Bacterial-Derived Plant Protection Metabolite 2,4-Diacetylphloroglucinol: Effects on Bacterial Cells at Inhibitory and Subinhibitory Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Purification of Antimicrobial Metabolite
2.2. Determination of Minimal Bactericidal Concentration
2.3. Time-Kill Assay
2.4. Permeabilization of the Cell Wall and Plasma Membrane
2.5. Microscopy Investigations
2.5.1. Epifluorescence Microscopy
2.5.2. Atomic Force Microscopy
2.6. Antibiofilm Activity of 2,4-DAPG (2,4-Diacetylphloroglucinol)
2.7. Sub-Inhibitory Action of 2,4-DAPG
2.7.1. SOS-Response and Protein Disturbance
2.7.2. Quenching of Bacterial Quorum Sensing by 2,4-DAPG
Investigation of the QS-Quenching Properties of 2,4-DAPG on P. carotvorum VKM-B1247
2.7.3. Quantification of Acyl-Homoserine Lactones Using a Bioluminescence-Based Assay
2.8. Statistical Analysis
3. Results
3.1. Inhibitory Activity of 2,4-DAPG
3.1.1. Spectra of Antimicrobial Activity of 2,4-DAPG
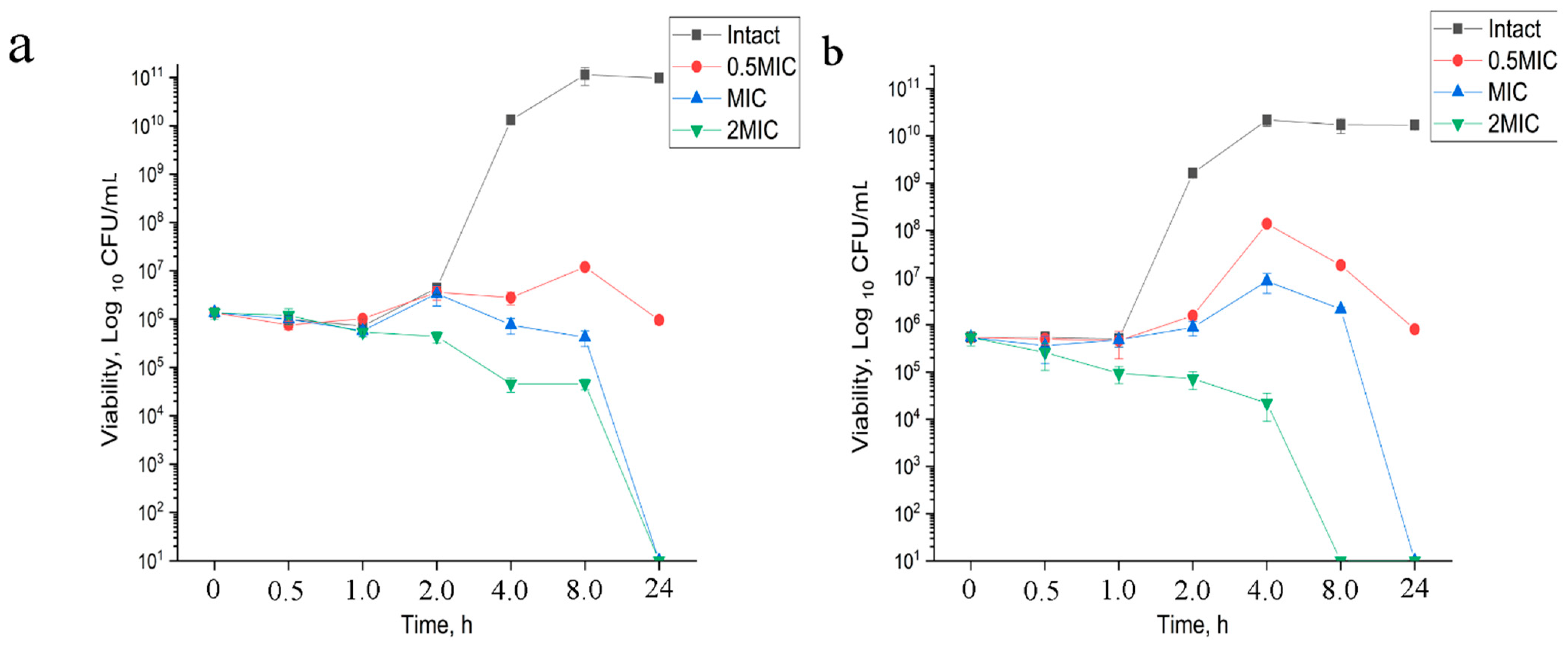
3.1.2. Time-Kill Kinetics
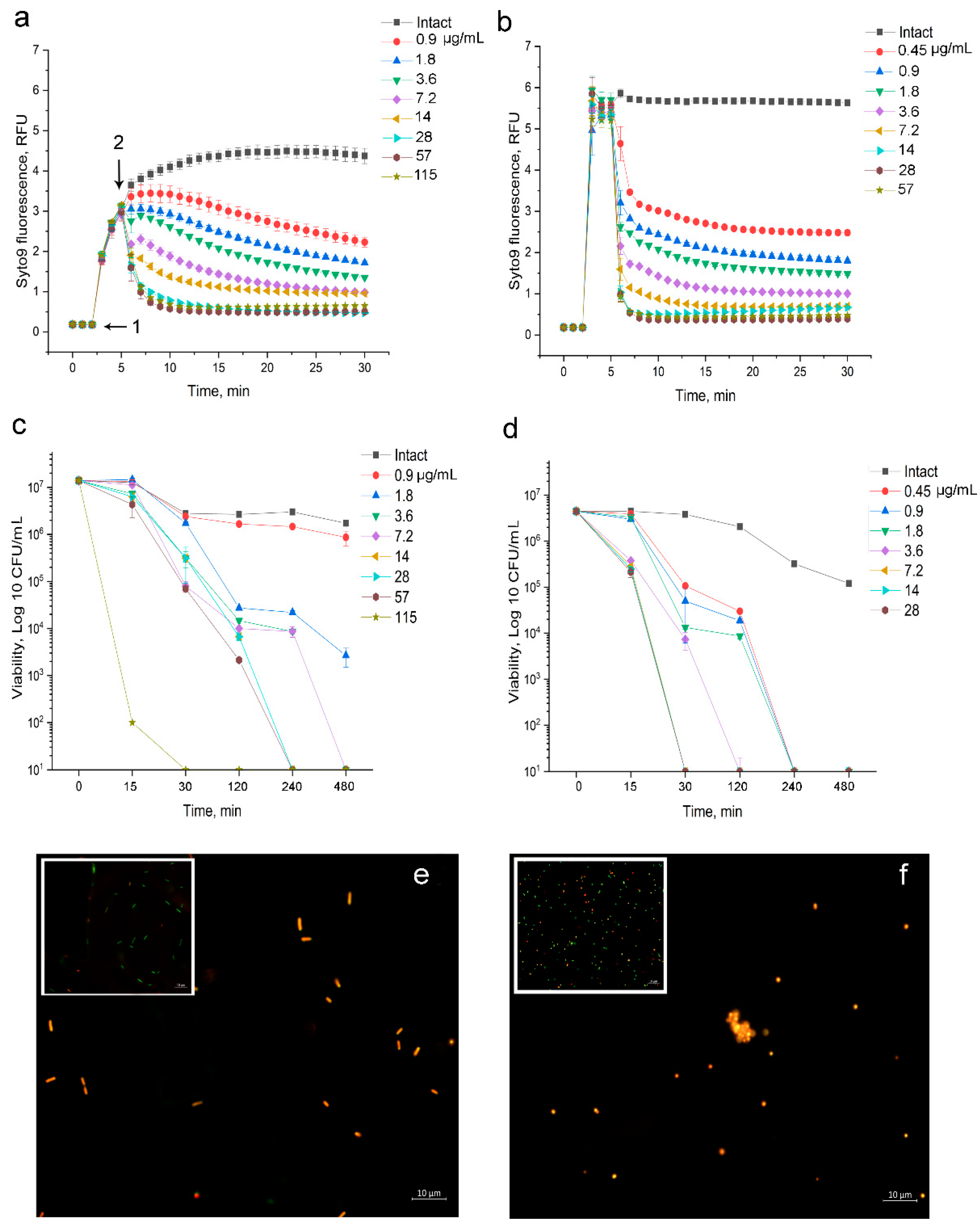
3.1.3. Fluorescent Investigation of 2,4-DAPG Action on E. coli K12
3.1.4. Fluorescent Investigation of 2,4-DAPG Action on S. aureus 209P
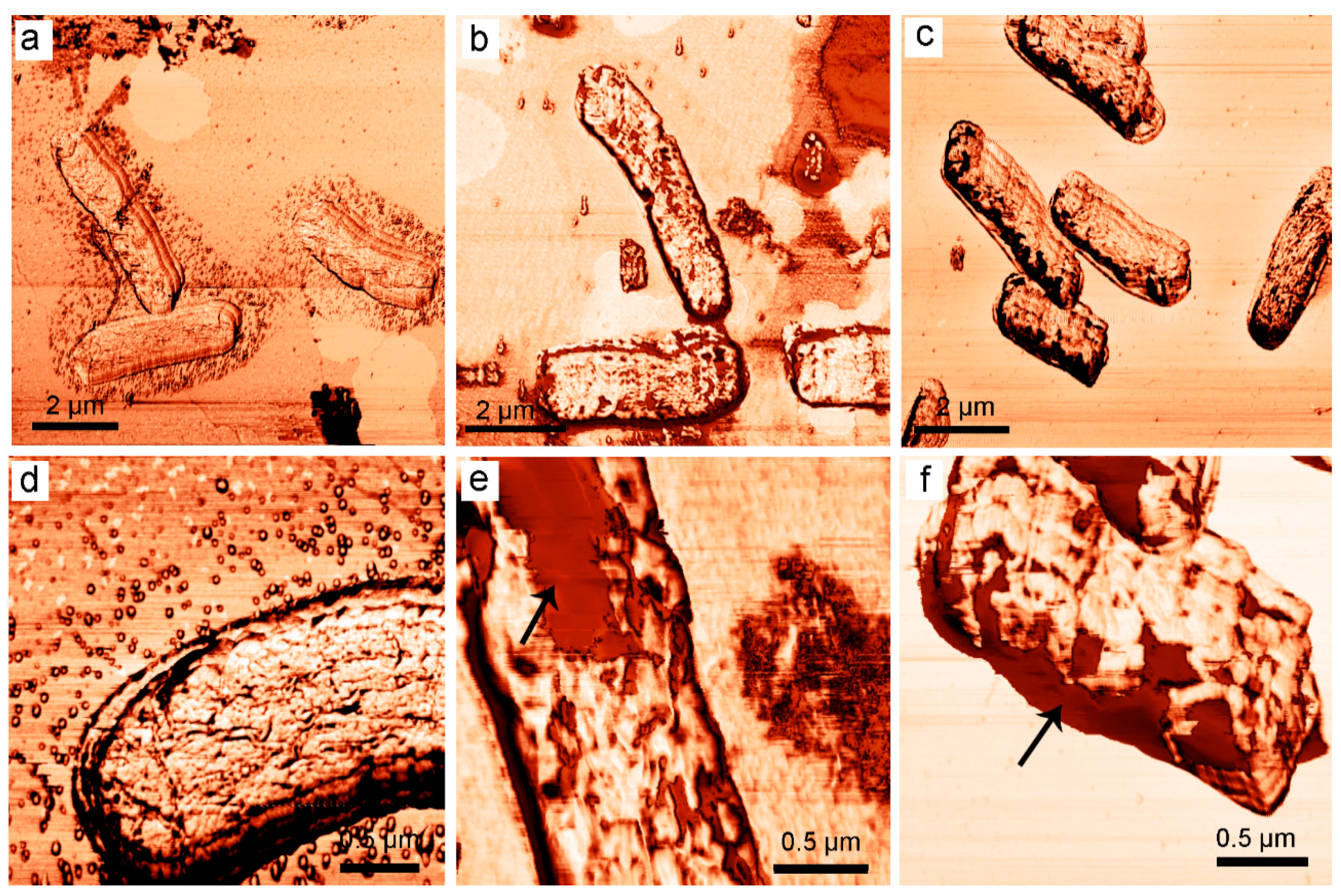
3.1.5. Atomic Force Microscopy of Bacterial Cells Treated with 2,4-DAPG
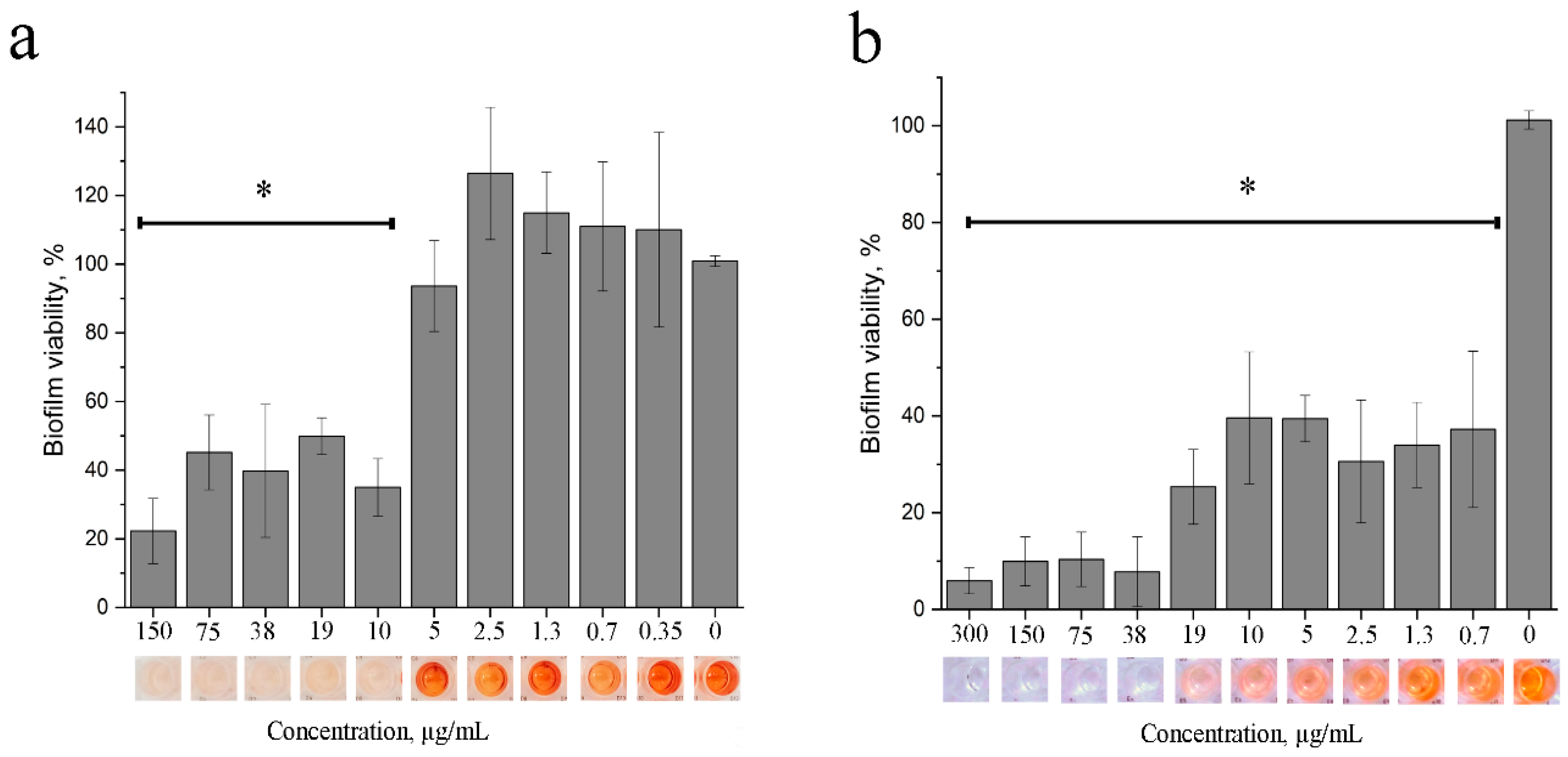
3.1.6. Anti-Biofilm Action of 2,4-DAPG
3.2. Sub-Inhibitory Activity of 2,4-DAPG
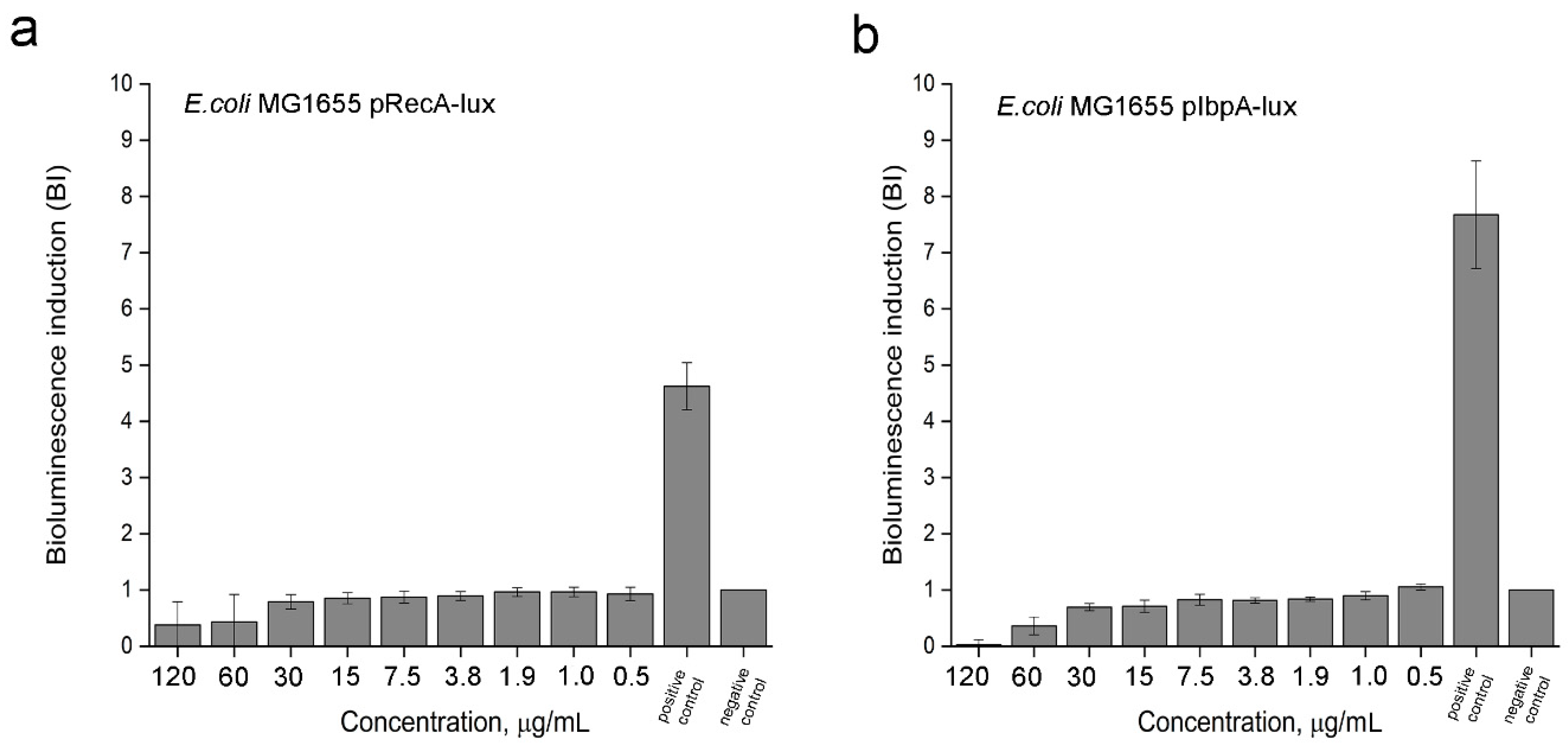
3.2.1. SOS-Response and Protein Damage of Bacteria under the 2,4-DAPG Action
3.2.2. Influence of 2,4-DAPG on the Production of Acyl-Homoserine Lactone Molecules by Pectobacterium carotovorum
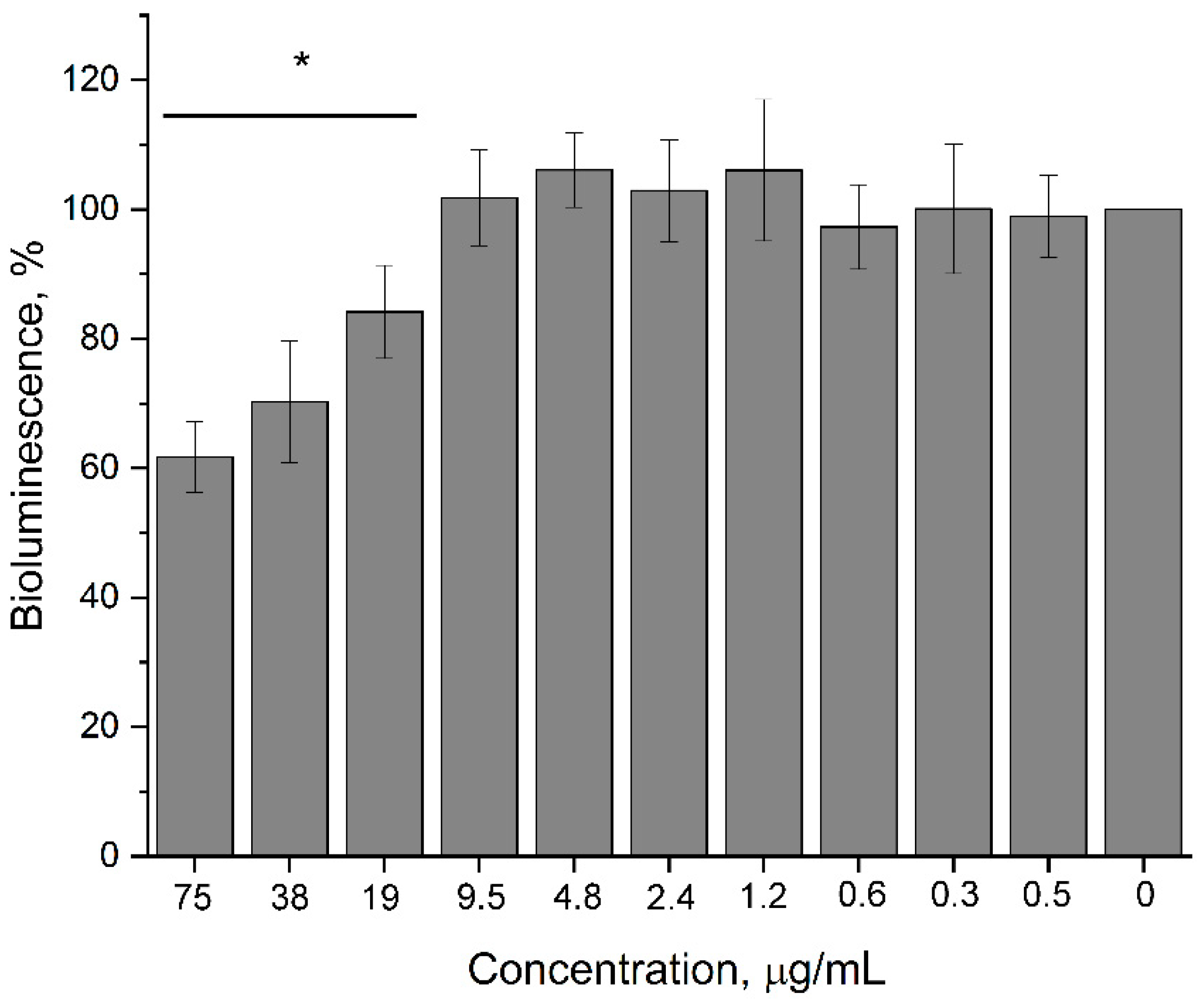
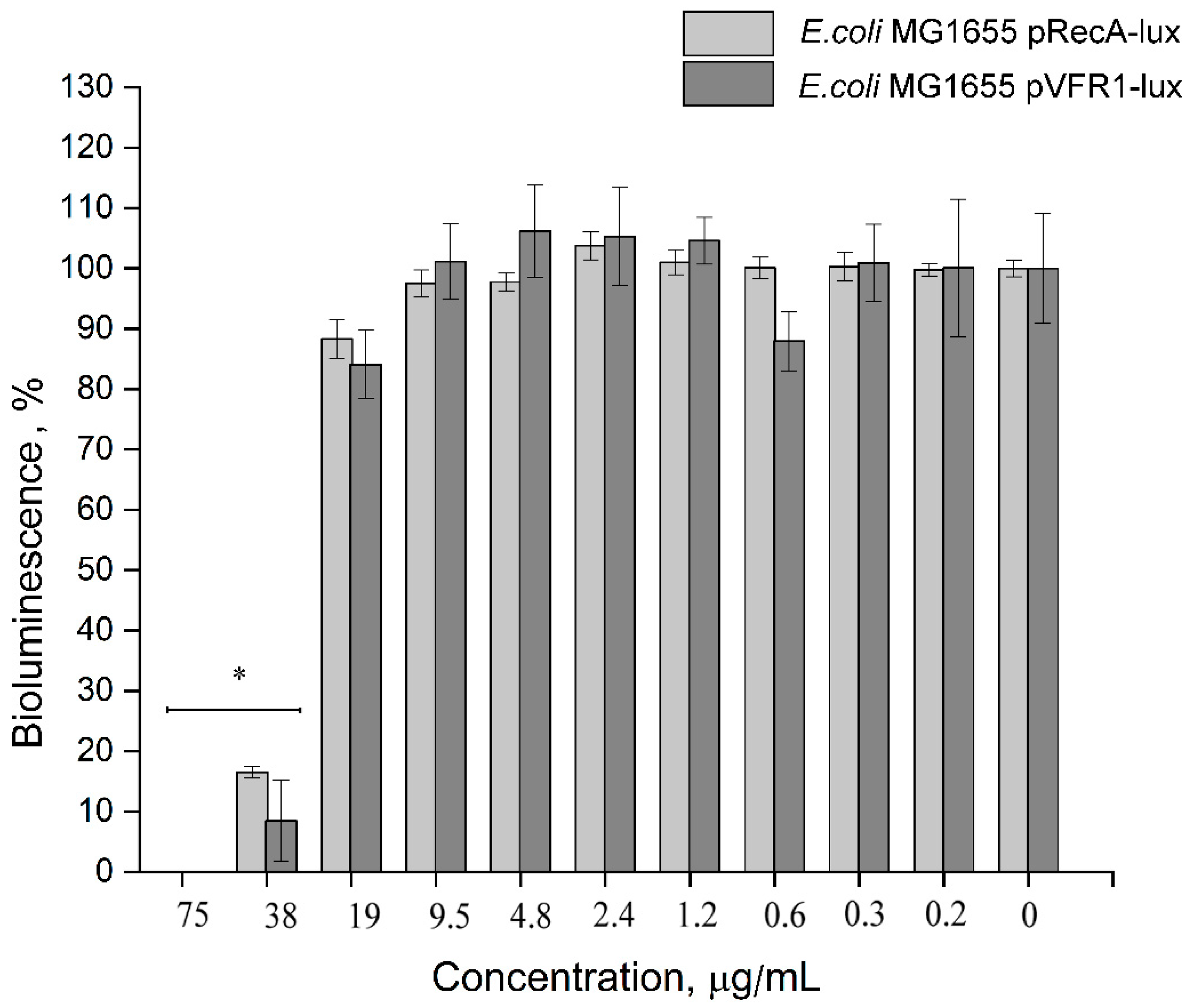
3.2.3. Impact of 2,4-DAPG on Quorum Sensing (QS)-Dependent Bioluminescence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biosecurity: An Integrated Approach to Manage Risk to Human, Animal and Plant Life and Health. Available online: https://www.who.int/foodsafety/areas_work/infosan/infosan_archives/en/ (accessed on 1 September 2020).
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.T.; Arnould, C.; Deulvot, C.; Lemanceau, P.; Gianinazzi-Pearson, V.; Raaijmakers, J.M. Effect of 2,4-Diacetylphloroglucinol on Pythium: Cellular Responses and Variation in Sensitivity among Propagules and Species. Phytopathology 2003, 93, 966–975. [Google Scholar] [CrossRef]
- Powers, M.J.; Sanabria-Valentín, E.; Bowers, A.A.; Shank, E.A. Inhibition of Cell Differentiation in Bacillus subtilis by Pseudomonas protegens. J. Bacteriol. 2015, 197, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Troppens, D.M.; Chu, M.; Holcombe, L.J.; Gleeson, O.M.; O’Gara, F.; Read, N.D.; Morrissey, J.P. The Bacterial Secondary Metabolite 2,4-Diacetylphloroglucinol Impairs Mitochondrial Function And Modulates Calcium Homeostasis in Neurospora Crassa. Fungal Genet. Biol. 2013, 56, 135–146. [Google Scholar] [CrossRef]
- Reddi, T.K.; Borovkov, A.V. Antibiotic Properties Of 2,4-Diacetylphloroglucinol Produced By Pseudomonas fluorescens Strain 26-0. Antibiotiki 1970, 15, 19–21. [Google Scholar]
- Kwak, Y.-S.; Han, S.; Thomashow, L.S.; Rice, J.T.; Paulitz, T.; Kim, D.; Weller, D. Saccharomyces cerevisiaeGenome-Wide Mutant Screen for Sensitivity to 2,4-Diacetylphloroglucinol, an Antibiotic Produced byPseudomonas fluorescens. Appl. Environ. Microbiol. 2010, 77, 1770–1776. [Google Scholar] [CrossRef]
- Gleeson, O.; O’Gara, F.; Morrissey, J.P. The Pseudomonas Fluorescens Secondary Metabolite 2,4 Diacetylphloroglucinol Impairs Mitochondrial Function in Saccharomyces Cerevisiae. Anton. Van Leeuw. 2010, 97, 261–273. [Google Scholar] [CrossRef]
- Schouten, A.; Maksimova, O.; Cuesta-Arenas, Y.; Van den Berg, G.; Raaijmakers, J.M. Involvement of the Abc Transporter Bcatrb and the Lactase Bclcc2 in Defence of Botrytis Cinerea against the Broad-Spectrum Antibiotic 2,4-Diacetylphloroglucinol. Environ. Microbiol. 2008, 10, 1145–1157. [Google Scholar] [CrossRef]
- Isnansetyo, A.; Horikawa, M.; Kamei, Y. In vitro Anti-MRSA Activity of 2,4 Diacetylphloroglucinol Produced by Pseudomonas sp. Amsn Which Was Isolated from a Marine Alga. J. Antimicrob. Chemother. 2007, 47, 724–725. [Google Scholar] [CrossRef]
- Joshi, J.R.; Khazanov, N.; Senderowitz, H.; Burdman, S.; Lipsky, A.; Yedidia, A. Plant Phenolic Volatiles Inhibit Quorum Sensing in Pectobacteria and Reduce Their Virulence by Potential Binding to Expi and Expr Proteins. Sci. Rep. 2016, 6, 38126. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; M26-A; NCCLS: Wayne, PA, USA, 1999. [Google Scholar]
- Cheng, M.; Huang, J.X.; Ramu, S.; Butler, M.S.; Cooper, M.A. Ramoplanin at bactericidal concentrations induces bacterial membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 6819–6827. [Google Scholar] [CrossRef] [PubMed]
- Belley, A.; Neesham-Grenon, E.; McKay, G.; Arhin, F.F.; Harris, R.; Beveridge, T.; Parr, T.R.; Moeck, G. Oritavancin Kills Stationary-Phase and Biofilm Staphylococcus aureus Cells In Vitro. Antimicrob. Agents Chemother. 2008, 53, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, E.A.; Sadykova, V.S.; Baranova, A.A.; Vasilchenko, A.S.; Lushpa, V.A.; Mineev, K.S.; Georgieva, M.L.; Kul’ko, A.B.; Krasheninnikov, M.E.; Lyundup, A.V.; et al. A Novel Lipopeptaibol Emericellipsin A with Antimicrobial and Antitumor Activity Produced by the Extremophilic Fungus Emericellopsis Alkalina. Molecules 2018, 23, 2785. [Google Scholar] [CrossRef]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R. Optimization Of Tetrazolium Salt Assay For Pseudomonas Aeruginosa Biofilm Using Microtiter Plate Method. J. Microbiol. Methods 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Kotova, V.Y.; Manukhov, I.V.; Zavilgelskii, G.B. Lux-biosensors for detection of SOS-response, heat shock, and oxidative stress. Appl. Biochem. Microbiol. 2010, 46, 781–788. [Google Scholar] [CrossRef]
- Manukhov, I.V.; Melkina, O.E.; Goryanin, I.I.; Baranova, A.V.; Zavilgelsky, G.B. The n-terminal Domain of Aliivibrio fischeri luxR is a Target of the groel Chaperonin. J. Bacteriol. 2010, 192, 5549–5551. [Google Scholar] [CrossRef]
- Manukhov, I.V.; Khrul’nova, S.A.; Baranova, A.; Zavilgelsky, G.B. Comparative Analysis of the Lux Operons in Aliivibrio Logei Kch1 (a Kamchatka Isolate) and Aliivibrio Salmonicida. J. Bacteriol. 2011, 193, 3998–4001. [Google Scholar] [CrossRef]
- Dorobantu, L.S.; Gray, M.R. Application of atomic force microscopy in bacterial research. Scanning 2010, 32, 74–96. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Comprehensive Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Gong, L.; Tan, H.; Chen, F.; Li, T.; Zhu, J.; Jian, Q.; Yuan, D.; Xu, L.; Hu, W.; Jiang, Y.; et al. Novel synthesized 2, 4-DAPG analogues: Antifungal activity, mechanism and toxicology. Sci. Rep. 2016, 6, 32266. [Google Scholar] [CrossRef] [PubMed]
- Stukalov, O.; Korenevsky, A.; Beveridge, T.J.; Dutcher, J.R. Use of atomic force microscopy and transmission electron microscopy for correlative studies of bacterial capsules. Appl. Environ. Microbiol. 2008, 74, 5457–5465. [Google Scholar] [CrossRef] [PubMed]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure–activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta BBA Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, S.-B.; Park, S.-J.; Seo, Y.-S.; Gong, R.; Choi, J.-G.; Shin, N.-W.; Rho, J.-R.; Kang, O.-H.; et al. The Mechanism of Antimicrobial Activity of Sophoraflavanone B against Methicillin-Resistant Staphylococcus Aureus. Foodborne Pathog Dis. 2014, 11, 234–249. [Google Scholar] [CrossRef]
- Rathinam, P.; Vijay Kumar, H.S.; Viswanathan, P. Eugenol Exhibits Anti-virulence Properties by Competitively Binding to Quorum Sensing Receptors. Biofouling 2017, 33, 624–639. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.; Woertman, J.; Veldhuizen, E.J. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef]
- Inchagova, K.S.; Duskaev, G.K.; Deryabin, D. Quorum Sensing Inhibition in Chromobacterium violaceum by Amikacin Combination with Activated Charcoal or Small Plant-Derived Molecules (Pyrogallol and Coumarin). Microbiology 2019, 88, 63–71. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, T.; Tan, X.; Sheng, J.; Jia, A. Can The Quorum Sensing Inhibitor Resveratrol Function as an Aminoglycoside Antibiotic Accelerant Against Pseudomonas aeruginosa? Int. J. Antimicrob. Agents 2018, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.C.; Buchanan, A.; Vining, O.; Kidarsa, T.A.; Chang, J.H.; McPhail, K.L.; Loper, J.E. Phloroglucinol Influences Gene Expression of P. protegens. Environ. Microbiol. 2016, 18, 3296–3308. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Lee, S.-J.; Park, N.-H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, A.; Suh, J.; Park, S.-C. Impact Of Phenolic Compounds in the Acyl Homoserine Lactone-Mediated Quorum Sensing Regulatory Pathways. Sci. Rep. 2017, 7, 10618. [Google Scholar] [CrossRef] [PubMed]
- Põllumaa, L.; Alamäe, T.; Mäe, A. Quorum Sensing and Expression of Virulence in Pectobacteria. Sensors 2012, 12, 3327–3349. [Google Scholar] [CrossRef]
- Crépin, A.; Barbey, C.; Beury-Cirou, A.; Hélias, V.; Taupin, L.; Reverchon, S.; Nasser, W.; Faure, D.; Dufour, A.; Orange, N.; et al. Quorum Sensing Signaling Molecules Produced by Reference and Emerging Soft-Rot Bacteria (Dickeya and Pectobacterium spp.). PLoS ONE 2012, 7, e35176. [Google Scholar] [CrossRef]
- Defoirdt, T.; Brackman, G.; Coenye, T. Quorum sensing inhibitors: How strong is the evidence? Trends Microbiol. 2013, 21, 619–624. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Rogozhin, E.A. Sub-inhibitory Effects of Antimicrobial Peptides. Front. Microbiol. 2019, 10, 1160. [Google Scholar] [CrossRef]


| Type of Cell Wall | Strains | MIC, µg/mL | MBC, µg/mL |
|---|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus 209 P | 2.0 | 2.0 |
| Clavibacter michiganensis VKM AS-1405 | 25 | 25 | |
| Enterococcus faecium ICIS 153 | 50 | 100 | |
| Gram-negative bacteria | Pectobacterium caratovorum VKM-B1247 | 90 | 90 |
| Chromobacterium violaceum ATCC 31532 | 24 | 48 | |
| Pseudomonas savastanoi VKM-1546 | >300 | >300 | |
| Escherichia coli K12 | 300 | 300 | |
| Pseudomonas aeruginosa ATCC 28753 | >300 | >300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julian, W.T.; Vasilchenko, A.V.; Shpindyuk, D.D.; Poshvina, D.V.; Vasilchenko, A.S. Bacterial-Derived Plant Protection Metabolite 2,4-Diacetylphloroglucinol: Effects on Bacterial Cells at Inhibitory and Subinhibitory Concentrations. Biomolecules 2021, 11, 13. https://doi.org/10.3390/biom11010013
Julian WT, Vasilchenko AV, Shpindyuk DD, Poshvina DV, Vasilchenko AS. Bacterial-Derived Plant Protection Metabolite 2,4-Diacetylphloroglucinol: Effects on Bacterial Cells at Inhibitory and Subinhibitory Concentrations. Biomolecules. 2021; 11(1):13. https://doi.org/10.3390/biom11010013
Chicago/Turabian StyleJulian, William T., Anastasia V. Vasilchenko, Daniil D. Shpindyuk, Darya V. Poshvina, and Alexey S. Vasilchenko. 2021. "Bacterial-Derived Plant Protection Metabolite 2,4-Diacetylphloroglucinol: Effects on Bacterial Cells at Inhibitory and Subinhibitory Concentrations" Biomolecules 11, no. 1: 13. https://doi.org/10.3390/biom11010013
APA StyleJulian, W. T., Vasilchenko, A. V., Shpindyuk, D. D., Poshvina, D. V., & Vasilchenko, A. S. (2021). Bacterial-Derived Plant Protection Metabolite 2,4-Diacetylphloroglucinol: Effects on Bacterial Cells at Inhibitory and Subinhibitory Concentrations. Biomolecules, 11(1), 13. https://doi.org/10.3390/biom11010013







