M.; Shimomura, K.; Sohn, J.; Fritch, M.R.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G.; Tuan, R.S. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, Q.; Wen, H.; Chen, J.; Liang, M.; Huang, H.; Lan, D.; Dong, H.; Cao, X. Injection and Self-Assembly of Bioinspired Stem Cell-Laden Gelatin/Hyaluronic Acid Hybrid Microgels Promote Cartilage Repair In Vivo. Adv. Funct. Mater. 2019, 29, 1906690. [Google Scholar] [CrossRef]
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ciftci, S.; Barthes, J.; Knopf-Marques, H.; Muller, C.B.; DeBry, C.; Vrana, N.E.; Ghaemmaghami, A. A composite Gelatin/hyaluronic acid hydrogel as an ECM mimic for developing mesenchymal stem cell-derived epithelial tissue patches. J. Tissue Eng. Regen. Med. 2020, 14, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-W.; Liu, C.; Wu, J.-H.; Zhao, D.-H.; Lin, L.-X.; Fan, H.-M.; Sun, Y.-L. In situ forming gelatin/hyaluronic acid hydrogel for tissue sealing and hemostasis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 790–797. [Google Scholar] [CrossRef]
- Kazemirad, S.; Heris, H.K.; Mongeau, L. Viscoelasticity of hyaluronic acid-gelatin hydrogels for vocal fold tissue engineering. J. Biomed. Mater. Res. Part B: Appl. Biomaterials 2016, 104, 283–290. [Google Scholar] [CrossRef]
- Slevin, M.; Kumar, S.; Gaffney, J. Angiogenic Oligosaccharides of Hyaluronan Induce Multiple Signaling Pathways Affecting Vascular Endothelial Cell Mitogenic and Wound Healing Responses. J. Biol. Chem. 2002, 277, 41046–41059. [Google Scholar] [CrossRef]
- Ebrahimi-Hosseinzadeh, B.; Pedram, M.; Hatamian-Zarmi, A.; Salahshour-Kordestani, S.; Rasti, M.; Mokhtari-Hosseini, Z.B.; Mir-Derikvand, M. In vivo evaluation of gelatin/hyaluronic acid nanofiber as Burn-wound healing and its comparison with ChitoHeal gel. Fibers Polym. 2016, 17, 820–826. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, Y.; Li, L.; Pan, L.; Njunge, L.W.; Dong, L.; Yang, L. Evaluation of emulsion electrospun polycaprolactone/hyaluronan/epidermal growth factor nanofibrous scaffolds for wound healing. J. Biomater. Appl. 2016, 30, 686–698. [Google Scholar] [CrossRef]
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: In-vitro and in-vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Z.; Liu, Y.; Wang, L.; Jiang, Z.; Li, T.; Zhang, W.; Liang, Y. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr. Polym. 2018, 192, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Yew, C.H.T.; Azari, P.; Choi, J.R.; Muhamad, F.; Murphy, B.P. Electrospun Polycaprolactone Nanofibers as a Reaction Membrane for Lateral Flow Assay. Polymers 2018, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín-Masiá, E.; Poveda-Reyes, S.; Ferrer, G.G. Extracellular matrix–inspired gelatin/hyaluronic acid injectable hydrogels. Int. J. Polym. Mater. 2016, 66, 280–288. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Chu, B.; Zhang, A.; Huang, J.; Peng, X.; You, L.; Wu, C.; Tang, S. Preparation and biological evaluation of a novel agarose-grafting-hyaluronan scaffold for accelerated wound regeneration. Biomed. Mater. 2020, 15, 045009. [Google Scholar] [CrossRef]
- Foot, M.; Mulholland, M. Classification of chondroitin sulfate A, chondroitin sulfate C, glucosamine hydrochloride and glucosamine 6 sulfate using chemometric techniques. J. Pharm. Biomed. Anal. 2005, 38, 397–407. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin sulfate in the treatment of osteoarthritis: From in vitro studies to clinical recommen-dations. Ther. Adv. Musculoskelet. Dis. 2010, 2, 335–348. [Google Scholar] [CrossRef]
- Hwang, N.S.; Varghese, S.; Lee, H.J.; Theprungsirikul, P.; Canver, A.; Sharma, B.; Elisseeff, J. Response of zonal chondrocytes to extracellular matrix-hydrogels. FEBS Lett. 2007, 581, 4172–4178. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, S.; Shu, X.Z.; Shelby, J.; Prestwich, G.D. Release of basic fibroblast growth factor from a crosslinked glycosaminoglycan hydrogel promotes wound healing. Wound Repair Regen. 2007, 15, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural polymers: Polysaccharides and their derivatives for biomedical applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; Chapter 4; pp. 67–89. [Google Scholar]
- Corradetti, B.; Taraballi, F.; Minardi, S.; Van Eps, J.L.; Cabrera, F.; Francis, L.W.; Gazze, S.A.; Ferrari, M.; Weiner, B.K.; Tasciotti, E. Chondroitin Sulfate Immobilized on a Biomimetic Scaffold Modulates Inflammation While Driving Chondrogenesis. Stem Cells Transl. Med. 2016, 5, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, Y.; Adoungotchodo, A.; Hui, E.; Zehtabi, F.; Lerouge, S. An injectable chitosan/chondroitin sulfate hydrogel with tunable mechanical properties for cell therapy/tissue engineering. Int. J. Biol. Macromol. 2018, 113, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Fang, W.; Tao, Y.; Zhao, T.; Xia, K.; Liang, C.; Hua, J.; Li, F.; Chen, Q. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018, 71, 496–509. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C.; et al. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2019, 14, 025006. [Google Scholar] [CrossRef]
- Piai, J.F.; Rubira, A.F.; Muniz, E.C. Self-assembly of a swollen chitosan/chondroitin sulfate hydrogel by outward diffusion of the chondroitin sulfate chains. Acta Biomater. 2009, 5, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Stanford, C.M.; Solursh, M.; Keller, J.C. Significant role of adhesion properties of primary osteoblast-like cells in early adhesion events for chondroitin sulfate and dermatan sulfate surface molecules. J. Biomed. Mater. Res. 1999, 47, 345–352. [Google Scholar] [CrossRef]
- Hempel, U.; Matthäus, C.; Preissler, C.; Moller, S.; Hintze, V.; Dieter, P. Artificial Matrices with High-Sulfated Glycosaminoglycans and Collagen Are Anti-Inflammatory and Pro-Osteogenic for Human Mesenchymal Stromal Cells. J. Cell. Biochem. 2014, 115, 1561–1571. [Google Scholar] [CrossRef]
- Andrews, S.; Cheng, A.; Stevens, H.; Logun, M.T.; Webb, R.; Jordan, E.; Xia, B.; Karumbaiah, L.; Guldberg, R.E.; Stice, S.L. Chondroitin Sulfate Glycosaminoglycan Scaffolds for Cell and Recombinant Protein-Based Bone Regeneration. Stem Cells Transl. Med. 2019, 8, 575–585. [Google Scholar] [CrossRef]
- Birdwhistell, K.E.; Karumbaiah, L.; Franklin, S.P. Sustained Release of Transforming Growth Factor-β1 from Platelet-Rich Chondroitin Sulfate Glycosaminoglycan Gels. J. Knee Surg. 2018, 31, 410–415. [Google Scholar] [CrossRef]
- Conovaloff, A.; Panitch, A. Characterization of a chondroitin sulfate hydrogel for nerve root regeneration. J. Neural Eng. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Karumbaiah, L.; Enam, S.F.; Brown, A.C.; Saxena, T.; Betancur, M.I.; Barker, T.H.; Bellamkonda, R. Chondroitin Sulfate Glycosaminoglycan Hydrogels Create Endogenous Niches for Neural Stem Cells. Bioconjugate Chem. 2015, 26, 2336–2349. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Bulnheim, U.; Diener, A.; Lüthen, F.; Teller, M.; Klinkenberg, E.-D.; Neumann, H.-G.; Nebe, B.; Liebold, A.; Steinhoff, G.; et al. Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J. Cell. Mol. Med. 2008, 12, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-R.V.; Hwang, Y.; Phadke, A.; Kang, H.; Hwang, N.S.; Caro, E.J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.C.; et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990–995. [Google Scholar] [CrossRef]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef]
- Jun, S.-H.; Lee, E.-J.; Jang, T.-S.; Kim, H.-E.; Jang, J.-H.; Koh, Y.-H. Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 773–782. [Google Scholar] [CrossRef]
- Kweon, H. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Benoit, D.S.; Tripodi, M.C.; Anseth, K.S. The effect of ethylene glycol methacrylate phosphate in PEG hydrogels on mineralization and viability of encapsulated hMSCs. Biomaterials 2006, 27, 1377–1386. [Google Scholar] [CrossRef]
- Kim, H.D.; Lee, E.A.; An, Y.-H.; Kim, S.L.; Lee, S.S.; Yu, S.J.; Jang, H.L.; Nam, K.T.; Im, S.G.; Hwang, N.S. Chondroitin Sulfate-Based Biomineralizing Surface Hydrogels for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 21639–21650. [Google Scholar] [CrossRef]
- Miyamoto, A.; Yoshikawa, M.; Maeda, H. Hard Tissue-Forming Ability and Ultra-Micro Structure of Newly Developed Sponges as Scaffolds Made with Sodium Alginate Gel and Chondroitin Sulfate. J. Biomed. Sci. Eng. 2018, 11, 289–306. [Google Scholar] [CrossRef][Green Version]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.; et al. Human Cartilage Repair with a Photoreactive Adhesive-Hydrogel Composite. Sci. Transl. Med. 2013, 5, 167ra6. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Moy, A.C.; Sydlik, S.A. Injectable amine functionalized graphene and chondroitin sulfate hydrogel with potential for cartilage regeneration. J. Mater. Chem. B 2019, 7, 2442–2453. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; E Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef]
- Kato, M.; Mrksich, M. Using model substrates to study the dependence of focal adhesion formation on the affinity of integrin-ligand complexes. Biochemistry 2004, 43, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Chunyang, Y. An Injectable Enzymatically Crosslinked Carboxymethylated Pullulan/Chondroitin Sulfate Hydrogel for Cartilage Tissue Engineering. Sci. Rep. 2016, 6, 20014. [Google Scholar] [CrossRef] [PubMed]
- Autissier, A.; Le Visage, C.; Pouzet, C.; Chaubet, F.; Letourneur, D. Fabrication of porous polysaccharide-based scaffolds using a combined freeze-drying/cross-linking process. Acta Biomater. 2010, 6, 3640–3648. [Google Scholar] [CrossRef]
- Bae, H.; Ahari, A.F.; Shin, H.; Nichol, J.W.; Hutson, C.B.; Masaeli, M.; Kim, S.-H.; Aubin, H.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter 2011, 7, 1903–1911. [Google Scholar] [CrossRef]
- Li, T.; Song, X.; Weng, C.; Wang, X.; Sun, L.; Gong, X.; Yang, L.; Chen, C. Self-crosslinking and injectable chondroitin sulfate/pullulan hydrogel for cartilage tissue engineering. Appl. Mater. Today 2018, 10, 173–183. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Chenite, A. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Nair, M.B.; Baranwal, G.; Vijayan, P.; Keyan, K.S.; Jayakumar, R. Composite hydrogel of chitosan-poly(hydroxybutyrate-co-valerate) with chondroitin sulfate nano-particles for nucleus pulposus tissue engineering. Colloids Surf. B Biointerfaces 2015, 136, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, A.; Blokzijl, M.; Mouser, V.; Marica, P.; Malda, J.; Hennink, W.; Vermonden, T. A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Pezeshki-Modaress, M.; Zandi, M. Electrospun polyvinyl alcohol/gelatin/chondroitin sulfate nano-fibrous scaffold: Fabrication and in vitro evaluation. Int. J. Biol. Macromol. 2018, 114, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Zandi, M.; Pezeshki-Modaress, M.; Rajabi, S. Tough, hybrid chondroitin sulfate nanofibers as a promising scaffold for skin tissue engineering. Int. J. Biol. Macromol. 2019, 132, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Na, K.; Putra, I.; Lee, H.J.; Hull, S.; Cheng, Y.; Blanco, I.J.; Eslani, M.; Djalilian, A.R.; Myung, D. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered Within a Viscoelastic Gel Carrier. Stem Cells Transl. Med. 2019, 8, 478–489. [Google Scholar] [CrossRef]
- Ni, Y.; Tang, Z.; Cao, W.; Lin, H.; Fan, Y.; Guo, L.; Zhang, X. Tough and elastic hydrogel of hyaluronic acid and chondroitin sulfate as potential cell scaffold materials. Int. J. Biol. Macromol. 2015, 74, 367–375. [Google Scholar] [CrossRef]
- Bhowmick, S.; Scharnweber, D.; Koul, V. Co-cultivation of keratinocyte-human mesenchymal stem cell (hMSC) on sericin loaded electrospun nanofibrous composite scaffold (cationic gelatin/hyaluronan/chondroitin sulfate) stimulates epithelial differentiation in hMSCs: In vitro study. Biomaterials 2016, 88, 83–96. [Google Scholar] [CrossRef]
- Sawatjui, N.; Damrongrungruang, T.; Leeanansaksiri, W.; Jearanaikoon, P.; Hongeng, S.; Limpaiboon, T. Silk fibroin/gelatin–chondroitin sulfate–hyaluronic acid effectively enhances in vitro chondrogen-esis of bone marrow mesenchymal stem cells. Mater. Sci. Eng. C 2015, 52, 90–96. [Google Scholar] [CrossRef]
- Huffman, F.G. Uronic acids. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5890–5896. [Google Scholar]
- Listik, E.; Xavier, E.G.; Pinhal, M.A.S.; Toma, L. Dermatan sulfate epimerase 1 expression and mislocalization may interfere with dermatan sulfate synthesis and breast cancer cell growth. Carbohydr. Res. 2020, 488, 107906. [Google Scholar] [CrossRef]
- Mizumoto, S.; Kosho, T.; Yamada, S.; Sugahara, K. Pathophysiological Significance of Dermatan Sulfate Proteoglycans Revealed by Human Genetic Disorders. Pharmaceuticals 2017, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Bakhtiyari, S.; Assadollahi, K.; Heidarizadi, S.; Moayeri, A.; Azizi, M. Evaluating Chondroitin Sulfate and Dermatan Sulfate Expression in Glial Scar to Determine Appro-priate Intervention Time in Rats. Basic Clin. Neurosci. 2020, 11, 31–40. [Google Scholar] [PubMed]
- Walimbe, T.; Panitch, A. Proteoglycans in Biomedicine: Resurgence of an Underexploited Class of ECM Molecules. Front. Pharmacol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, T.; Thai, P.N.; Sodhi, H.; Ren, L.; Sirish, P.; Nader, C.E.; Timofeyev, V.; Overton, J.L.; Li, X.; Lam, K.S.; et al. Selectin-Targeting Glycosaminoglycan-Peptide Conjugate Limits Neutrophil Mediated Cardiac Reperfusion Injury. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, F.; Yu, Y.; Zhangb, Q. A dermatan sulfate-functionalized biomimetic nanocarrier for melanoma targeted chemotherapy. Carbohydr. Polym. 2020, 235, 115983. [Google Scholar] [CrossRef] [PubMed]
- Blachman, A.; Funez, F.; Birocco, A.M.; Saavedra, S.L.; Lázaro-Martinez, J.M.; Camperi, S.A.; Glisoni, R.; Sosnik, A.; Calabrese, G.C. Targeted anti-inflammatory peptide delivery in injured endothelial cells using dermatan sul-fate/chitosan nanomaterials. Carbohydr. Polym. 2020, 230, 115610. [Google Scholar] [CrossRef]
- Rasente, R.Y.; Imperiale, J.C.; Algarra, M.; Gualco, L.; Oberkersch, R.; Sosnik, A.; Calabrese, G.C. Dermatan sulfate/chitosan polyelectrolyte complex with potential application in the treatment and diagnosis of vascular disease. Carbohydr. Polym. 2016, 144, 362–370. [Google Scholar] [CrossRef]
- Persson, A.; Tykesson, E.; Westergren-Thorsson, G.; Malmström, A.; Ellervik, U.; Mani, K. Xyloside-primed Chondroitin Sulfate/Dermatan Sulfate from Breast Carcinoma Cells with a Defined Disaccharide Composition Has Cytotoxic Effects in Vitro. J. Biol. Chem. 2016, 291, 14871–14882. [Google Scholar] [CrossRef]
- Jyothsna, K.M.; Sarkar, P.; Jha, K.K.; Raghunathan, V.; Bhat, R. Differential levels of dermatan sulfate generate distinct Collagen I gel architectures. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hayder, J.; Chaouch, M.A.; Amira, N.; Ben Mansour, M.; Majdoub, H.; Chaubet, F.; Maaroufi, R.M. Co-immobilization of chitosan and dermatan sulfate from Raja montagui skin on polyethylene ter-ephthalate surfaces: Characterization and antibiofilm activity. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 277–287. [Google Scholar] [CrossRef]
- Ogura, C.; Hirano, K.; Mizumoto, S.; Yamada, S.; Nishihara, S. Dermatan sulphate promotes neuronal differentiation in mouse and human stem cells. J. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.T.; Walker, A. Molecular Distinctions between Heparan-Sulfate and Heparin—Analysis of Sulfation Patterns Indicates That Heparan-Sulfate and Heparin Are Separate Families of N-Sulfated Polysaccharides. Biochem. J. 1985, 230, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xiao, C.; Tan, H.; Yuan, G.; Li, J.; Li, S.; Jia, Y.; Xiong, D.; Hu, X.; Niu, X. Covalently injectable chitosan/chondroitin sulfate hydrogel integrated gelatin/heparin microspheres for soft tissue engineering. Int. J. Polym. Mater. 2019, 1–9. [Google Scholar] [CrossRef]
- Mammadov, B.; Sever, M.; Gecer, M.; Zor, F.; Ozturk, S.; Akgun, H.; Ulas, U.H.; Orhan, Z.; Guler, M.O.; Tekinay, A.B. Sciatic Nerve Regeneration Induced by Glycosaminoglycan and Laminin Mimetic Peptide Nan-ofiber Gels. RSC Adv. 2016, 6, 110535–110547. [Google Scholar] [CrossRef]
- Murali, S.; Rai, B.; Dombrowski, C.; Lee, J.; Lim, Z.; Bramono, D.; Ling, L.; Bell, T.; Hinkley, S.; Nathan, S.; et al. Affinity-selected heparan sulfate for bone repair. Biomaterials 2013, 34, 5594–5605. [Google Scholar] [CrossRef]
- Wang, C.; Poon, S.; Murali, S.; Koo, C.Y.; Bell, T.J.; Hinkley, S.F.; Yeong, H.; Bhakoo, K.; Nurcombe, V.; Cool, S. Engineering a vascular endothelial growth factor 165-binding heparan sulfate for vascular therapy. Biomaterials 2014, 35, 6776–6786. [Google Scholar] [CrossRef]
- Lee, J.H.; Luo, X.; Ren, X.; Tan, T.C.; Smith, R.A.; Swaminathan, K.; Sekar, S.; Bhakoo, K.; Nurcombe, V.; Hui, J.H.P.; et al. A Heparan Sulfate Device for the Regeneration of Osteochondral Defects. Tissue Eng. Part A 2018, 25, 352–363. [Google Scholar] [CrossRef]
- Sefkow-Werner, J.; Machillot, P.; Sales, A.; Castro-Ramirez, E.; Degardin, M.; Boturyn, D.; Cavalcanti-Adam, E.A.; Albiges-Rizo, C.; Picart, C.; Migliorini, E. Heparan sulfate co-immobilized with cRGD ligands and BMP2 on biomimetic platforms promotes BMP2-mediated osteogenic differentiation. Acta Biomater. 2020, 114, 90–103. [Google Scholar] [CrossRef]
- Casella, A.; Panitch, A.; Leach, J.K. Endogenous Electric Signaling as a Blueprint for Conductive Materials in Tissue Engineering. Bioelectricity 2020. [Google Scholar] [CrossRef]
- Melrose, J. Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease: The importance ofKS-glycodynamics and interactive capability with neuroregulatory ligands. J. Neurochem. 2019, 149, 170–194. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.; Porell, R.N.; Fernandes, S.M.; Wei, Y.; Yu, H.; Carroll, D.J.; McBride, R.; Paulson, J.C.; Tiemeyer, M.; Aoki, K.; et al. Sialylated keratan sulfate proteoglycans are Siglec-8 ligands in human airways. Glycobiology 2018, 28, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Kiwamoto, T.; Brummet, M.E.; Wu, F.; Aoki, K.; Zhu, Z.; Bochner, B.S.; Tiemeyer, M. Airway glycomic and allergic inflammatory consequences resulting from keratan sulfate galactose 6-O-sulfotransferase (CHST1) deficiency. Glycobiology 2018, 28, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.; Kesimer, M. Membrane-bound mucins of the airway mucosal surfaces are densely decorated with keratan sulfate: Revisiting their role in the Lung’s innate defense. Glycobiology 2020. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Fujinawa, R.; Yoshida, T.; Ueno, M.; Ota, F.; Kizuka, Y.; Hirayama, T.; Korekane, H.; Kitazume, S.; Maeno, T.; et al. A keratan sulfate disaccharide prevents inflammation and the progression of emphysema in murine models. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L268–L276. [Google Scholar] [CrossRef]
- Zheng, T.; Zhao, C.; Zhao, B.; Liu, H.; Wang, S.; Wang, L.; Liu, P. Impairment of the autophagy-lysosomal pathway and activation of pyroptosis in macular corneal dystrophy. Cell Death Discov. 2020, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leiphrakpam, P.D.; Patil, P.P.; Remmers, N.; Swanson, B.; Grandgenett, P.M.; Qiu, F.; Yu, F.; Radhakrishnan, P. Role of keratan sulfate expression in human pancreatic cancer malignancy. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hadley, J.A.; Horvat-Gordon, M.; Kim, W.K.; Praul, C.A.; Burns, D.; Leach, R.M., Jr. Bone sialoprotein keratan sulfate proteoglycan (BSP-KSPG) and FGF-23 are important physiolog-ical components of medullary bone. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 194, 1–7. [Google Scholar] [CrossRef]
- Melrose, J. Mucin-like glycopolymer gels in electrosensory tissues generate cues which direct electrolocation in am-phibians and neuronal activation in mammals. Neural Regen. Res. 2019, 14, 1191–1195. [Google Scholar] [CrossRef]
- Fu, L.; Sun, X.; He, W.; Cai, C.; Onishi, A.; Zhang, F.; Linhardt, R.J.; Liu, Z. Keratan sulfate glycosaminoglycan from chicken egg white. Glycobiology 2016, 26, 693–700. [Google Scholar] [CrossRef]
- Restaino, O.F.; Finamore, R.; Diana, P.; Marseglia, M.; Vitiello, M.; Casillo, A.; Bedini, E.; Parrilli, M.; Corsaro, M.M.; Trifuoggi, M.; et al. A multi-analytical approach to better assess the keratan sulfate contamination in animal origin chondroitin sulfate. Anal. Chim. Acta 2017, 958, 59–70. [Google Scholar] [CrossRef]
- Da Cunha, A.L.; de Oliveira, L.G.; Maia, L.F.; de Oliveira, L.F.C.; Michelacci, Y.M.; de Aguiar, J.A.K. Pharmaceutical grade chondroitin sulfate: Structural analysis and identification of contaminants in different commercial preparations. Carbohydr. Polym. 2015, 134, 300–308. [Google Scholar] [CrossRef]
- Bottelli, S.; Grillo, G.; Barindelli, E.; Nencioni, A.; Di Maria, A.; Fossati, T. Validated high-performance anion-exchange chromatography with pulsed amperometric detection method for the determination of residual keratan sulfate and other glucosamine impurities in sodium chondroitin sulfate. J. Chromatogr. A 2017, 1505, 43–49. [Google Scholar] [CrossRef]

| Glycosaminoglycan | Hexuronic Acid | Hexosamine |
|---|---|---|
| Chondroitin Sulfate [1] | glucuronic Acid | N-acetylgalactosamine |
| Dermatan Sulfate [1] | Iduronic Acid | N-acetylgalactosamine |
| Keratan Sulfate [1] | galactose | N-acetylglucosamine |
| Heparan Sulfate [1] | glucuronic Acid | N-acetylglucosamine |
| Hyaluronic Acid [1] | glucuronic Acid Unsulfated | N-acetyl-D-glucosamine Unsulfated |
| HA Type | Copolymer Type | Biological Testing | Biological Outcome |
|---|---|---|---|
| Unmodified HA46 | Epoxy-Agarose |
|
|
| Unmodified HA40 | Polycaprolactone |
|
|
| Methacrylated HA22 |
|
| |
| Tyramine-HA43 | Silk Fibroin |
|
|
| HA-tyramine37 | Gelatin-Tyramine |
|
|
| HA + Dithiol linker peptide26 |
|
| |
| HA + catecholamine16 |
|
| |
| Esterified HA23 |
|
| |
| HA-hydrazine17 | HA-aldehyde, HA-aldehyde, and/or collagen |
|
|
| HA-monoaldehyde33 | Carbohydrazide gelatin |
|
|
| Electrospun HA39 | Electrospun gelatin |
|
|
| CS Type | Copolymer Type | Biological Testing | Biological Outcome |
|---|---|---|---|
| Unmodified CS51 | Collagen |
|
|
| Unmodified CS53 | Collagen II + Genipin |
|
|
| CS + chitosan nanoparticle79 | chitosan–Poly(hydroxybutyrate-co-valerate) |
|
|
| Unmodified CS52 | Chitosan + SHC* + BGP* |
|
|
| Unmodified CS54 | Hydroxy–Butyl–Chitosan |
|
|
| Unmodified CS68 | Polyethylene glycol + CS binding peptide + crosslinker peptide |
|
|
| Methacrylated CS58 |
|
| |
| Methacrylated CS68 | Polyethylene glycol |
|
|
| Unmodified CS69 | Alginate |
|
|
| CS + Tyramine75 | Hydroxymethyl Pullulan |
|
|
| Oxidized CS76 | Pullulan-adipic hydrazide |
|
|
| Unmodified CS81 | Polyvinyl alcohol and gelatin |
|
|
| Methacrylated CS59 | pHPMAlac-PEG triblock polymer* |
|
|
| CS-Graphene Oxide72 |
|
| |
| Unmodified CS80 | PDMAEA-Q* |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2021, 11, 29. https://doi.org/10.3390/biom11010029
Sodhi H, Panitch A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules. 2021; 11(1):29. https://doi.org/10.3390/biom11010029
Chicago/Turabian StyleSodhi, Harkanwalpreet, and Alyssa Panitch. 2021. "Glycosaminoglycans in Tissue Engineering: A Review" Biomolecules 11, no. 1: 29. https://doi.org/10.3390/biom11010029
APA StyleSodhi, H., & Panitch, A. (2021). Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules, 11(1), 29. https://doi.org/10.3390/biom11010029






