Phellodendron amurense Extract Protects Human Keratinocytes from PM2.5-Induced Inflammation via PAR-2 Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment of the Cells with DPM, PAE, and 4-O-Feruloylquinic Acid (FQA)
2.2. Measurement of [Ca2+]i
2.3. Quantitative Real-Time PCR
2.4. Western Blot Analysis
2.5. Isolation of Active Compounds in PAE
2.6. Statistical Analysis
3. Results
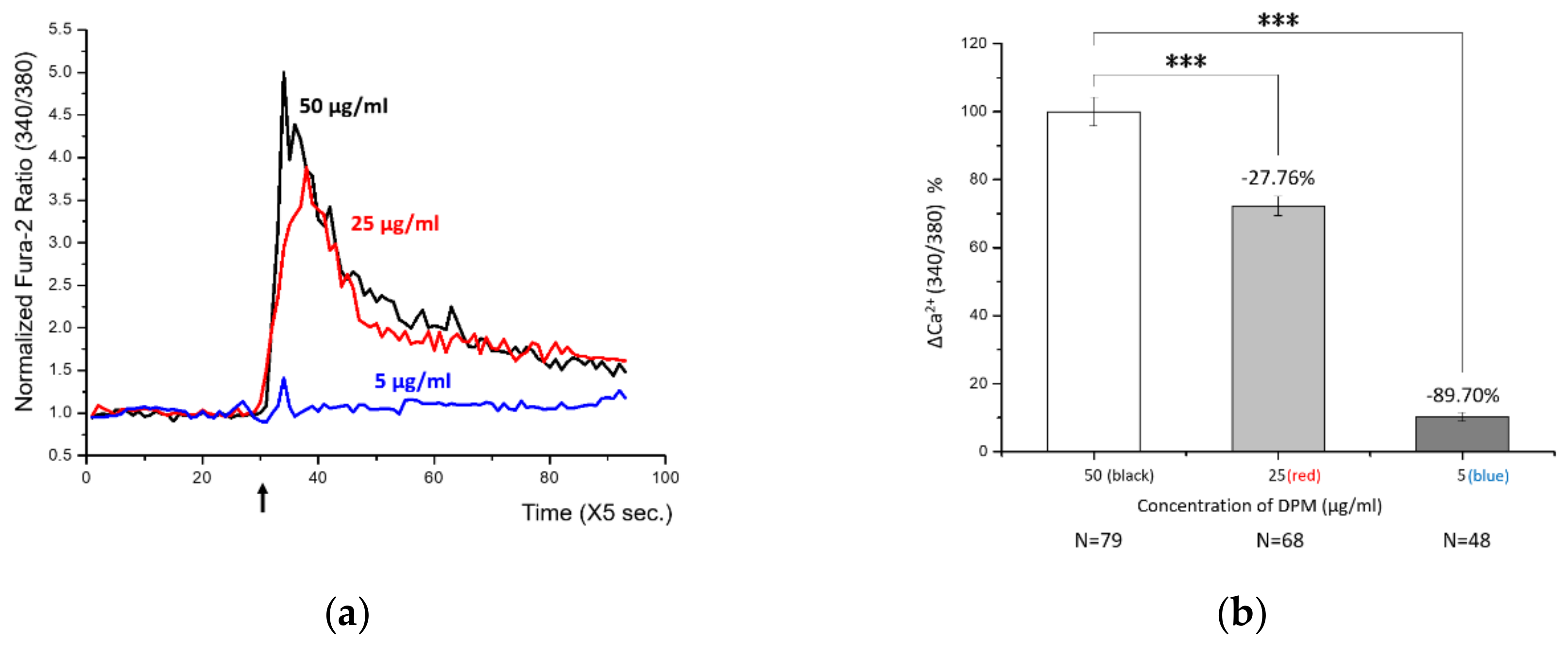
3.1. Identification of a Pollutant-Induced Intracellular Signal
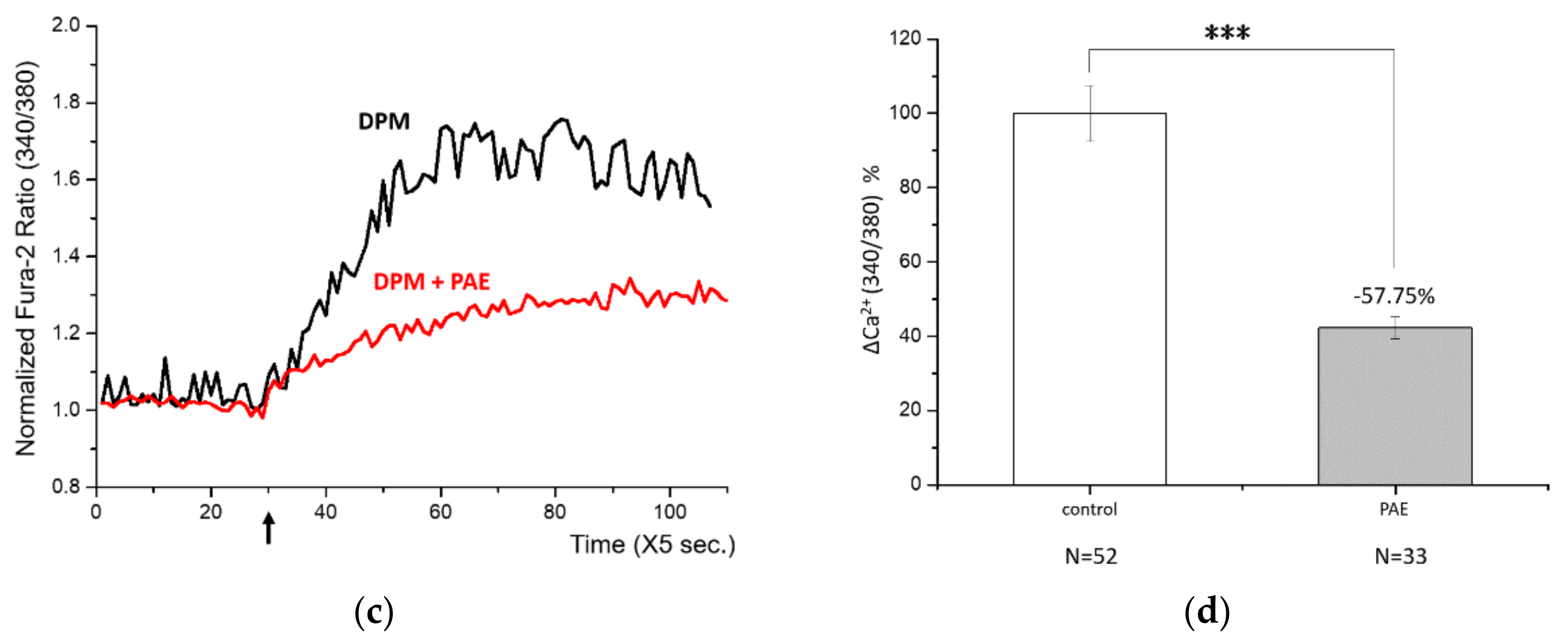
3.2. Control of DPM-Induced Ca2+ Influx by PAE
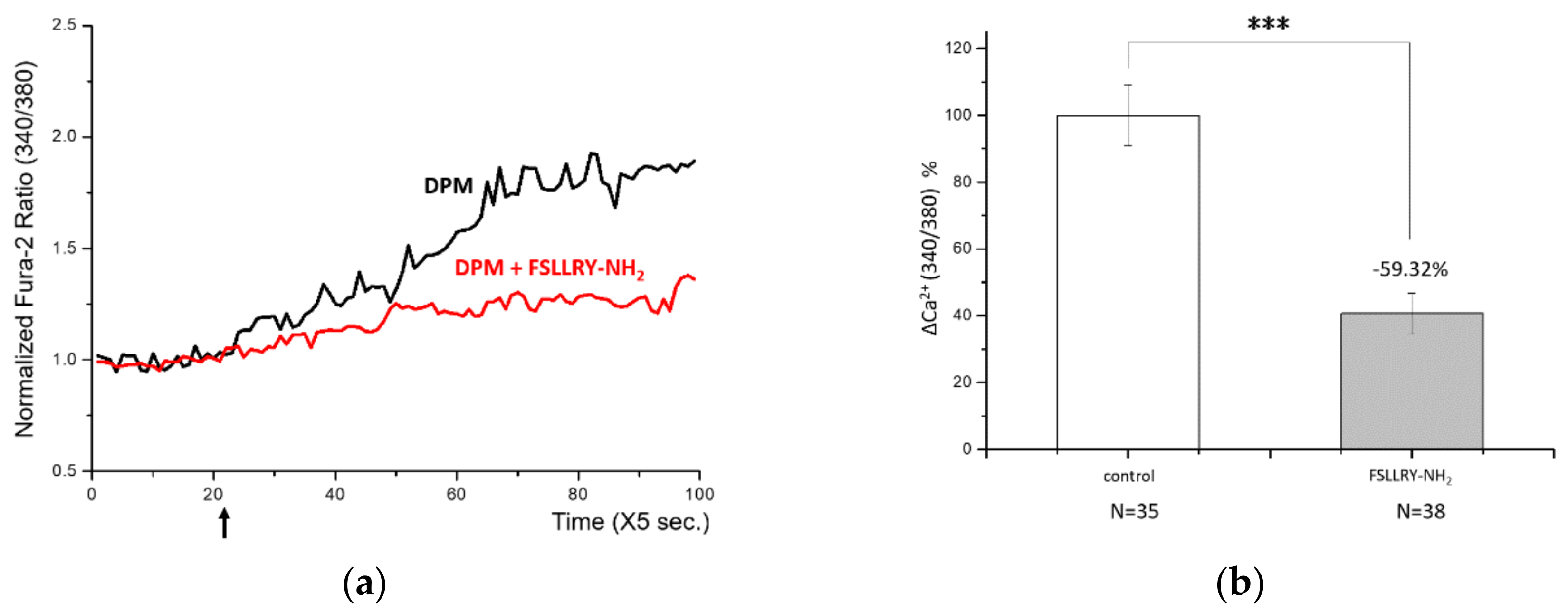
3.3. PAR-2: A Mediator of DPM-Induced Ca2+ Influx
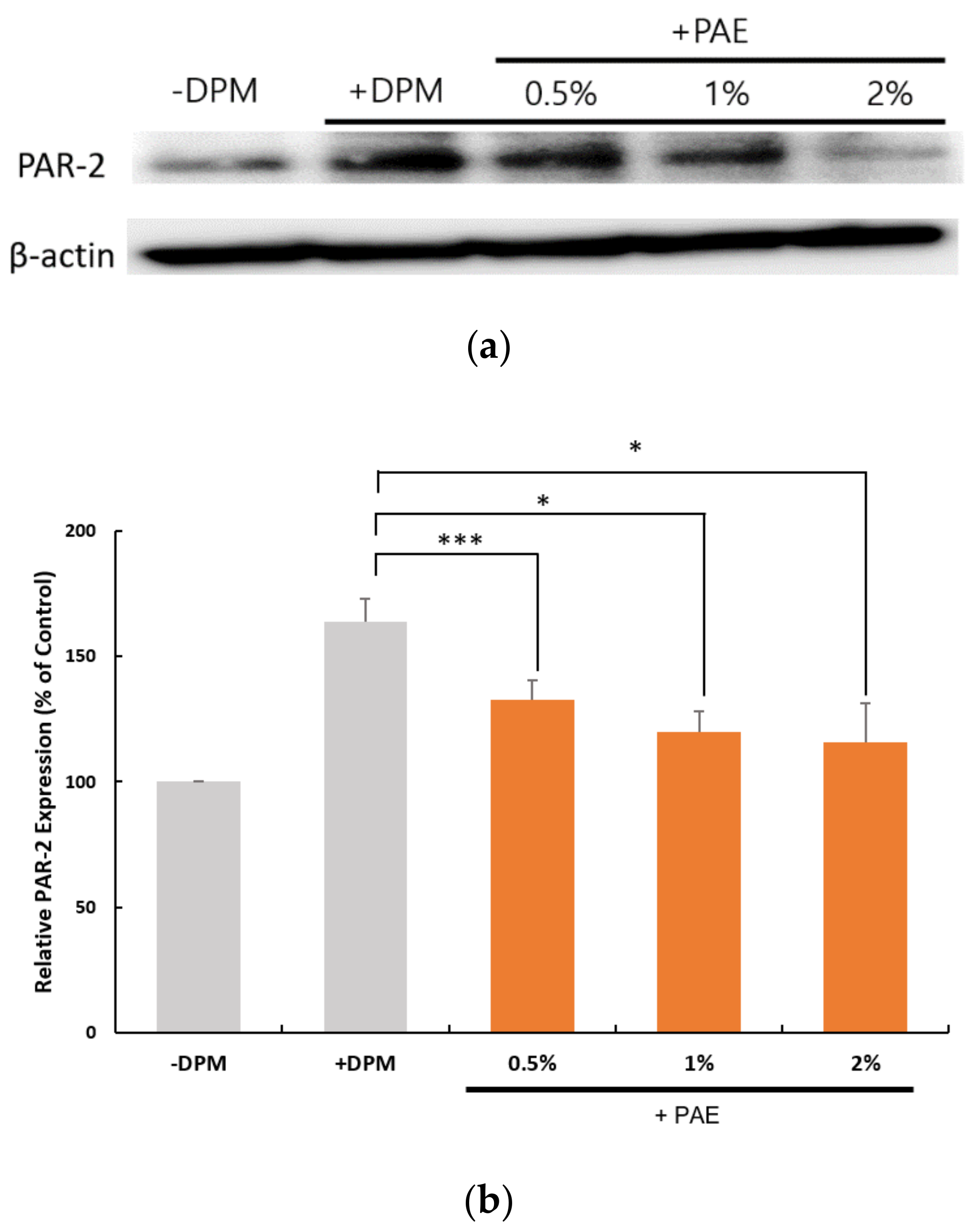
3.4. Regulation of PAR-2 Expression
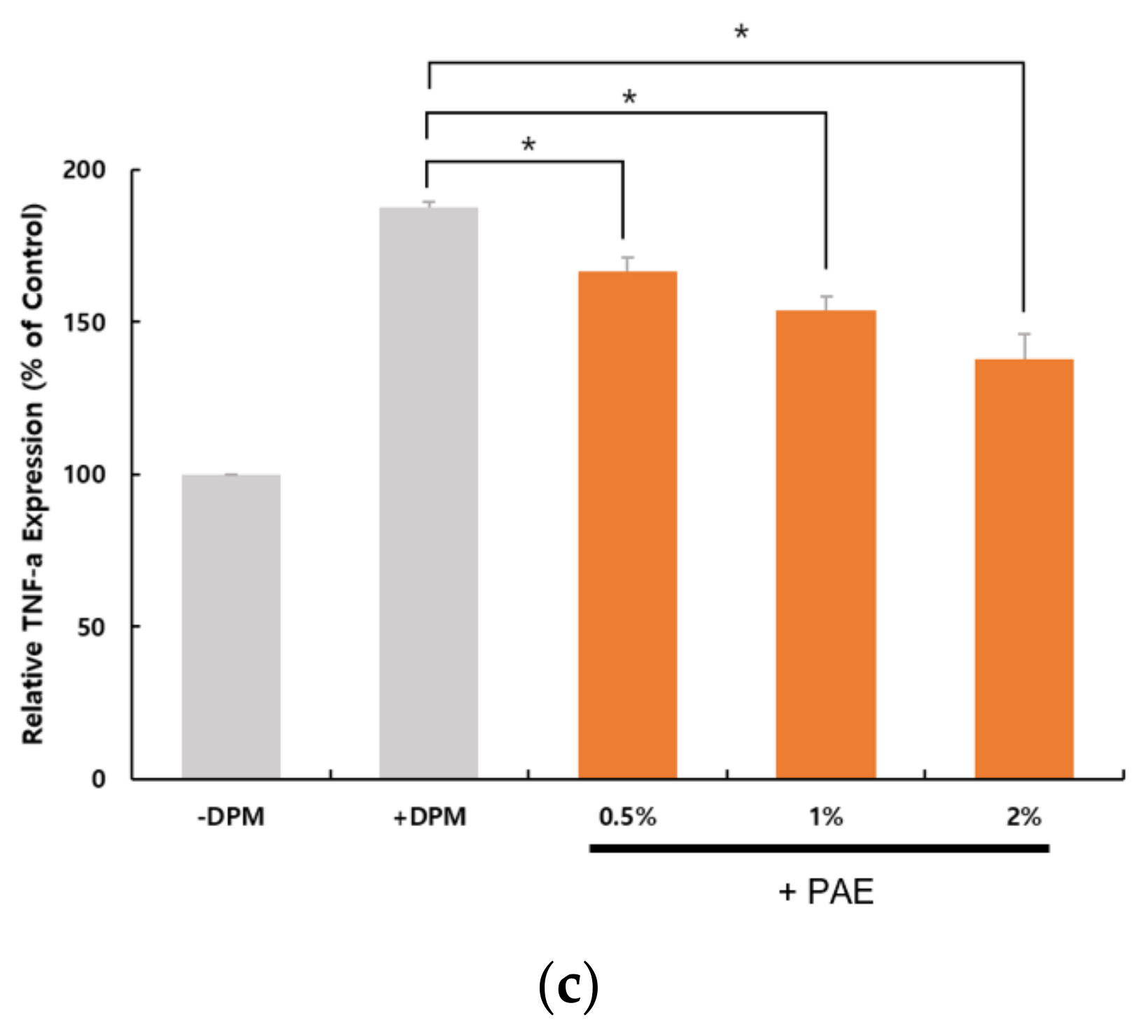
3.5. Inflammatory Conditions as an Outcome of DPM Challenge
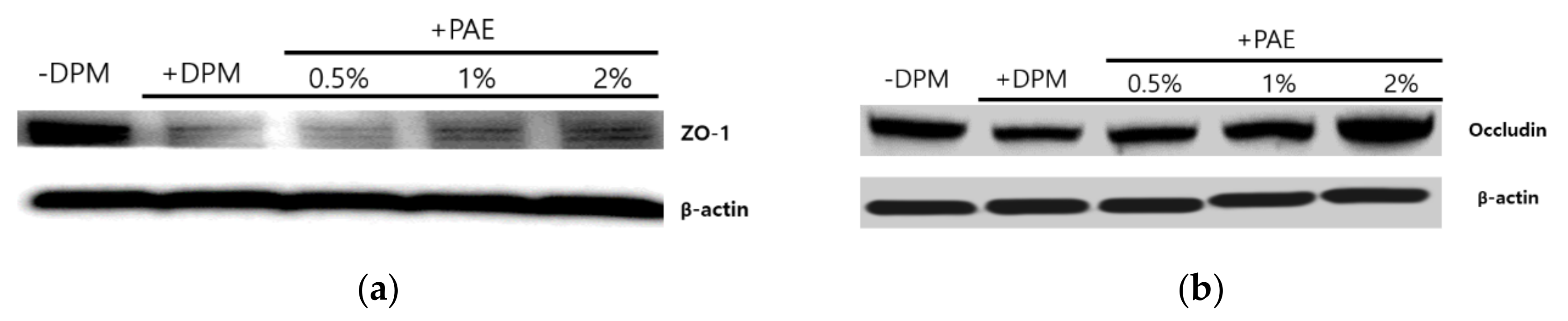
3.6. Skin Barrier Weakened by DPM and Protected by PAE
3.7. FQA: A Novel Antipollution Agent
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsouyanni, K. Ambient air pollution and health. Br. Med. Bull. 2003, 68, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Cori, L.; Donzelli, G.; Gorini, F.; Banchi, F.; Curzio, O. Risk Perception of Air Pollution: A Systematic Review Focused on Particulate Matter Exposure. Int. J. Environ. Res. Public Health 2020, 17, 6424. [Google Scholar] [CrossRef] [PubMed]
- Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 10 May 2020).
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A., III; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. New Eng. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air Pollution and Mortality in the Medicare Population. New Eng. J. Med. 2017, 376, 2513–2522. [Google Scholar]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.S.Z.S.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. New Eng. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- Baudouin, C.; Charveron, M.; Tarroux, R.; Gall, Y. Environmental pollutants and skin cancer. Cell Biol. Toxicol. 2002, 18, 341–348. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.A.; Schwarze, P.E. Activation of Proinflammatory Responses in Cells of the Airway Mucosa by Particulate Matter: Oxidant- and Non-Oxidant-Mediated Triggering Mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef]
- Park, S.-Y.; Byun, E.J.; Lee, J.D.; Kim, S.; Kim, H.S. Air Pollution, Autophagy, and Skin Aging: Impact of Particulate Matter (PM10) on Human Dermal Fibroblasts. Int. J. Mol. Sci. 2018, 19, 2727. [Google Scholar] [CrossRef]
- Li, Q.; Kang, Z.; Jiang, S.; Zhao, J.; Yan, S.; Xu, F.; Xu, J. Effects of Ambient Fine Particles PM2.5 on Human HaCaT Cells. Int. J. Environ. Res. Public Health 2017, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, R.; Yang, D.; Zhao, J.; Kan, H.; Tan, J.; Guan, M.; Kang, Z.; Xu, F. Fine Particulate Matter (PM2.5) upregulates expression of Inflammasome NLRP1 via ROS/NF-κB signaling in HaCaT Cells. Int. J. Med. Sci. 2020, 17, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Cai, X. Evolution of acidic Ca2+ stores and their resident Ca2+-permeable channels. Cell Calcium 2015, 57, 222–230. [Google Scholar] [CrossRef]
- Li, J.; Kanju, P.; Patterson, M.; Chew, W.-L.; Cho, S.-H.; Gilmour, I.; Oliver, T.; Yasuda, R.; Ghio, A.; Simon, S.A.; et al. TRPV4-Mediated Calcium Influx into Human Bronchial Epithelia upon Exposure to Diesel Exhaust Particles. Environ. Health Perspect. 2011, 119, 784–793. [Google Scholar] [CrossRef]
- Ferrell, W.R.; Lockhart, J.C.; Kelso, E.B.; Dunning, L.; Plevin, R.; Meek, S.E.; Smith, A.J.H.; Hunter, G.D.; McLean, J.S.; McGarry, F.; et al. Essential role for proteinase-activated receptor-2 in arthritis. J. Clin. Investig. 2003, 111, 35–41. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lim, J.-Y.; Jo, E.H.; Noh, H.M.; Park, S.; Park, M.C.; Kim, D.-K. Chijabyukpi-Tang Inhibits Pro-Inflammatory Cytokines and Chemokines via the Nrf2/HO-1 Signaling Pathway in TNF-α/IFN-γ-Stimulated HaCaT Cells and Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice. Front. Pharmacol. 2020, 11, 1018. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, L.; Yu, G.; Li, D.; Peng, T.; Luo, D.; Xu, J. Cathepsin S silencing induces apoptosis of human hepatocellular carcinoma cells. Am. J. Transl. Res. 2015, 7, 100–110. [Google Scholar]
- Mikirova, N.; Scimeca, R.C. Intravenous high-dose ascorbic acid reduces the expression of inflammatory markers in peripheral mononuclear cells of subjects with metabolic syndrome. J. Transl. Sci. 2016, 2, 188–195. [Google Scholar] [CrossRef][Green Version]
- Messerschmidt, L.; Fischer, S.; Wiedemann, P.; Bringmann, A.; Hollborn, M. Osmotic induction of cyclooxygenase-2 in RPE cells: Stimulation of inflammasome activation. Mol. Vis. 2019, 25, 329–344. [Google Scholar]
- Furue, K.; Ito, T.; Tanaka, Y.; Yumine, A.; Hashimoto-Hachiya, A.; Takemura, M.; Murata, M.; Yamamura, K.; Tsuji, G.; Furue, M. Cyto/chemokine profile of in vitro scratched keratinocyte model: Implications of significant upregulation of CCL20, CXCL8 and IL36G in Koebner phenomenon. J. Dermatol. Sci. 2019, 94, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.; Mohr, M.; Zänker, K.S.; Dittmar, T. Matrix metalloproteinase-9 (MMP9) is involved in the TNF-α-induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells. Cell Commun. Signal. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Shin, W.-S.; Kwon, Y.-I.; Bang, B.-H. Isolation and Purification of Antibacterial Components in Cortex Phellodendri. J. Korean Soc. Food Nutr. 2013, 26, 547–552. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Lee, B.W.; Kang, N.S.; Yang, M.S.; Park, K.H. Alkaloids from the Stem Bark of Phellodendron amurense Rupr. J. Life Sci. 2005, 15, 423–426. [Google Scholar] [CrossRef]
- Dokli, I.; Navarini, L.; Hameršak, Z. Syntheses of 3-, 4-, and 5-O-feruloylquinic acids. Tetrahedron Asymmetry 2013, 24, 785–790. [Google Scholar] [CrossRef]
- Katoga, S.; Maruyama, K.; McGuire, J.J. Characterization and Functions of Protease-Activated Receptor 2 in Obesity, Diabetes, and Metabolic Syndrome: A Systematic Review. BioMed Res. Int. 2016, 2016, 3130496. [Google Scholar]
- Mayati, A.; Le, F.E.; Holme, J.A.; Fardel, O.; Lagadic-Gossmann, D.; Øvrevik, J. Calcium signaling and beta2-adrenergic receptors regulate 1-nitropyrene induced CXCL8 responses in BEAS-2B cells. Toxicol. In Vitro 2014, 28, 1153–1157. [Google Scholar] [CrossRef]
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66 (Suppl. 1), 8–16. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Shin, Y.-S.; Kim, H.W.; Kim, C.D.; Kim, H.-W.; Park, J.W.; Jung, S.; Lee, J.-H.; Ko, Y.-K.; Lee, Y.H. Protease-activated receptor-2 is associated with terminal differentiation of epidermis and eccrine sweat glands. Ann. Dermatol. 2015, 27, 364–370. [Google Scholar] [CrossRef]
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The Impact of Pollution on Skin and Proper Efficacy Testing for Anti-Pollution Claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef]
- Jung, H.W.; Kim, K.H.; Park, Y.K. Inhibitory effect of the extract of Phellodendron amurense ruprecht root on collagen-induced arthritis in mice. Chin. J. Integr. Med. 2017, 23, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, S.; Shin, B.K. Synergistic topical application of salt-processed Phellodendron amurense and Sanguisorba officinalis Linne alleviates atopic dermatitis symptoms by reducing levels of immunoglobulin E and pro-inflammatory cytokines in NC/Nga mice. Mol. Med. Rep. 2015, 12, 7657–7664. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer Sequence (5′ to 3′) | References | |
|---|---|---|---|
| PAR-2 | Forward | CTGTGGGTCTTTCTTTTCCGAA | [19] |
| Reverse | CAAGGGGAACCAGATGACAGA | ||
| IL-6 | Forward | AGTCCTGATCCAGTTCCTGC | [20] |
| Reverse | AAGCTGCGCAGAATGAGATG | ||
| IL-8 | Forward | TGAGCATCTACGGTTTGCTG | [21] |
| Reverse | TGCTTGTCTGGAACAACTGC | ||
| TNF0-α | Forward | GAGGCCAAGCCCTGGTATG | [22] |
| Reverse | CGGGCCGATTGATCTCAGC | ||
| β-actin | Forward | CCTCGCCTTTGCCGATCC | [23] |
| Reverse | CGCGGCGATATCATCATCC | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Moon, M.Y.; Han, G.Y.; Chang, M.S.; Yang, D.; Cha, J. Phellodendron amurense Extract Protects Human Keratinocytes from PM2.5-Induced Inflammation via PAR-2 Signaling. Biomolecules 2021, 11, 23. https://doi.org/10.3390/biom11010023
Choi J, Moon MY, Han GY, Chang MS, Yang D, Cha J. Phellodendron amurense Extract Protects Human Keratinocytes from PM2.5-Induced Inflammation via PAR-2 Signaling. Biomolecules. 2021; 11(1):23. https://doi.org/10.3390/biom11010023
Chicago/Turabian StyleChoi, Jiyoung, Mi Yeon Moon, Gi Yeon Han, Moon Sik Chang, Dongki Yang, and Joonseok Cha. 2021. "Phellodendron amurense Extract Protects Human Keratinocytes from PM2.5-Induced Inflammation via PAR-2 Signaling" Biomolecules 11, no. 1: 23. https://doi.org/10.3390/biom11010023
APA StyleChoi, J., Moon, M. Y., Han, G. Y., Chang, M. S., Yang, D., & Cha, J. (2021). Phellodendron amurense Extract Protects Human Keratinocytes from PM2.5-Induced Inflammation via PAR-2 Signaling. Biomolecules, 11(1), 23. https://doi.org/10.3390/biom11010023







