Spatial Structure and Activity of Synthetic Fragments of Lynx1 and of Nicotinic Receptor Loop C Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Linear and Disulfide-Stabilized Fragments of Human Lynx1, of the Lymnaea Stagnalis AChBP Loop C and of the Combinatorial Peptide HAP
2.2. Activity of Synthetic Fragments of Lynx1 and of Nicotinic Receptor Loop C Models in Radioligand Assay
2.3. CD Analysis of the Synthetic Peptides
2.4. NMR Spectroscopy
3. Results
3.1. Synthesis of Linear and Disulfide-Stabilized Fragments of Human Lynx1 Loop II, of the L. stagnalis AChBP Loop C and of the Combinatorial Peptide HAP
3.2. Activity of Synthetic Fragments of Lynx1 and of Nicotinic Receptor Loop C Models in Radioligand Assay
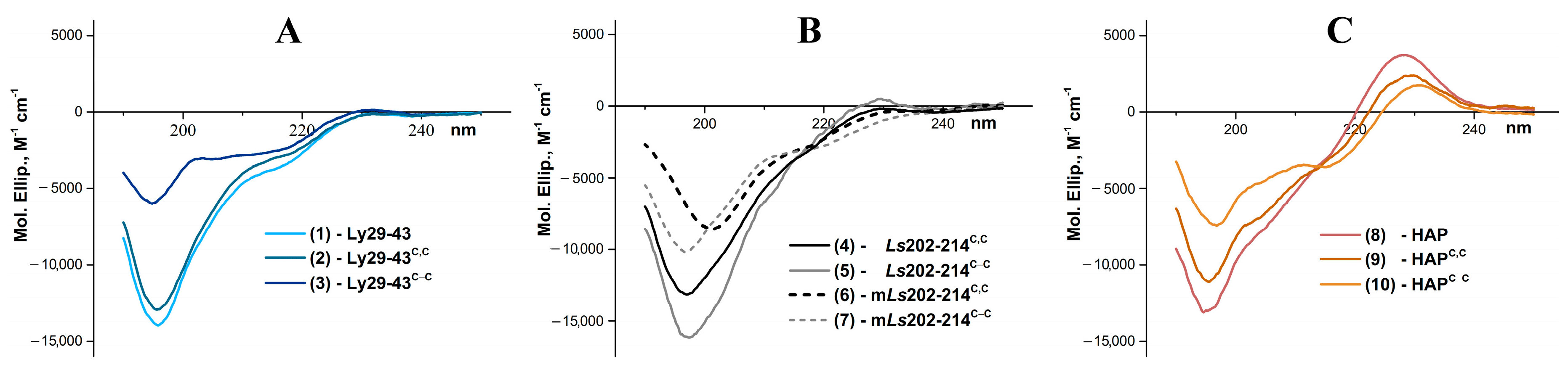
3.3. CD Analysis of the Synthetic Peptides
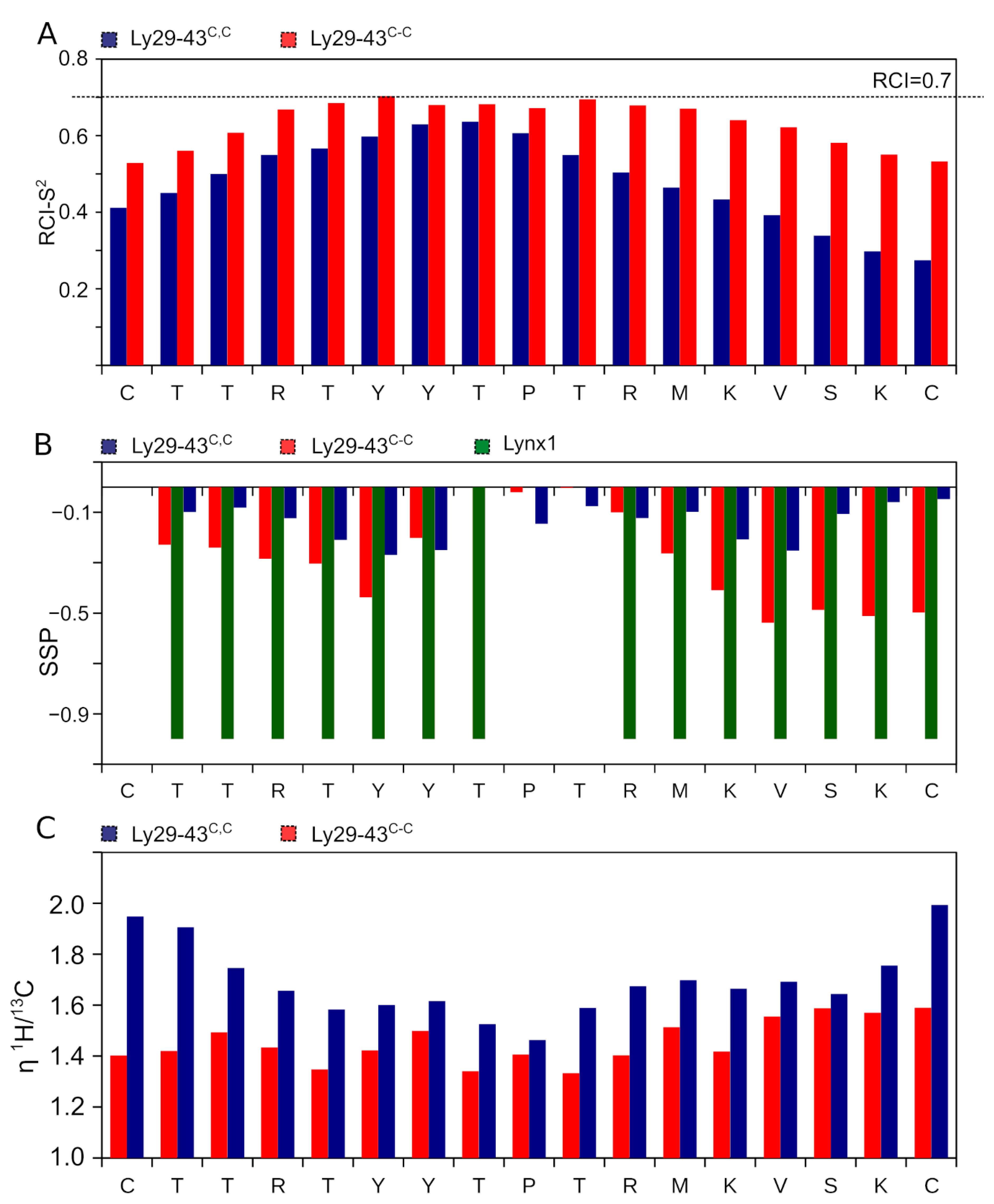
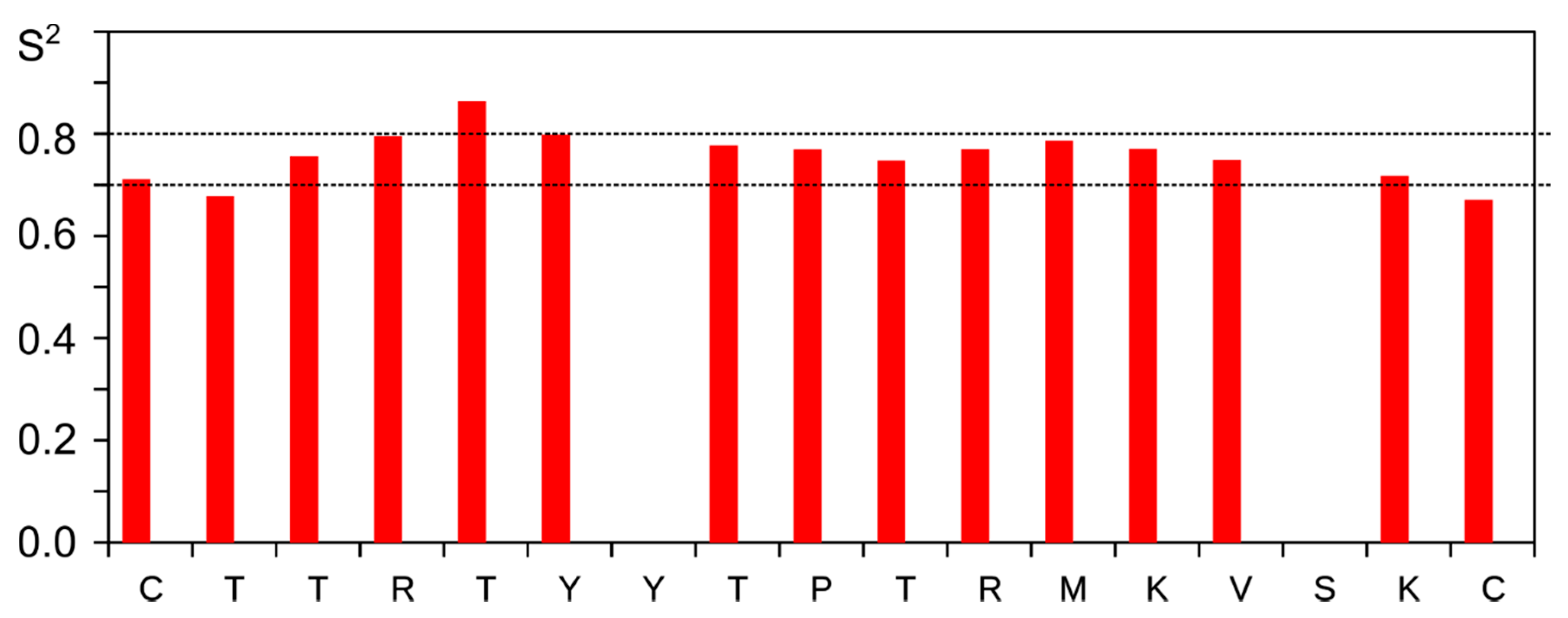
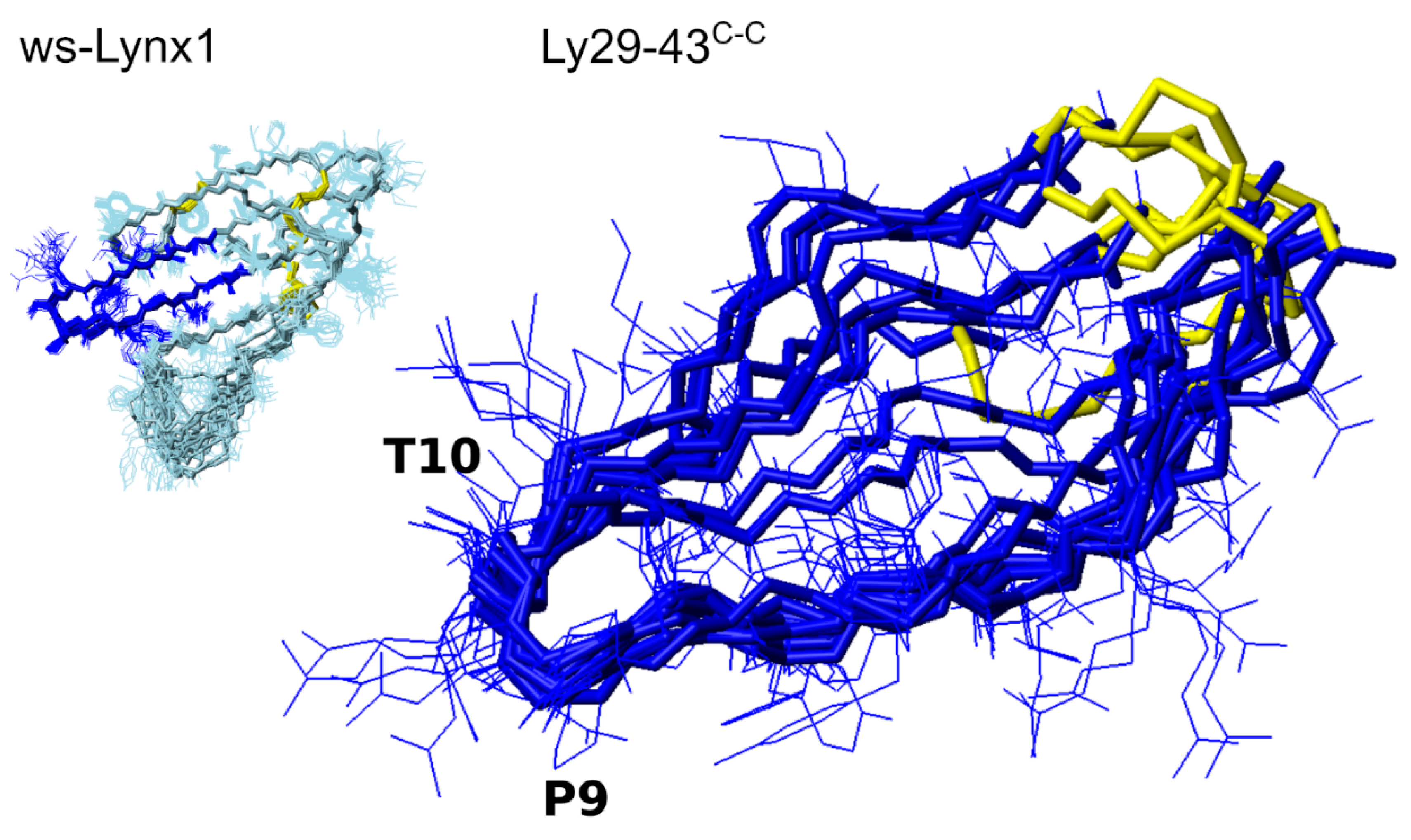
3.4. NMR Analysis of Synthetic Lynx1 Fragments
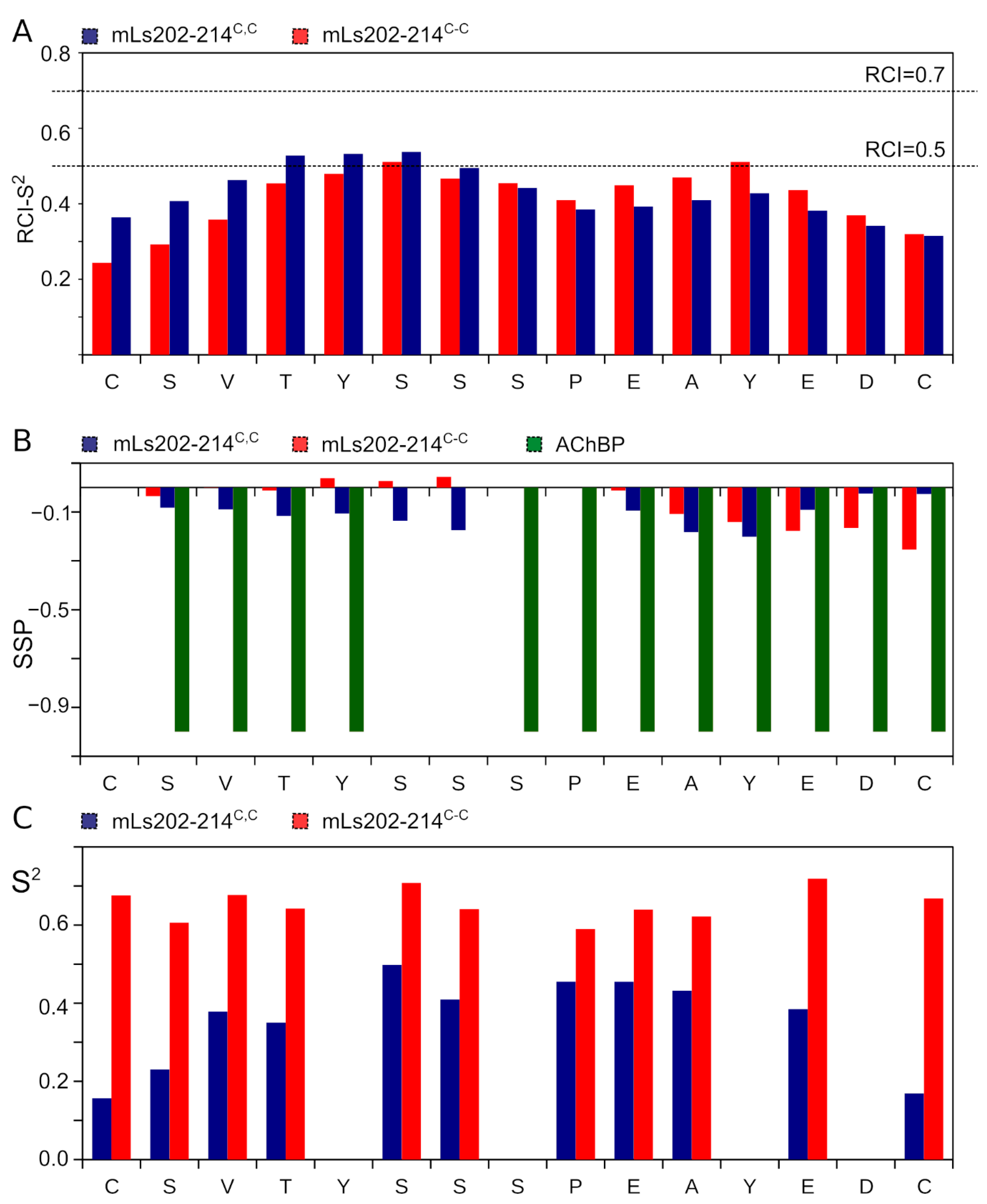
3.5. NMR Analysis of Synthetic Analogs of the L. stagnalis AChBP Loop C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nirthanan, S. Snake three-finger α-neurotoxins and nicotinic acetylcholine receptors: Molecules, mechanisms and medicine. Biochem. Pharmacol. 2020, 114168. [Google Scholar] [CrossRef] [PubMed]
- Tsetlin, V.I.; Kasheverov, I.E.; Utkin, Y.N. Three-finger proteins from snakes and humans acting on nicotinic receptors: Old and new. J. Neurochem. 2020. [Google Scholar] [CrossRef]
- Kini, R.M. Toxins for decoding interface selectivity in nicotinic acetylcholine receptors. Biochem. J. 2019, 476, 1515–1520. [Google Scholar] [CrossRef]
- Miwa, J.M.; Anderson, K.R.; Hoffman, K.M. Lynx prototoxins: Roles of endogenous mammalian neurotoxin-like proteins in modulating nicotinic acetylcholine receptor function to influence complex biological processes. Front. Pharmacol. 2019, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, N.A.; Loktyushov, E.V.; Bychkov, M.L.; Shenkarev, Z.O.; Lyukmanova, E.N. Three-finger proteins from the Ly6/uPAR family: Functional diversity within one structural motif. Biochemistry 2017, 82, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Tsetlin, V.I. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: Pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 2015, 36, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Kasheverov, I.E.; Utkin, Y.N.; Tsetlin, V.I. Naturally occurring and synthetic peptides acting on nicotinic acetylcholine receptors. Curr. Pharm. Des. 2009, 15, 2430–2452. [Google Scholar] [CrossRef] [PubMed]
- Tsetlin, V.I.; Utkin, Y.N.; Kasheverov, I.E. Polypeptide and peptide toxins, magnifying lenses for binding sites in nicotinic acetylcholine receptors. Biochem. Pharmacol. 2009, 78, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Giastas, P.; Zouridakis, M.; Tzartos, S.J. Understanding structure-function relationships of the human neuronal acetylcholine receptor: Insights from the first crystal structures of neuronal subunits. Br. J. Pharmacol. 2018, 175, 1880–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryukova, E.V.; Egorova, N.S.; Kudryavtsev, D.S.; Lebedev, D.S.; Spirova, E.N.; Zhmak, M.N.; Garifulina, A.I.; Kasheverov, I.E.; Utkin, Y.N.; Tsetlin, V.I. From synthetic fragments of endogenous three-finger proteins to potential drugs. Front. Pharmacol. 2019, 10, 748. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.T.; Lentz, T.L.; Hawrot, E. Determination of the primary amino acid sequence specifying the α-bungarotoxin binding site on the α subunit of the acetylcholine receptor from Torpedo californica. Proc. Natl. Acad. Sci. USA 1985, 82, 8790–8794. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.T.; Hawrot, E.; Lentz, T.L. Distribution of α-bungarotoxin binding sites over residues 173-204 of the α subunit of the acetylcholine receptor. Mol. Pharmacol. 1988, 34, 643–650. [Google Scholar] [PubMed]
- Marinou, M.; Tzartos, S.J. Identification of regions involved in the binding of α-bungarotoxin to the human α7 neuronal nicotinic acetylcholine receptor using synthetic peptides. Biochem. J. 2003, 372, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, M.; Spiga, O.; Ciutti, A.; Bernini, A.; Bracci, L.; Lelli, B.; Lozzi, L.; Calamandrei, D.; Di Maro, D.; Klein, S.; et al. NMR structure of α-bungarotoxin free and bound to a mimotope of the nicotinic acetylcholine receptor. Biochemistry 2002, 41, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Kasher, R.; Balass, M.; Scherf, T.; Fridkin, M.; Fuchs, S.; Katchalski-Katzir, E. Design and synthesis of peptides that bind α-bungarotoxin with high affinity. Chem. Biol. 2001, 8, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Harel, M.; Kasher, R.; Nicolas, A.; Guss, J.M.; Balass, M.; Fridkin, M.; Smit, A.B.; Brejc, K.; Sixma, T.K.; Katchalski-Katzir, E.; et al. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between α-bungarotoxin and a mimotope peptide. Neuron 2001, 32, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Scherf, T.; Kasher, R.; Balass, M.; Fridkin, M.; Fuchs, S.; Katchalski-Katzir, E. A β-hairpin structure in a 13-mer peptide that binds α-bungarotoxin with high affinity and neutralizes its toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 6629–6634. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.B.; Hibbs, R.E. Structure of the native muscle-type nicotinic receptor and inhibition by snake venom toxins. Neuron 2020, 106, 952–962. [Google Scholar] [CrossRef]
- Dellisanti, C.D.; Yao, Y.; Stroud, J.C.; Wang, Z.Z.; Chen, L. Crystal structure of the extracellular domain of nAChR α1 bound to α-bungarotoxin at 1.94 Å resolution. Nat. Neurosci. 2007, 10, 953–962. [Google Scholar] [CrossRef]
- Bourne, Y.; Talley, T.T.; Hansen, S.B.; Taylor, P.; Marchot, P. Crystal structure of a Cbtx–AChBP complex reveals essential interactions between snake α-neurotoxins and nicotinic receptors. EMBO J. 2005, 24, 1512–1522. [Google Scholar] [CrossRef] [Green Version]
- Samson, A.; Scherf, T.; Eisenstein, M.; Chill, J.; Anglister, J. The mechanism for acetylcholine receptor inhibition by alpha-neurotoxins and species-specific resistance to alpha-bungarotoxin revealed by NMR. Neuron 2002, 35, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Hawrot, E. NMR-based binding screen and structural analysis of the complex formed between α-cobratoxin and an 18-mer cognate peptide derived from the α1 subunit of the nicotinic acetylcholine receptor from Torpedo californica. J. Biol. Chem. 2002, 277, 37439–37445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Li, S.-X.; Bren, N.; Cheng, K.; Gomoto, R.; Chen, L.; Sine, S.M. Complex between α-bungarotoxin and an α7 nicotinic receptor ligand-binding domain chimaera. Biochem. J. 2013, 454, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouridakis, M.; Giastas, P.; Zarkadas, E.; Chroni-Tzartou, D.; Bregestovski, P.; Tzartos, S.J. Crystal structures of free and antagonist-bound states of human α9 nicotinic receptor extracellular domain. Nat. Struct. Mol. Biol. 2014, 21, 976–980. [Google Scholar] [CrossRef]
- Yi, M.; Tjong, H.; Zhou, H.X. Spontaneous conformational change and toxin binding in alpha7 acetylcholine receptor: Insight into channel activation and inhibition. Proc. Natl. Acad. Sci. USA 2008, 105, 8280–8285. [Google Scholar] [CrossRef] [Green Version]
- Lyukmanova, E.N.; Shenkarev, Z.O.; Shulepko, M.A.; Mineev, K.S.; D’Hoedt, D.; Kasheverov, I.E.; Filkin, S.Y.; Krivolapova, A.P.; Janickova, H.; Dolezal, V.; et al. NMR structure and action on nicotinic acetylcholine receptors of water-soluble domain of human Lynx1. J. Biol. Chem. 2011, 286, 10618–10627. [Google Scholar] [CrossRef] [Green Version]
- Utkin, Y.N.; Kuch, U.; Kasheverov, I.E.; Lebedev, D.S.; Cederlund, E.; Molles, B.E.; Polyak, I.; Ivanov, I.A.; Prokopev, N.A.; Ziganshin, R.H.; et al. Novel long-chain neurotoxins from Bungarus candidus distinguish the two binding sites in muscle-type nicotinic acetylcholine receptors. Biochem. J. 2019, 476, 1285–1302. [Google Scholar] [CrossRef]
- Berjanskii, M.V.; Wishart, D.S. A simple method to predict protein flexibility using secondary chemical shifts. J. Am. Chem. Soc. 2005, 127, 14970–14971. [Google Scholar] [CrossRef]
- Marsh, J.A.; Singh, V.K.; Jia, Z.; Forman-Kay, J.D. Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: Implications for fibrillation. Prot. Sci. 2006, 15, 2795–2804. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Delaglio, F.; Cornilescu, G.; Bax, A. TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomolec. NMR 2009, 44, 213–223. [Google Scholar] [CrossRef]
- Güntert, P.; Buchner, L. Combined automated NOE assignment and structure calculation with CYANA. J. Biomol. NMR 2015, 62, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Jarvet, J.; Allard, P.; Ehrenberg, A.; Graslund, A. Spectral-density mapping of 13Cα-1Hα vector dynamics using dipolar relaxation rates measured at several magnetic fields. J. Magn. Reson. B. 1996, 111, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kemple, M.D.; Buckley, P.; Yuan, P.; Prendergast, F.G. Main chain and side chain dynamics of peptides in liquid solution from 13C NMR: melittin as a model peptide. Biochemistry 1997, 36, 1678–1688. [Google Scholar] [CrossRef]
- Chou, J.J.; Baber, J.L.; Bax, A. Characterization of phospholipid mixed micelles by translational diffusion. J. Biomolec. NMR 2004, 29, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Price, W.S. Simultaneous convection compensation and solvent suppression in biomolecular NMR diffusion experiments. J. Biomolec. NMR 2009, 45, 295. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Tyn, M.T.; Gusek, T.W. Prediction of diffusion coefficients of proteins. Biotechnol. Bioeng. 1990, 35, 327–338. [Google Scholar] [CrossRef]
- Kudryavtsev, D.S.; Tabakmakher, V.M.; Budylin, G.S.; Egorova, N.S.; Efremov, R.G.; Ivanov, I.A.; Belukhina, S.Y.; Jegorov, A.V.; Kasheverov, I.E.; Kryukova, E.V.; et al. Complex approach for analysis of snake venom α-neurotoxins binding to HAP, the high-affinity peptide. Sci. Rep. 2020, 10, 3861. [Google Scholar] [CrossRef]
- McLane, K.E.; Wu, X.D.; Diethelm, B.; Conti-Tronconi, B.M. Structural determinants of α-bungarotoxin binding to the sequence segment 181–200 of the muscle nicotinic acetylcholine receptor α subunit: Effects of cysteine/cystine modification and species-specific amino acid substitutions. Biochemistry 1991, 30, 4925–4934. [Google Scholar] [CrossRef]
- Klukas, O.; Peshenko, I.A.; Rodionov, I.L.; Teliakova, O.V.; Utkin, Y.N.; Tsetlin, V.I. Fragments 183–198 and 125-145 of the α-subunits of the Torpedo californica nicotinic acetylcholinergic receptor binds α-bungarotoxin and neurotoxin II from Naja naja oxiana. Bioorg. Khim. 1995, 21, 152–155. [Google Scholar]
- Lamthanh, H.; Jegou-Matheron, C.; Servent, D.; Menez, A.; Lancelin, J.M. Minimal conformation of the α-conotoxin ImI for the α7 neuronal nicotinic acetylcholine receptor recognition: Correlated CD, NMR and binding studies. FEBS Lett. 1999, 454, 293–298. [Google Scholar] [PubMed] [Green Version]
- Price-Carter, M.; Hull, M.S.; Goldenberg, D.P. Roles of individual disulfide bonds in the stability and folding of an ω-conotoxin. Biochemistry 1998, 37, 9851–9861. [Google Scholar] [CrossRef] [PubMed]
- Kasheverov, I.E.; Zhmak, M.N.; Maslennikov, I.V.; Utkin, Y.N.; Tsetlin, V.I. Comparative study on selectivity of α-conotoxins GI and ImI using their synthetic analogues and derivatives. Neurochem. Res. 2003, 28, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Spindel, E.R. Cholinergic targets in lung cancer. Curr. Pharm. Des. 2016, 22, 2152–2159. [Google Scholar] [CrossRef]
- Bychkov, M.; Shenkarev, Z.; Shulepko, M.; Shlepova, O.; Kirpichnikov, M.; Lyukmanova, E. Water-soluble variant of human Lynx1 induces cell cycle arrest and apoptosis in lung cancer cells via modulation of α7 nicotinic acetylcholine receptors. PLoS ONE 2019, 14, e0217339. [Google Scholar] [CrossRef]


| № | Protein Fragment | Sequence | Theoretical Mass/Measured Mass, Da | Abbreviation |
|---|---|---|---|---|
| (1) | Human Lynx1 fragment 29–43 (Loop II). | TTRTYYTPTRMKVSK | 1833.0/1832.9 | Ly29–43 |
| (2) | Human Lynx1 fragment 29–43 with cysteines at N- and C- termini, linear form. | CTTRTYYTPTRMKVSKC | 2039.0/2039.0 | Ly29–43C,C |
| (3) | Human Lynx1 fragment 29–43 with cysteines at N- and C-termini, cyclized form. | 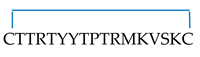 | 2037.0/2037.1 | Ly29–43C-C |
| (4) | L. stagnalis AChBP fragment 202–214 (Loop C), linear form. | SVTYSCCPEAYED | 1466.5/1466.4 | Ls202–214C,C |
| (5) | L. stagnalis AChBP fragment 202–214 (Loop C), cyclized form. | 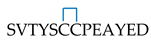 | 1464.5/1464.6 | Ls202–214C-C |
| (6) | L. stagnalis AChBP fragment 202–214 with cysteines 207–208 mutated to serines and cysteines added at N- and C- termini, linear form. | CSVTYSSSPEAYEDC | 1640.6/1640.6 | mLs202–214C,C |
| (7) | L. stagnalis AChBP fragment 202–214 with cysteines 207–208 mutated to serines and cysteines added at N- and C- termini, cyclized form. |  | 1638.6/1638.6 | mLs202–214C-C |
| (8) | Combinatorial high-affinity peptide HAP. | WRYYESSLLPYPD | 1688.8/1688.7 | HAP |
| (9) | Combinatorial high-affinity peptide HAP with cysteines at N- and C- termini, linear form. | CWRYYESSLLPYPDC | 1894.8/1894.8 | HAPC,C |
| (10) | Combinatorial high-affinity peptide HAP with cysteines at N- and C- termini, cyclized form. |  | 1892.8/1892.9 | HAPC-C |
| Peptide | α-Helix, % | β-Structure, % | β-Turn, % | Random, % | NRMSD 1 |
|---|---|---|---|---|---|
| (1) | 5.2 | 31.5 | 22.6 | 40.7 | 0.02 |
| (2) | 5.1 | 32.6 | 22.5 | 39.8 | 0.02 |
| (3) | 4.1 | 38.5 | 22.5 | 34.9 | 0.03 |
| (4) | 5.9 | 32.4 | 23.4 | 38.2 | 0.02 |
| (5) | 6.1 | 30.9 | 23.7 | 39.2 | 0.02 |
| (6) | 6.4 | 36.1 | 23.0 | 34.5 | 0.03 |
| (7) | 5.9 | 33.6 | 22.4 | 38.0 | 0.02 |
| (8) | 3.3 | 39.5 | 22.6 | 34.6 | 0.03 |
| (9) | 3.9 | 38.5 | 22.3 | 35.3 | 0.03 |
| (10) | 3.7 | 39.9 | 21.7 | 34.7 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mineev, K.S.; Kryukova, E.V.; Kasheverov, I.E.; Egorova, N.S.; Zhmak, M.N.; Ivanov, I.A.; Senko, D.A.; Feofanov, A.V.; Ignatova, A.A.; Arseniev, A.S.; et al. Spatial Structure and Activity of Synthetic Fragments of Lynx1 and of Nicotinic Receptor Loop C Models. Biomolecules 2021, 11, 1. https://doi.org/10.3390/biom11010001
Mineev KS, Kryukova EV, Kasheverov IE, Egorova NS, Zhmak MN, Ivanov IA, Senko DA, Feofanov AV, Ignatova AA, Arseniev AS, et al. Spatial Structure and Activity of Synthetic Fragments of Lynx1 and of Nicotinic Receptor Loop C Models. Biomolecules. 2021; 11(1):1. https://doi.org/10.3390/biom11010001
Chicago/Turabian StyleMineev, Konstantin S., Elena V. Kryukova, Igor E. Kasheverov, Natalia S. Egorova, Maxim N. Zhmak, Igor A. Ivanov, Dmitry A. Senko, Alexey V. Feofanov, Anastasia A. Ignatova, Alexander S. Arseniev, and et al. 2021. "Spatial Structure and Activity of Synthetic Fragments of Lynx1 and of Nicotinic Receptor Loop C Models" Biomolecules 11, no. 1: 1. https://doi.org/10.3390/biom11010001
APA StyleMineev, K. S., Kryukova, E. V., Kasheverov, I. E., Egorova, N. S., Zhmak, M. N., Ivanov, I. A., Senko, D. A., Feofanov, A. V., Ignatova, A. A., Arseniev, A. S., Utkin, Y. N., & Tsetlin, V. I. (2021). Spatial Structure and Activity of Synthetic Fragments of Lynx1 and of Nicotinic Receptor Loop C Models. Biomolecules, 11(1), 1. https://doi.org/10.3390/biom11010001







