The Usefulness of Diagnostic Panels Based on Circulating Adipocytokines/Regulatory Peptides, Renal Function Tests, Insulin Resistance Indicators and Lipid-Carbohydrate Metabolism Parameters in Diagnosis and Prognosis of Type 2 Diabetes Mellitus with Obesity
Abstract
1. Introduction
2. Experimental Section
3. Results
4. Discussion
4.1. Adropin Secretory Profile in Obese Controls and T2DM—The Influence of Six-Month Metformin Therapy, Sex Differences, Correlation With Biochemical Variables, Mechanism of Action
4.2. Irisin Secretory Profile in Obese Controls and T2DM—Mode of Action, the Influence of Six-Month Metformin Therapy, Factors Affecting Plasma Irisin Level (BMI, Sex Differences), Correlation with Biochemical Variables
4.3. Vaspin Secretory Profile in Obese Controls and T2DM—Gender-Related Differences, the Influence of 6-Month Metformin Therapy, Correlation With Biochemical Variables, Mechanism of Action
4.4. Vaspin—Mode of Action
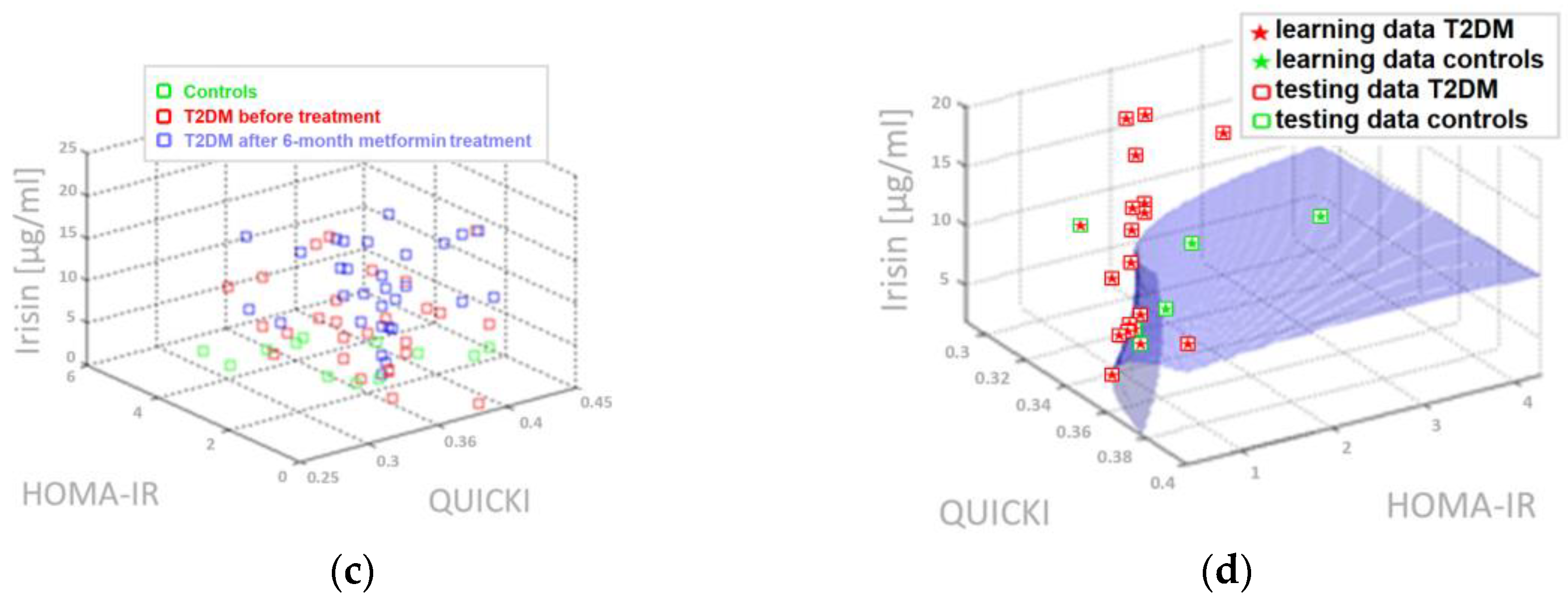
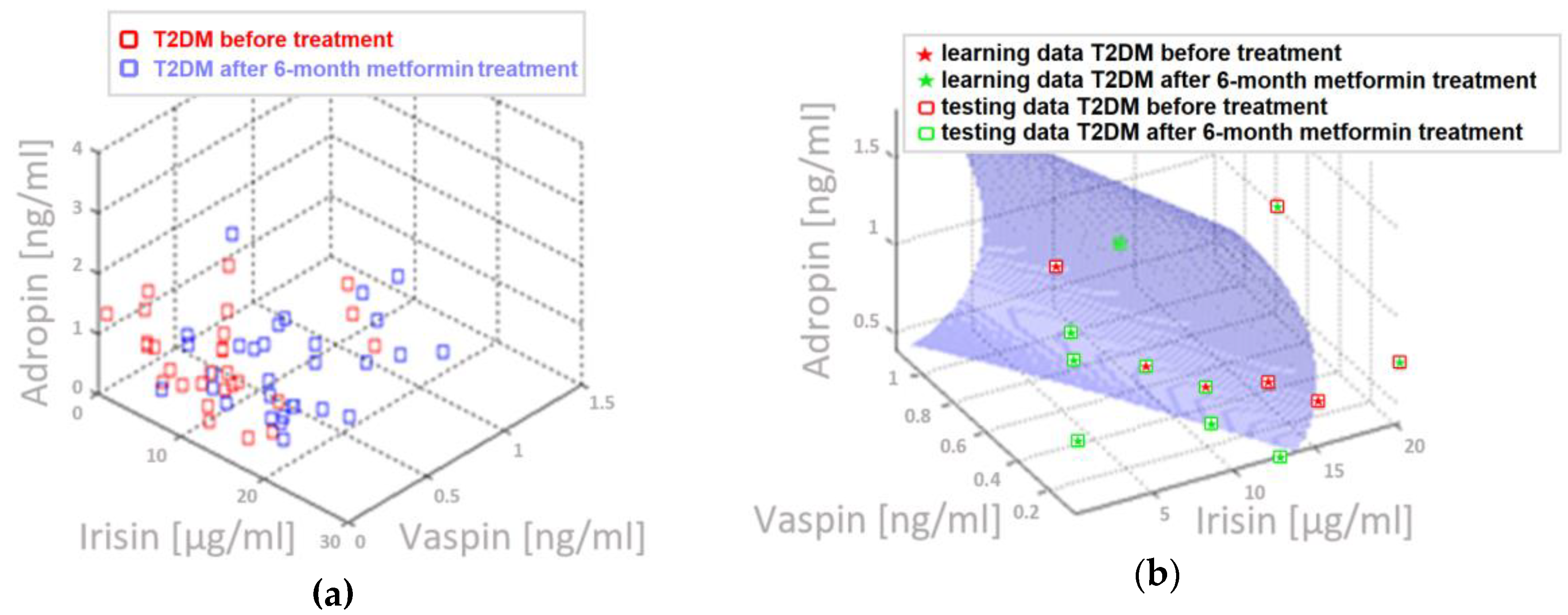
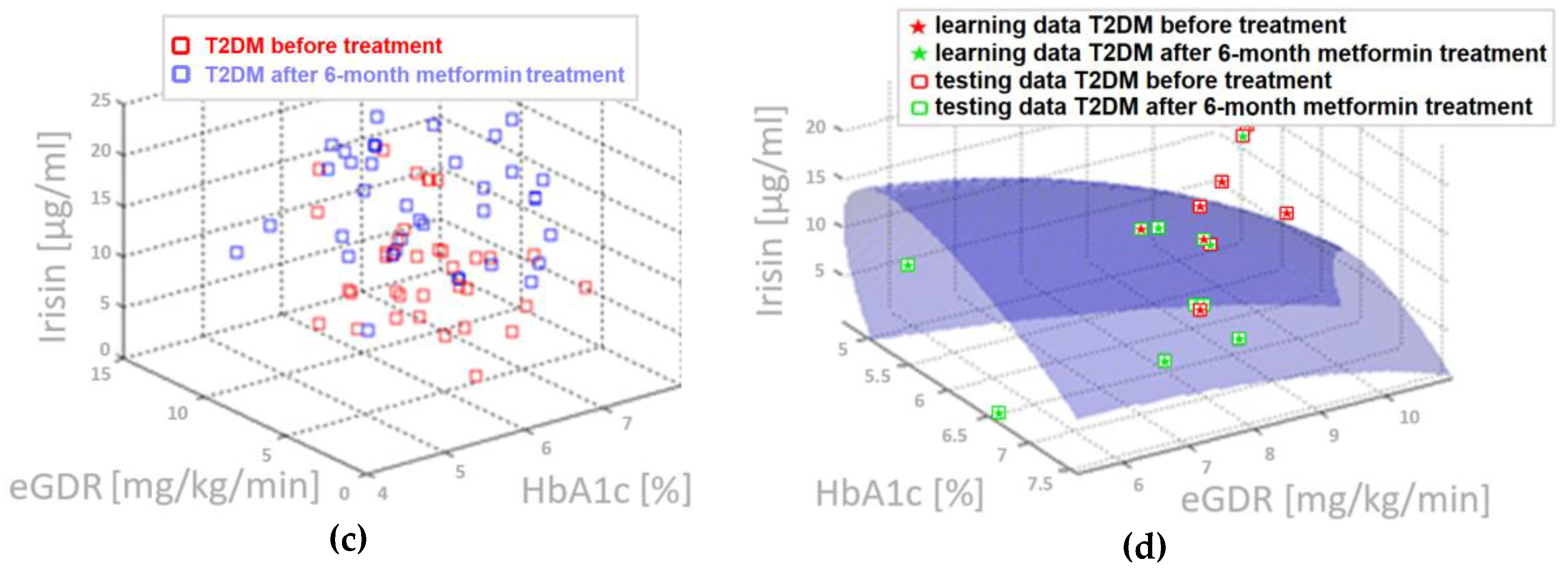
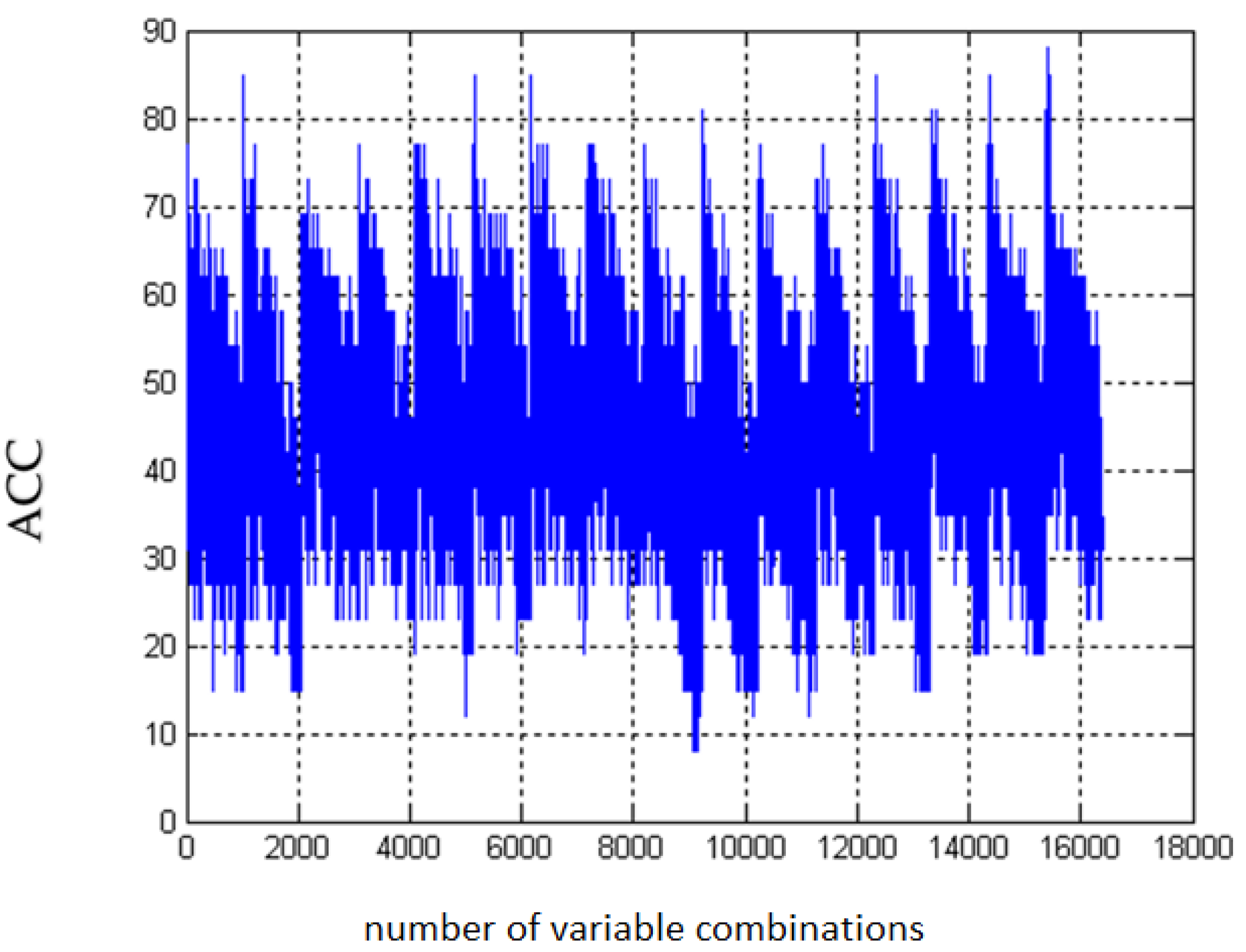
4.5. Three-Parameter Assessment and Visualization of the Relationship Between Biochemical Variables—Diagnostic Panels Useful in Early Detection of T2DM and Differentiating the Obesity Phenotype with Normal Metabolic Profile from T2DM Obese Patients
4.6. Multifactorial Discriminant Analysis—Diagnostic Panels Based on Irisin and Vaspin Plasma Levels Contribute Clinically Relevant Information Concerning the Effectiveness of Metformin Treatment in T2D Patients
4.7. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thuzar, M.; Ho, K.K. Mechanisms in endocrinology: Brown adipose tissue in humans: Regulation and metabolic significance. Eur. J. Endocrinol. 2016, 175, R11–R25. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Câmara, N.O.; Moraes-Vieira, P.M. Adipokines as drug targets in diabetes and underlying disturbances. J. Diabetes Res. 2015, 2015, 681612. [Google Scholar] [CrossRef]
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Schleinitz, D. Genetic determination of serum levels of diabetes-associated adipokines. Rev. Diabet. Stud. 2015, 12, 277–298. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; St-Onge, M.P.; Siebert, E.A.; Medici, V.; Stanhope, K.L.; Havel, P.J. Differential responses of plasma adropin concentrations to dietary glucose or fructose consumption in humans. Sci. Rep. 2015, 5, 14691. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, A.; Shalaby, S.M.; El-Arabi Bdeer, S.; Mahmoud, A.A.; Mousa, M.M.; Khalifa, A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life 2016, 68, 544–556. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.J.; Yuan, G.Y.; Wang, D.; Chen, J.J. Changes and clinical significance of serum vaspin levels in patients with type 2 diabetes. Genet. Mol. Res. 2015, 14, 11356–11361. [Google Scholar] [CrossRef]
- Ganesh, K.K.; Zhang, J.; Gao, S.; Rossi, J.; McGuinness, O.P.; Halem, H.H.; Culler, M.D.; Mynatt, R.L.; Butler, A.A. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity 2012, 20, 1394–1402. [Google Scholar] [CrossRef]
- Butler, A.; Tam, C.T.; Stanhope, K.L.; Wolfe, B.M.; Ali, M.R.; O’Keeffe, M.; St-Onge, M.P.; Ravussin, E.; Havel, P.J. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J. Clin. Endocrinol. Metab. 2012, 97, 3783–3791. [Google Scholar] [CrossRef]
- Altincik, A.; Sayin, O. Evaluation of the relationship between serum adropin levels and blood pressure in obese children. J. Pediatr. Endocrinol. Metab. 2015, 28, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Kocaoglu, C.; Buyukinan, M.; Erdem, S.S.; Ozel, A. Are obesity and metabolic syndrome associated with plasma adropin levels in children? J. Pediatr. Endocrinol. Metab. 2015, 28, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, I.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef]
- Youn, B.S.; Klöting, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008, 57, 372–377. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wu, Y.; Duan, R.; Zhang, J.; Du, F.; Zhang, Q.; Li, Y.; Li, N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS ONE 2017, 12, e0189722. [Google Scholar] [CrossRef]
- Saboori, S.; Hosseinzadeh-Attar, M.J.; Yousefi Rad, E.; Hosseini, M.; Miraei, K.; Ahmadivand, Z. The comparison of serum vaspin and visfatin concentrations in obese and normal weight women. Diabetes Metab. Syndr. 2015, 9, 320–323. [Google Scholar] [CrossRef]
- Liu, S.; Duan, R.; Wu, Y.; Du, F.; Zhang, J.; Li, X.; Guo, S.; Wang, M.; Zhang, Q.; Li, Y.; et al. Effects of vaspin on insulin resistance in rats and underlying mechanisms. Sci. Rep. 2018, 8, 13542. [Google Scholar] [CrossRef] [PubMed]
- Flehmig, G.; Scholz, M.; Klöting, N.; Fasshauer, M.; Tönjes, A.; Stumvoll, M.; Youn, B.S.; Blüher, M. Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PLoS ONE 2014, 9, e99785. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Peng, W.; Xiao, S.; Li, H.; Jin, J.; Qin, L.; Dong, Y.; Su, Q. Role of serum vaspin in progression of type 2 diabetes: A 2-year cohort study. PLoS ONE 2014, 9, e94763. [Google Scholar] [CrossRef]
- Li, Q.; Chen, R.; Moriya, J.; Yamakawa, J.; Sumino, H.; Kanda, T.; Takahashi, T. A novel adipocytokine, visceral adipose tissue-derived serine protease inhibitor (vaspin), and obesity. J. Int. Med. Res. 2008, 36, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Auguet, T.; Quintero, Y.; Riesco, D.; Morancho, B.; Terra, X.; Crescenti, A.; Broch, M.; Aguilar, C.; Olona, M.; Porras, J.A.; et al. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med. Genet. 2011, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Huang, F.; Lu, W.J.; Jiang, G.J.; Deng, Y.P.; Shen, F.M. Metformin promotes irisin release from murine skeletal muscle independently of AMP-activated protein kinase activation. Acta Physiol. 2015, 213, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, X.; Chen, Y.; Zhao, Q. PGC-1 mediates the regulation of metformin in muscle irisin expression and function. Am. J. Transl. Res. 2015, 7, 1850–1859. [Google Scholar]
- Tan, B.K.; Heutling, D.; Chen, J.; Farhatullah, S.; Adya, R.; Keay, S.D.; Kennedy, C.R.; Lehnert, H.; Randeva, H.S. Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes 2008, 57, 1501–1507. [Google Scholar] [CrossRef]
- Gonzalez, C.R.; Caminos, J.E.; Vázquez, M.J.; Garces, M.F.; Cepeda, L.A.; Angel, A.; Gonzalez, A.C.; García-Rendueles, M.E.; Sangiao-Alvarellos, S.; Lopez, M.; et al. Regulation of visceral adipose tissue-derived serine protease inhibitor by nutritional status, metformin, gender and pituitary factors in rat white adipose tissue. J. Physiol. 2009, 587, 3741–3750. [Google Scholar] [CrossRef]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef]
- Gao, S.; Ghoshal, S.; Zhang, L.; Stevens, J.R.; McCommis, K.S.; Finck, B.N.; Lopaschuk, G.D.; Butler, A.A. The peptide hormone adropin regulates signal transduction pathways controlling hepatic glucose metabolism in a mouse model of diet-induced obesity. J. Biol. Chem. 2019, 294, 13366–13377. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; McMillan, R.P.; Zhu, Q.; Lopaschuk, G.D.; Hulver, M.W.; Butler, A.A. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 2015, 4, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, M.; Anandakumar, P.; Debela, T. A review on the role of irisin in insulin resistance and type 2 diabetes mellitus. J. Pharmacopunct. 2017, 20, 235–242. [Google Scholar]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A hope in understanding and managing obesity and metabolic syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, L. Association of serum adropin concentrations with diabetic nephropathy. Mediat. Inflamm. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, K.; Liu, Q.C.; Guo, Z.; Zhang, S.; Chen, X.R.; Lin, J.H.; Wen, J.P.; Zhao, C.F.; Lin, X.H.; et al. Adropin deficiency worsens HFD-induced metabolic defects. Cell Death Dis. 2017, 8, e3008. [Google Scholar] [CrossRef]
- Wu, L.; Fang, J.; Chen, L.; Zhao, Z.; Luo, Y.; Lin, C.; Fan, L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 751–758. [Google Scholar] [CrossRef]
- Hosseini, A.; Shanaki, M.; Emamgholipour, S.; Nakhjavani, M.; Razi, F.; Golmohammadi, T. Elevated serum levels of adropin in patients with type 2 diabetes mellitus and its association with insulin resistance. J. Biol. Today’s World 2016, 5, 44–49. [Google Scholar] [CrossRef]
- Ugur, K.; Oz, B.; Ozkan, Y.; Sener, S.Y.; Orhan, B.; Aydin, S. Microalbuminuria by concentration serum and urine levels of adropin in patients with type 2 diabetes mellitus. Endocr. Abstr. 2015, 37, EP446. [Google Scholar] [CrossRef]
- Butler, A.A.; Zhang, J.; Price, C.A.; Stevens, J.R.; Graham, J.L.; Stanhope, K.L.; King, S.; Krauss, R.M.; Bremer, A.A.; Havel, P.J. Low plasma adropin concentrations increase risks of weight gain and metabolic dysregulation in response to a high-sugar diet in male nonhuman primates. J. Biol. Chem. 2019, 294, 9706–9719. [Google Scholar] [CrossRef]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as a fat-burning hormone with multiple functions-review of a decade of research. Molecules 2020, 25, 549. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Stevens, J.R.; Billon, C.; Girardet, C.; Sitaula, S.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Rankinen, T.; Bouchard, C.; et al. Adropin: An endocrine link between the biological clock and cholesterol homeostasis. Mol. Metab. 2018, 8, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.N.; Yim, J.E. Plasma Adropin as a Potential Marker Predicting Obesity and Obesity-associated Cancer in Korean Patients With Type 2 Diabetes Mellitus. J. Cancer Prev. 2018, 23, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Rana, K.S.; Pararasa, C.; Afzal, I.; Nagel, D.A.; Hill, E.J.; Bailey, C.J.; Griffiths, H.R.; Kyrou, I.; Randeva, H.S.; Bellary, S.; et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc. Diabetol. 2017, 16, 147. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768. [Google Scholar] [CrossRef]
- Liu, S.; Du, F.; Li, X.; Wang, M.; Duan, R.; Zhang, J.; Wu, Y.; Zhang, Q. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS ONE 2017, 12, e0175498. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, T.; Leung, P.S. Irisin ameliorates glucolipotoxicity-associated β-Cell dysfunction and apoptosis via AMPK signaling and anti-inflammatory actions. Cell Physiol. Biochem. 2018, 51, 924–937. [Google Scholar] [CrossRef]
- Pang, Y.; Zhu, H.; Xu, J.; Yang, L.; Liu, L.; Li, J. β-arrestin-2 is involved in irisin induced glucose metabolism in type 2 diabetes via p38 MAPK signaling. Exp. Cell Res. 2017, 360, 199–204. [Google Scholar] [CrossRef]
- Mai, S.; Grugni, G.; Mele, C.; Vietti, R.; Vigna, L.; Sartorio, A.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Irisin levels in genetic and essential obesity: Clues for a potential dual role. Sci. Rep. 2020, 10, 1020. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Y.; Zhang, H.; Xu, Y.; Wang, G. Exenatide treatment increases serum irisin levels in patients with obesity and newly diagnosed type 2 diabetes. J. Diabetes Complicat. 2016, 30, 1555–1559. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, M.K.; Bae, K.H.; Seo, H.A.; Jeong, J.Y.; Lee, W.K.; Kim, J.G.; Lee, I.K.; Park, K.G. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 96–101. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Shelbaya, S.; Abushady, M.M.; Nasr, M.S.; Bekhet, M.M.; Mageed, Y.A.; Abbas, M. Study of irisin hormone level in type 2 diabetic patients and patients with diabetic nephropathy. Curr. Diabetes Rev. 2018, 14, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, I.L.; Chacon-Mikahil, M.P.; Brunelli, D.T.; Gáspari, A.F.; Duft, R.G.; Oliveira, A.G.; Araujo, T.G.; Saad, M.J.; Cavaglieri, C.R. Obese with higher FNDC5/Irisin levels have a better metabolic profile, lower lipopolysaccharide levels and type 2 diabetes risk. Arch. Endocrinol. Metab. 2017, 61, 524–533. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alokail, M.S.; Rahman, S.; Amer, O.E.; Al-Attas, O.S.; Alfawaz, H.; Tripathi, G.; Sabico, S.; Chrousos, G.P.; McTernan, P.G.; et al. Habitual physical activity is associated with circulating irisin in healthy controls but not in subjects with diabetes mellitus type 2. Eur. J. Clin. Investig. 2015, 45, 75–81. [Google Scholar] [CrossRef]
- García-Fontana, B.; Reyes-García, R.; Morales-Santana, S.; Ávila-Rubio, V.; Muñoz-Garach, A.; Rozas-Moreno, P.; Muñoz-Torres, M. Relationship between myostatin and irisin in type 2 diabetes mellitus: A compensatory mechanism to an unfavourable metabolic state? Endocrine 2016, 52, 54–62. [Google Scholar] [CrossRef]
- Saleh, O.; Majeed, M.J.; Oreaby, G.M. Descriptive consideration of serum irisin levels various factors, obesity, type 2 diabetes mellitus, pre-diabetic status, gender, and athletics. J. Diabetes Metab. 2014, 5, 12. [Google Scholar]
- Lopez-Legarrea, P.; de la Iglesia, R.; Crujeiras, A.B.; Pardo, M.; Casanueva, F.F.; Zulet, M.A.; Martinez Alfredo, M. Higher baseline irisin concentrations are associated with greater reductions in glycemia and insulinemia after weight loss in obese subjects. Nutr. Diabetes 2014, 4, e110. [Google Scholar] [CrossRef]
- Zügel, M.; Qiu, S.; Laszlo, R.; Bosnyák, E.; Weigt, C.; Müller, D.; Diel, P.; Steinacker, J.M.; Schumann, U. The role of sex, adiposity, and gonadectomy in the regulation of irisin secretion. Endocrine 2016, 54, 101–110. [Google Scholar] [CrossRef]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdes, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lin, M.; Liu, C.; Xiao, F.; Liu, Y.; Huang, P.; Zeng, X.; Yan, B.; Liu, S.; Li, X.; et al. Elevated circulating irisin is associated with lower risk of insulin resistance: Association and path analyses of obese Chinese adults. BMC Endocr. Disord. 2016, 16, 44. [Google Scholar] [CrossRef]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Elbelt, U.; Kobelt, P.; Klapp, B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity–correlation with body mass index. Peptides 2013, 39, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Alis, R.; Pareja-Galeano, H.; Sola, E.; Victor, V.M.; Rocha, M.; Hernandez-Mijares, A.; Romagnoli, M. Circulating irisin levels are not correlated with BMI, age, and other biological parameters in obese and diabetic patients. Endocrine 2014, 46, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Jameel, F.; Thota, N.; Wood, L.; Plunkett, B.; Garg, M. Sex-dependent association between circulating irisin levels and insulin resistance in healthy adults. J. Nutr. Intermed. Metab. 2015, 2, 86–92. [Google Scholar] [CrossRef][Green Version]
- Yuksel, M.A.; Oncul, M.; Tuten, A.; Imamoglu, M.; Acikgoz, A.S.; Kucur, M.; Madazli, R. Maternal serum and fetal cord blood irisin levels in gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2014, 104, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Shi, X.; Zhang, H.; Pan, L.; Ma, Z.; Liu, S.; Liu, Y.; Li, X.; Yang, S.; Li, Z. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS ONE 2014, 9, e94235. [Google Scholar] [CrossRef]
- Fukushima, Y.; Kurose, S.; Shinno, H.; Thu, H.C.T.; Tamanoi, A.; Tsutsumi, H.; Hasegawa, T.; Nakajima, T.; Kimura, Y. Relationships between serum irisin levels and metabolic parameters in Japanese patients with obesity. Obes. Sci. Pract. 2016, 2, 203–209. [Google Scholar] [CrossRef]
- Klöting, N.; Berndt, J.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Vaspin gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 2006, 339, 430–436. [Google Scholar] [CrossRef]
- Ye, Y.; Hou, X.H.; Pan, X.P.; Lu, J.X.; Jia, W.P. Serum vaspin level in relation to postprandial plasma glucose concentration in subjects with diabetes. Chin. Med. J. 2009, 122, 2530–2533. [Google Scholar] [PubMed]
- Dai, R.; Dong, Z.; Qian, Y.; Han, Y. Obese type 2 diabetes mellitus patients have higher serum vaspin concentrations. J. Diabetes 2016, 8, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Zhang, H.; Zhu, J.; Kuang, H.; Yu, Q.; Bai, M.; Mu, J. Association between vaspin level and coronary artery disease in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 113, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Y.; Tian, T.; Wang, L. Serum vaspin concentration in elderly type 2 diabetes mellitus patients with differing body mass index: A cross-sectional study. Biomed. Res. Int. 2017, 2017, 4875026. [Google Scholar] [CrossRef] [PubMed]
- Escoté, X.; Gómez-Zorita, S.; López-Yoldi, M.; Milton-Laskibar, I.; Fernández-Quintela, A.; Martínez, J.A.; Moreno-Aliaga, M.J.; Portillo, M.P. Role of omentin, vaspin, cardiotrophin-1, TWEAK and NOV/CCN3 in obesity and diabetes development. Int. J. Mol. Sci. 2017, 18, 1770. [Google Scholar] [CrossRef]
- Yan, M.; Su, B.; Peng, W.; Li, L.; Li, H.; Zhuang, J.; Lu, Y.; Jian, W.; Wei, Y.; Li, W.; et al. Association of serum vaspin and adiponectin levels with renal function in patients with or without type 2 diabetes mellitus. J. Diabetes Res. 2014, 2014, 868732. [Google Scholar] [CrossRef]
- Castro, C.A.; da Silva, K.A.; Buffo, M.M.; Pinto, K.N.; Duarte, F.O.; Nonaka, K.O.; Aníbal, F.F.; Duarte, A.C. Experimental type 2 diabetes induction reduces serum vaspin, but not serum omentin, in Wistar rats. Int. J. Exp. Pathol. 2017, 98, 26–33. [Google Scholar] [CrossRef]
- Schindler, K.; Vila, G.; Hoppichler, F.; Lechleitner, M.; Luger, A.; Anderwald, C.; Hoefler, J.; Tomasec, G.; Kautzky-Willer, A.; Ludvik, B. The impact of type 2 diabetes on circulating adipokines in patients with metabolic syndrome. Obes. Facts 2012, 5, 270–276. [Google Scholar] [CrossRef]
- Yan, T.; Li, L.; Wang, H.; Wang, J.; Cai, D. Correlation between adipocytokines levels and metabolic syndrome in type 2 diabetes mellitus. Nan Fang Yi Ke Da Xue Xue Bao 2014, 34, 275–278. [Google Scholar]
- Seeger, J.; Ziegelmeier, M.; Bachmann, A.; Lössner, U.; Kratzsch, J.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Serum levels of the adipokine vaspin in relation to metabolic and renal parameters. J. Clin. Endocrinol. Metab. 2008, 93, 247–251. [Google Scholar] [CrossRef]
- Moradi, S.; Mirzaei, K.; Abdurahman, A.A.; Keshavarz, S.A.; Hossein-Nezhad, A. Mediatory effect of circulating vaspin on resting metabolic rate in obese individuals. Eur. J. Nutr. 2016, 55, 1297–1305. [Google Scholar] [CrossRef]
- Handisurya, A.; Riedl, M.; Vila, G.; Maier, C.; Clodi, M.; Prikoszovich, T.; Ludvik, B.; Prager, G.; Luger, A.; Kautzky-Willer, A. Serum vaspin concentrations in relation to insulin sensitivity following RYGB-induced weight loss. Obes. Surg. 2010, 20, 198–203. [Google Scholar] [CrossRef]
- Chang, H.M.; Park, H.S.; Park, C.Y.; Song, Y.S.; Jang, Y.J. Association between serum vaspin concentrations and visceral adipose tissue in Korean subjects. Metabolism 2010, 59, 1276–1281. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, W.P.; Wang, L.P.; Lü, Q.H.; Zhou, X.H. Calorie control increased vaspin levels of serum and periepididymal adipose tissue in diet-induced obese rats in association with serum free fatty acid and tumor necrosis factor alpha. Chin. Med. J. 2010, 123, 936–941. [Google Scholar]
- Breitfeld, J.; Tönjes, A.; Böttcher, Y.; Schleinitz, D.; Wiele, N.; Marzi, C.; Brockhaus, C.; Rathmann, W.; Huth, C.; Grallert, H.; et al. Genetic variation in the vaspin gene affects circulating serum vaspin concentrations. Int. J. Obes. 2013, 37, 861–866. [Google Scholar] [CrossRef]
- Pala, D.; Carlos-Cândido, A.P.; Leandro-da-Cruz, L.; Oliveira-Barbosa, P.; Teixeira-Silva, C.; Pinheiro-Volp, A.C.; Lins-Machado-Coelho, G.L.; Nascimento-de-Freitas, R. Vaspin association with insulin resistance is related to physical activity and body fat in Brazilian adolescents—A cross-sectional study. Nutr. Hosp. 2016, 33, 265. [Google Scholar]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef]
- Wada, J. Vaspin: A novel serpin with insulin-sensitizing effects. Expert Opin. Investig. Drugs 2008, 17, 327–333. [Google Scholar] [CrossRef]
- Wada, J. Vaspin and insulin resistance. Rinsho Byori. 2008, 56, 705–711. [Google Scholar]
- Zieger, K.; Weiner, J.; Kunath, A.; Gericke, M.; Krause, K.; Kern, M.; Stumvoll, M.; Klöting, N.; Blüher, M.; Heiker, J.T. Ablation of kallikrein 7 (KLK7) in adipose tissue ameliorates metabolic consequences of high fat diet-induced obesity by counteracting adipose tissue inflammation in vivo. Cell Mol. Life Sci. 2018, 75, 727–742. [Google Scholar] [CrossRef]
- Zieger, K.; Weiner, J.; Krause, K.; Schwarz, M.; Kohn, M.; Stumvoll, M.; Blüher, M.; Heiker, J.T. Vaspin suppresses cytokine-induced inflammation in 3T3-L1 adipocytes via inhibition of NFκB pathway. Mol. Cell Endocrinol. 2018, 460, 181–188. [Google Scholar] [CrossRef]
- Mihanfar, A.; Rahmati-Yamchi, M.; Mota, A.; Abediazar, S.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Serum levels of vaspin and its correlation with nitric oxide in type 2 diabetic patients with nephropathy. Curr. Diabetes Rev. 2018, 14, 162–167. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, W.J.; Hwang, J.Y.; Lee, M.J.; Seol, S.M.; Kim, Y.M.; Lee, Y.L.; Kim, H.S.; Kim, M.S.; Park, J.Y. Vaspin increases nitric oxide bioavailability through the reduction of asymmetric dimethylarginine in vascular endothelial cells. PLoS ONE 2012, 7, e52346. [Google Scholar] [CrossRef]

| Parameters | Control Subjects | Obese Patients with Type 2 Diabetes Mellitus | [%] Change Following Therapy | |
|---|---|---|---|---|
| Before Metformin Therapy | After the 6-Month Metformin Treatment | |||
| Female/Male (n) | 20 (11F/9M) | 40 (24F/16M) | 40 (24F/16M) | |
| Age (yr) | 56.05 ± 4.99 | 59.28 ± 7.29 | 59.88 ± 7.29 | |
| BMI (kg/m2) | 29.59 ± 6.22 | 32.76 ± 4.83 a | 32.13 ± 5.06 a | −1.92 |
| WHR (cm2) | 0.95 ± 0.07 | 0.98 ± 0.06 | 0.97 ± 0.06 | −1.02 |
| Glucose (mg/dL) | 90.50 ± 5.09 | 113.94 ± 12.06 a | 114.22 ± 14.38 a | +0.25 |
| HbA1c (%) | 5.21 ± 0.39 | 6.26 ± 0.61 a | 6.33 ± 0.79 a | +1.12 |
| Triglycerides (mg/dL) | 162.07 ± 50.01 | 176.84 ± 93.14 | 142.42 ± 66.25 | −19.46 |
| Cholesterol (mg/dL) | 181.23 ± 27.27 | 196.66 ± 44.87 | 182.29 ± 29.10 d | −7.31 |
| HDL (mg/dL) | 46.80 ± 8.53 | 50.10 ± 14.77 | 51.59 ± 14.55 | +2.97 |
| LDL (mg/dL) | 115.78 ± 28.44 | 113.79 ± 35.54 | 99.21 ± 27.17 b,c | −12.81 |
| TG/HDL ratio | 3.65 ± 1.47 | 4.16 ± 2.61 | 3.12 ± 1.98 | −25.00 |
| sCr (mg/dL) | 0.85 ± 0.10 | 0.79 ± 0.13 | 0.85 ± 0.17 d | +7.59 |
| uCr (mg/dL) | 110.60 ± 65.43 | 100.74 ± 56.90 | 105.51 ± 52.54 | +4.73 |
| uAlbumin (μg/mL) | 4.66 ± 3.81 | 5.99 ± 5.32 | 6.05± 5.65 | +1.00 |
| ACR (μg/mg Cr) | 5.63 ± 4.51 | 6.94 ± 5.39 | 6.02± 4.56 | −13.26 |
| eGFR (mL/min/1.73m2) | 90.89 ± 13.74 | 90.37 ± 13.84 | 84.33± 16.69 d | −6.68 |
| Insulin (μIU/mL) | 13.15 ± 9.72 | 10.53 ± 6.43 | 9.52 ± 5.12 | −9.59 |
| HOMA-IR2 | 1.16 ± 0.73 | 1.46 ± 0.83 | 1.29 ± 0.68 d | −11.64 |
| HOMA-B | 135.59 ± 58.60 | 78.16 ± 37.88 a | 69.06 ± 32.86 a | −11.64 |
| HOMA-S | 85.05 ± 61.96 | 93.50 ± 55.60 | 99.44 ± 54.01 | +6.35 |
| QUICKI | 0.36 ± 0.06 | 0.35 ± 0.05 | 0.35 ± 0.04 | 0.00 |
| eGDR | 9.78 ± 0.93 | 7.46 ±1.77 a | 7.88 ± 1.98 a | +5.63 |
| Systolic pressure [mmHg] | 122.50 ± 6.98 | 135.69 ± 10.71 a | 131.67 ± 10.95 a | −2.96 |
| Diastolic pressure [mmHg] | 81.25 ±3.41 | 81.67 ± 5.07 | 78.06 ± 7.39 d | −4.42 |
| Parameters | Control Subjects | Obese Patients with Type 2 Diabetes Mellitus | [%] Change Following Therapy | |
|---|---|---|---|---|
| Before the Implementation of Metformin Therapy | After the 6-Month of Metformin Treatment | |||
| Adropin (ng/mL) | ||||
| All | 0.75 (0.62–1.06) | 0.86 (0.61–1.07) | 0.74 (0.52–0.93) | −13.95 |
| F | 0.56 (0.40–0.70) | 0.88 (0.62–1.06) | 0.74 (0.51–0.90) | −15.91 |
| M | 0.79 (0.71–1.13) e | 0.76 (0.52–1.08) | 0.74 (0.52–0.97) | −2.63 |
| Irisin (μg/mL) | ||||
| All | 4.99 (3.29–6.16) | 8.83 (5.63–12.39) a | 15.28 (11.39–19.78) a,d | +73.05 |
| F | 4.99 (3.45–6.28) | 8.78 (5.81–13.91) | 18.10 (12.20–20.14) | +106.15 |
| M | 4.73 (3.28–5.91) | 9.57 (5.04–11.06) | 13.86 (10.02–15.80) | +44.83 |
| Vaspin (ng/mL) | ||||
| All | 0.35 (0.21–0.99) | 0.12 (0.07–0.29) b | 0.29 (0.17–0.66) c | +141.67 |
| F | 0.38 (0.21–0.53) | 0.11 (0.07–0.21) | 0.29 (0.20–0.45) | +163.64 |
| M | 0.31 (0.11–0.82) | 0.15 (0.07–0.38) | 0.31 (0.13–0.70) | +106.67 |
| Parameters | Obese T2DM Patients Before Metformin Therapy | ||
|---|---|---|---|
| Adropin (ng/mL) | Irisin (μg/mL) | Vaspin (ng/mL) | |
| Weight (kg) | −0.086 NS | −0.217 NS | 0.151 NS |
| BMI (kg/m2) | 0.127 NS | −0.111 NS | −0.044 NS |
| ACR (mg/mg Cr) | −0.014 NS | −0.264 NS | −0.209 NS |
| eGFR (mL/min/1.73m2) | 0.1 NS | −0.304 (p = 0.045) | −0.251 NS |
| Insulin (mIU/mL) | 0.1 NS | 0.222 NS | 0.491(p = 0.007) |
| HOMA-IR2 | 0.164 NS | 0.177 NS | 0.437 (p = 0.02) |
| HOMA-S | −0.152 NS | −0.160 NS | −0.445 (p = 0.017) |
| HOMA-B | 0.167 NS | 0.089 NS | 0.352 (p = 0.006) |
| QUICKI | −0.144 NS | −0.104 NS | −0.292 NS |
| eGDR | −0.124 NS | 0.073 NS | −0.084 NS |
| Glucose (mg/dL) | −0.018 NS | −0.008 NS | −0.029 NS |
| HbA1c (%) | 0.255 NS | −0.430 (p = 0.008) | 0.172 NS |
| Cholesterol (mg/dL) | 0.124 NS | −0.014 NS | 0.177 NS |
| HDL (mg/dL) | −0.161 NS | 0.137 NS | −0.081 NS |
| LDL (mg/dL) | −0.132 NS | −0.042 NS | 0.135 NS |
| Triglycerides (mg/dL) | 0.479 (p = 0.003) | −0.193 NS | 0.278 NS |
| TG/HDL | 0.382 (p = 0.021) | −0.203 NS | 0.213 NS |
| Parameters | Obese T2DM Patients After the 6-Month Metformin Therapy | ||
| Adropin (ng/mL) | Irisin (μg/mL) | Vaspin (ng/mL) | |
| Weight (kg) | 0.143 NS | −0.253 NS | 0.435 (p = 0.008) |
| BMI (kg/m2) | −0,087 NS | −0,045 NS | 0,244 NS |
| ACR (mg/mg Cr) | −0.222 NS | 0.131 NS | −0.0154 NS |
| eGFR (mL/min/1.73m2) | −0.261 NS | −0.072 NS | −0.219 NS |
| Insulin (mIU/mL) | 0.102 NS | −0.115 NS | 0.347 NS |
| HOMA-IR2 | 0.108 NS | −0.087 NS | 0.349 NS |
| HOMA-S | −0.097 NS | 0.082 NS | −0.345 NS |
| HOMA-B | −0.004 NS | −0.249 NS | 0.305 NS |
| QUICKI | −0.054 NS | 0.349 (p = 0.039) | −0.372 (p = 0.025) |
| eGDR | −0.015 NS | 0.125 NS | −0.04 NS |
| Glucose (mg/dL) | 0.193 NS | 0.241 NS | −0.059 NS |
| HbA1c (%) | −0.232 NS | −0.195 NS | 0.055 NS |
| Cholesterol (mg/dL) | −0.015 NS | −0.006 NS | −0.091 NS |
| HDL (mg/dL) | 0.03 NS | −0.004 NS | −0.021 NS |
| LDL (mg/dL) | −0.213 NS | −0.124 NS | −0.081 NS |
| Triglycerides (mg/dL) | 0.025 NS | −0.033 NS | 0.188 NS |
| TG/HDL | 0.026 NS | 0.018 NS | 0.092 NS |
| Part A | Part B | ||
|---|---|---|---|
| Three-Parameter Sets of Variables Which Allow to Differentiate the Healthy Subjects and T2DM Patients | ACC [%] | Three-Parameter Sets of Variables Which Allow to Differentiate the T2DM Patients before and after 6-Month Metformin Therapy | ACC [%] |
| adropin, irisin and vaspin | 94 | adropin, irisin and vaspin | 69 |
| adropin, QUICKI and HOMA-IR | 71 | adropin, QUICKI and HOMA-IR | 48 |
| irisin, QUICKI and HOMA-IR | 90 | irisin, QUICKI and HOMA-IR | 57 |
| vaspin, QUICKI and HOMA-IR | 63 | vaspin, QUICKI and HOMA-IR | 59 |
| adropin, ACR and eGFR | 80 | adropin, ACR and eGFR | 44 |
| irisin, ACR and eGFR | 75 | irisin, ACR and eGFR | 68 |
| vaspin, ACR and eGFR | 73 | vaspin, ACR and eGFR | 50 |
| adropin, HbA1c and eGDR | 86 | adropin, HbA1c and eGDR | 57 |
| irisin, HbA1c and eGDR | 88 | irisin, HbA1c and eGDR | 80 |
| vaspin, HbA1c and eGDR | 82 | vaspin, HbA1c and eGDR | 56 |
| adropin, HOMA-B and HOMA-S | 95 | adropin, HOMA-B and HOMA-S | 27 |
| irisin, HOMA-B and HOMA-S | 75 | irisin, HOMA-B and HOMA-S | 50 |
| vaspin, HOMA-B and HOMA-S | 67 | vaspin, HOMA-B and HOMA-S | 50 |
| adropin, insulin and TG/HDL | 77 | adropin, insulin and TG/HDL | 59 |
| irisin, insulin and TG/HDL | 66 | irisin, insulin and TG/HDL | 45 |
| vaspin, insulin and TG/HDL | 80 | vaspin, insulin and TG/HDL | 63 |
| Variables in the Diagnostic Panel | ACC [%] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaspin | - | HbA1c | HDL | LDL | TG | Insulin | - | HOMA-B | - | - | - | - | 88 |
| Vaspin | Irisin | - | - | - | - | - | QUICKI | - | - | - | - | eGDR | 86 |
| Vaspin | Irisin | - | - | LDL | - | - | - | - | HOMA-S | ACR | eGFR | - | 86 |
| Vaspin | Irisin | - | - | - | - | - | - | - | - | - | - | - | 82 |
| Vaspin | Irisin | HbA1c | HDL | LDL | TG | - | QUICKI | - | - | - | - | - | 85 |
| Vaspin | - | - | - | TG | - | - | - | - | - | - | - | 85 | |
| Vaspin | - | - | HDL | - | TG | - | QUICKI | - | - | - | - | - | 85 |
| Vaspin | - | - | HDL | LDL | - | - | QUICKI | - | - | - | - | eGDR | 85 |
| Vaspin | - | HbA1c | HDL | LDL | TG | - | QUICKI | - | - | - | - | - | 85 |
| Vaspin | - | HbA1c | HDL | LDL | TG | Insulin | QUICKI | HOMA-B | - | - | - | - | 85 |
| Vaspin | - | HbA1c | HDL | - | TG | - | QUICKI | - | - | - | - | - | 85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komosinska-Vassev, K.; Gala, O.; Olczyk, K.; Jura-Półtorak, A.; Olczyk, P. The Usefulness of Diagnostic Panels Based on Circulating Adipocytokines/Regulatory Peptides, Renal Function Tests, Insulin Resistance Indicators and Lipid-Carbohydrate Metabolism Parameters in Diagnosis and Prognosis of Type 2 Diabetes Mellitus with Obesity. Biomolecules 2020, 10, 1304. https://doi.org/10.3390/biom10091304
Komosinska-Vassev K, Gala O, Olczyk K, Jura-Półtorak A, Olczyk P. The Usefulness of Diagnostic Panels Based on Circulating Adipocytokines/Regulatory Peptides, Renal Function Tests, Insulin Resistance Indicators and Lipid-Carbohydrate Metabolism Parameters in Diagnosis and Prognosis of Type 2 Diabetes Mellitus with Obesity. Biomolecules. 2020; 10(9):1304. https://doi.org/10.3390/biom10091304
Chicago/Turabian StyleKomosinska-Vassev, Katarzyna, Olga Gala, Krystyna Olczyk, Agnieszka Jura-Półtorak, and Paweł Olczyk. 2020. "The Usefulness of Diagnostic Panels Based on Circulating Adipocytokines/Regulatory Peptides, Renal Function Tests, Insulin Resistance Indicators and Lipid-Carbohydrate Metabolism Parameters in Diagnosis and Prognosis of Type 2 Diabetes Mellitus with Obesity" Biomolecules 10, no. 9: 1304. https://doi.org/10.3390/biom10091304
APA StyleKomosinska-Vassev, K., Gala, O., Olczyk, K., Jura-Półtorak, A., & Olczyk, P. (2020). The Usefulness of Diagnostic Panels Based on Circulating Adipocytokines/Regulatory Peptides, Renal Function Tests, Insulin Resistance Indicators and Lipid-Carbohydrate Metabolism Parameters in Diagnosis and Prognosis of Type 2 Diabetes Mellitus with Obesity. Biomolecules, 10(9), 1304. https://doi.org/10.3390/biom10091304






