Pharmacological Effects of Agastache rugosa against Gastritis Using a Network Pharmacology Approach
Abstract
1. Introduction
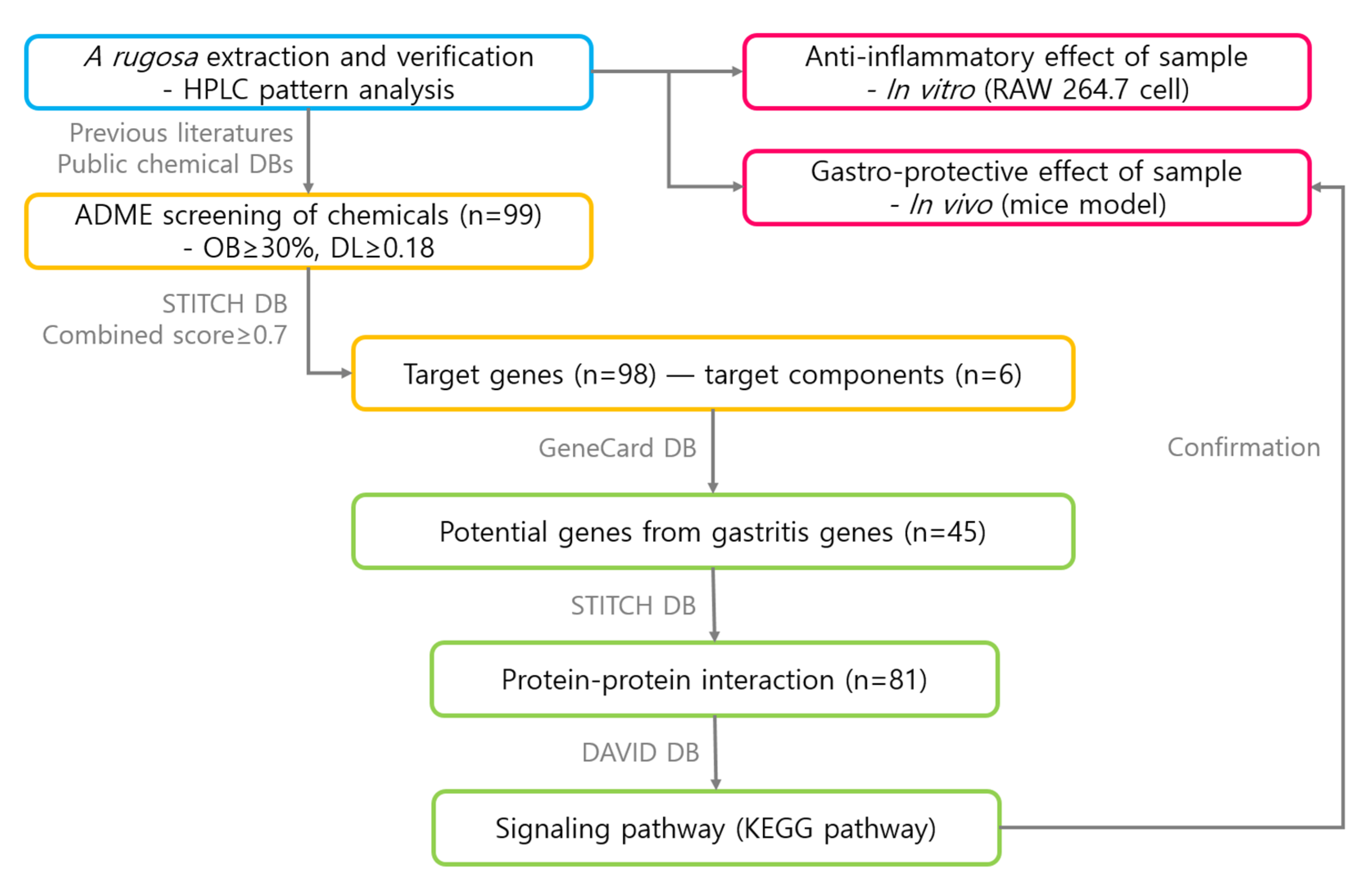
2. Materials and Methods
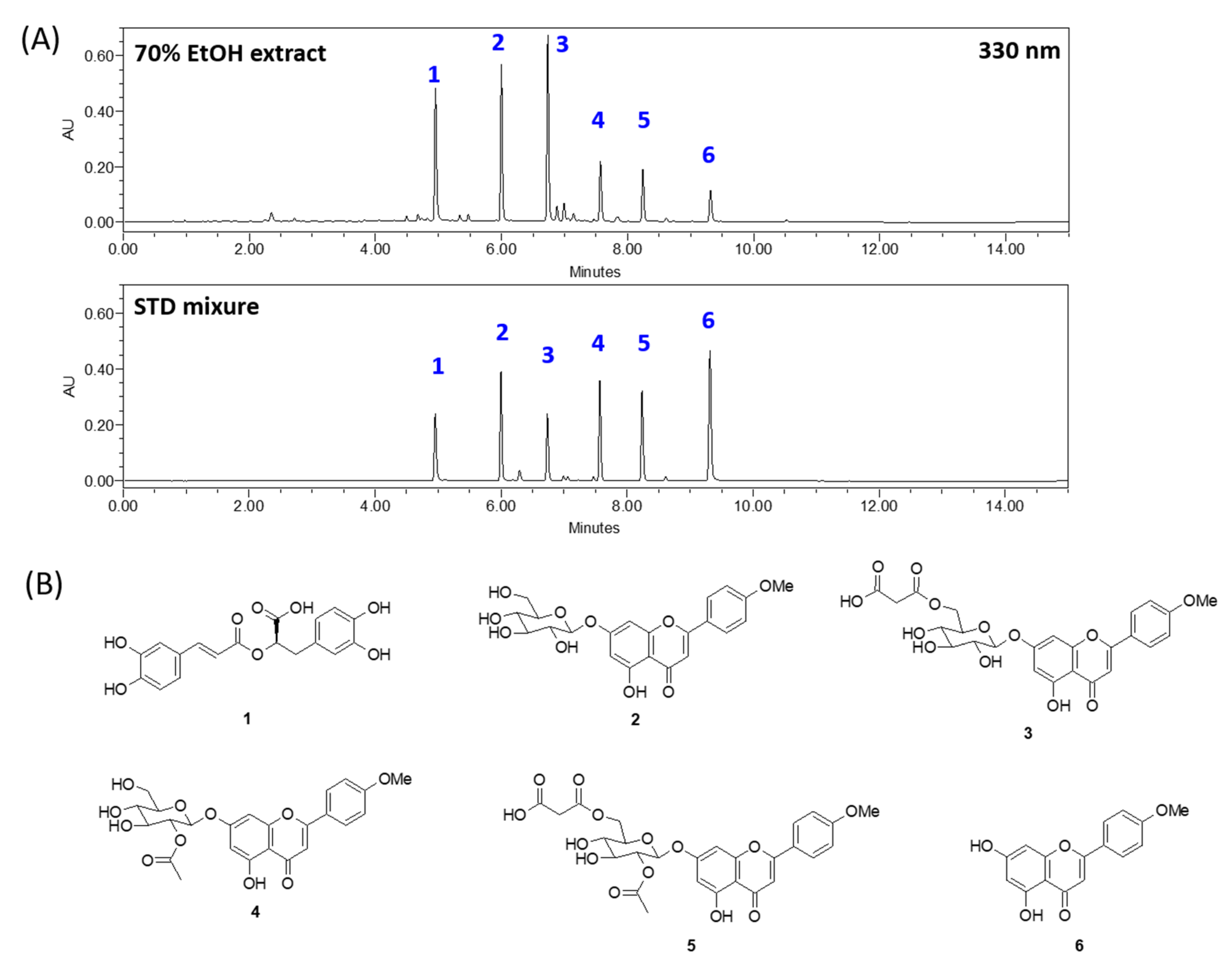
2.1. 70% Ethanol Extract of A. rugosa and Chemical Profiling
2.2. Cell Culture
2.3. Cell Cytotoxicity and NO Production
2.4. Level of the Anti-Inflammatory Proteins
2.5. Animals
2.6. HCl/EtOH-Induced Gastritis Mouse Model
2.7. Histological Analysis
2.8. Searching Chemical Components in A. Rugosa
2.9. Filtering Strategy for Components
2.10. Target Genes
2.11. Potential Target Genes
2.12. Protein–Protein Interaction
2.13. Signaling Pathway Analyses
2.14. Statistical Analysis
3. Results
3.1. Chemical Profile of A. rugosa
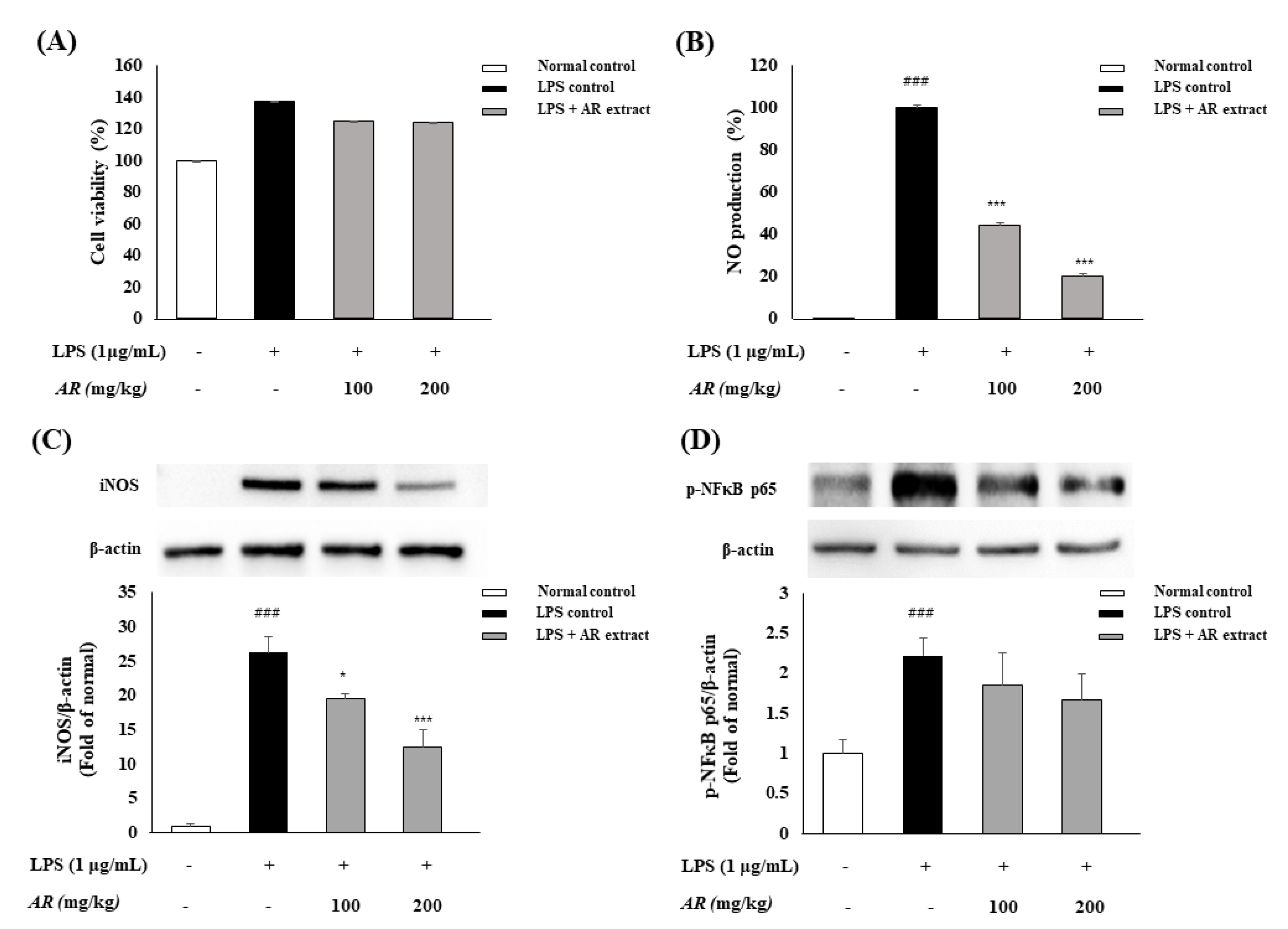
3.2. Anti-Inflammatory Effects of A. rugosa on LPS-Activated Macrophages
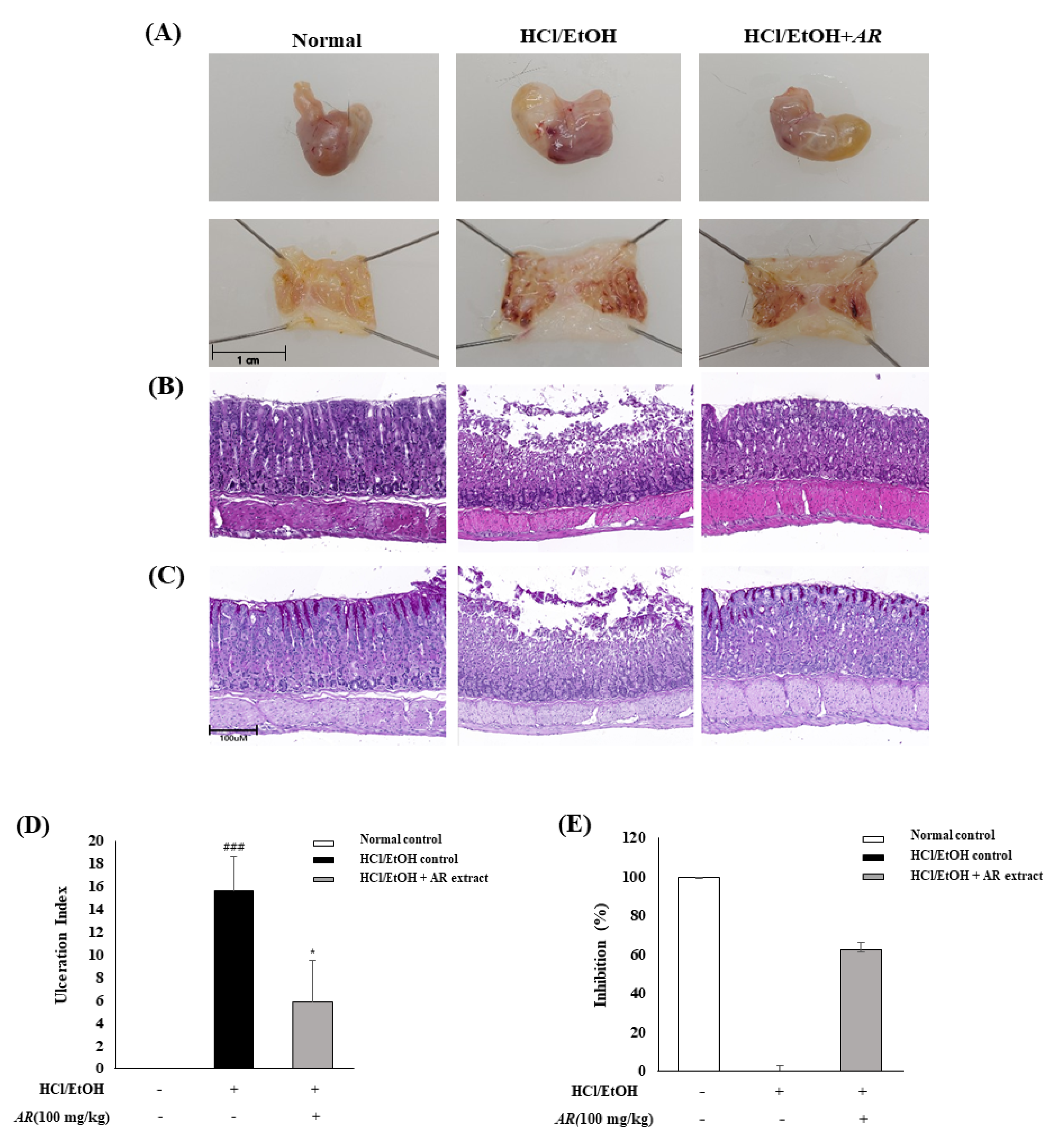
3.3. Gastro-Protective Effects of A. rugosa on HCl/EtOH-Induced Gastric Injury
3.4. ADME Screening of Components
3.5. Target Genes Linked to Target Components
3.6. Potential Target Genes Selected from Gastritis-Related Genes
3.7. Protein–Protein Interaction Network
3.8. Signaling Pathway Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tian, G.; Wu, C.; Li, J.; Liang, B.; Zhang, F.; Fan, X.; Li, Z.; Wang, Y.; Li, Z.; Liu, D.; et al. Network pharmacology based investigation into the effect and mechanism of Modified Sijunzi Decoction against the subtypes of chronic atrophic gastritis. Pharmacol. Res. 2019, 144, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, W.; Wang, X.; Xu, M.; Zhang, L.; Ding, L.; Guo, R.; Shi, Y. Network pharmacology-based strategy to investigate pharmacological mechanisms of Zuojinwan for treatment of gastritis. BMC Complement. Altern. Med. 2018, 18, 292. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.D.C.J.; Pantoja, P.D.S.; Matos, V.E.A.; Silva, R.O.; Damasceno, S.R.B.; Franco, Á.X.; Alves, R.C.; Justino, P.F.C.; De Souza, M.H.L.P.; Feitosa, J.P.A.; et al. Galactomannan from the seeds of Caesalpinia pulcherrima prevents indomethacin-induced gastrointestinal damage via neutrophil migration. Int. J. Biol. Macromol. 2019, 141, 68–75. [Google Scholar] [CrossRef]
- Azer, S.A.; Akhondi, H. Gastritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Marengo, A.; Fumagalli, M.; Sanna, C.; Maxia, A.; Piazza, S.; Cagliero, C.; Rubiolo, P.; SanGiovanni, E.; Dell’Agli, M. The hydro-alcoholic extracts of Sardinian wild thistles (Onopordum spp.) inhibit TNFα-induced IL-8 secretion and NF-κB pathway in human gastric epithelial AGS cells. J. Ethnopharmacol. 2018, 210, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-J.; Han, G.; Kim, S.-K.; Seo, J.-G.; Chung, W.-S.; Ryu, B.; Kim, J.; Yeo, I.; Lee, B.-J.; Lee, J.-M.; et al. Effect of Korean Herbal Medicine Combined with a Probiotic Mixture on Diarrhea-Dominant Irritable Bowel Syndrome: A Double-Blind, Randomized, Placebo-Controlled Trial. Evid. Based Complement. Altern. Med. 2013, 2013, 824605. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Kim, O.-S.; Yoo, S.-R.; Seo, C.-S.; Kim, Y.; Shin, H.-K. Anti-inflammatory and antioxidant activity of the traditional herbal formula Gwakhyangjeonggi-san via enhancement of heme oxygenase-1 expression in RAW264.7 macrophages. Mol. Med. Rep. 2016, 13, 4365–4371. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kwon, H.S.; Lee, H.Y. Effect of inhibition on tyrosinase and melanogenesis of Agastache rugosa Kuntze by lactic acid bacteria fermentation. J. Cosmet. Dermatol. 2016, 16, 407–415. [Google Scholar] [CrossRef]
- Seo, Y.H.; Kang, S.-Y.; Shin, J.-S.; Ryu, S.M.; Lee, A.Y.; Choi, G.; Moon, B.C.; Jang, D.S.; Shim, S.H.; Lee, D.; et al. Chemical Constituents from the Aerial Parts of Agastache rugosa and Their Inhibitory Activities on Prostaglandin E2 Production in Lipopolysaccharide-Treated RAW 264.7 Macrophages. J. Nat. Prod. 2019, 82, 3379–3385. [Google Scholar] [CrossRef]
- Seo, H.; Kim, C.; Kim, M.-B.; Hwang, J.-K. Anti-Photoaging Effect of Korean Mint (Agastache rugosa Kuntze) Extract on UVB-Irradiated Human Dermal Fibroblasts. Prev. Nutr. Food Sci. 2019, 24, 442–448. [Google Scholar] [CrossRef]
- Wei, J.-F.; Cao, P.; Wang, J.; Kang, W. Analysis of tilianin and acacetin in Agastache rugosa by high-performance liquid chromatography with ionic liquids-ultrasound based extraction. Chem. Cent. J. 2016, 10, 76. [Google Scholar] [CrossRef]
- Cao, P.; Xie, P.; Wang, X.; Wang, J.; Wei, J.-F.; Kang, W. Chemical constituents and coagulation activity of Agastache rugosa. BMC Complement. Altern. Med. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Baskar, T.B.; Park, Y.E.; Park, J.S.; Lee, S.Y.; Park, S.U. In Vitro Antioxidant and Antimicrobial Properties of Flower, Leaf, and Stem Extracts of Korean Mint. Antioxidants 2019, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Lim, H.-W.; Huang, Y.-H.; Kwon, H.-S.; Jin, C.-D.; Kim, K.; Lim, C.-J. Attenuating properties of Agastache rugosa leaf extract against ultraviolet-B-induced photoaging via up-regulating glutathione and superoxide dismutase in a human keratinocyte cell line. J. Photochem. Photobiol. B Biol. 2016, 163, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Haiyan, G.; Lijuan, H.; Shaoyu, L.; Chen, Z.; Ashraf, M.A. Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi J. Biol. Sci. 2016, 23, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Jiang, Z.-M.; Chen, Y.; Xiao, P.-T.; Wang, Z.-Y.; Huang, T.-Q.; Liu, E.-H. Network pharmacology approach to elucidate possible action mechanisms of Sinomenii Caulis for treating osteoporosis. J. Ethnopharmacol. 2020, 257, 112871. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Lee, C.-Y.; Kim, Y.-S.; Kim, C.-E. The Methodological Trends of Traditional Herbal Medicine Employing Network Pharmacology. Biomolecules 2019, 9, 362. [Google Scholar] [CrossRef]
- Li, S.; Xue, X.; Yang, X.; Zhou, S.; Wang, S.; Meng, J. A Network Pharmacology Approach Used to Estimate the Active Ingredients of Moutan Cortex Charcoal and the Potential Targets in Hemorrhagic Diseases. Biol. Pharm. Bull. 2019, 42, 432–441. [Google Scholar] [CrossRef]
- Yu, G.; Luo, Z.; Zhou, Y.; Zhang, L.; Wu, Y.; Ding, L.; Shi, Y. Uncovering the pharmacological mechanism of Carthamus tinctorius L. on cardiovascular disease by a systems pharmacology approach. Biomed. Pharmacother. 2019, 117, 109094. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, Y.-S.; Li, S.; Tan, H.-Y.; Wang, N.; Mu, S.; Hao, X.-J.; Feng, Y. A Network Pharmacology-Based Study on the Hepatoprotective Effect of Fructus Schisandrae. Molecules 2017, 22, 1617. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, W.; Lu, Y.; Hu, S.; Gao, R.; Sun, Z.; Chen, X.; Ma, J.; Guo, S.; Du, S.; et al. Network pharmacology-based identification of protective mechanism of Panax Notoginseng Saponins on aspirin induced gastrointestinal injury. Biomed. Pharmacother. 2018, 105, 159–166. [Google Scholar] [CrossRef]
- Huang, A.; Fang, G.; Pang, Y.; Pang, Z. A Network Pharmacology Approach to Explore Mechanism of Action of Longzuan Tongbi Formula on Rheumatoid Arthritis. Evid. Based Complement. Altern. Med. 2019, 2019, 5191362. [Google Scholar] [CrossRef] [PubMed]
- Azab, S.S.; Jaleel, G.A.A.; Eldahshan, O.A. Anti-inflammatory and gastroprotective potential of leaf essential oil of Cinnamomum glanduliferum in ethanol-induced rat experimental gastritis. Pharm. Biol. 2017, 55, 1654–1661. [Google Scholar] [CrossRef]
- Lee, A.Y.; Park, W.; Kang, T.-W.; Cha, M.H.; Chun, J.M. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. J. Ethnopharmacol. 2018, 221, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-X.; Zhang, Y.-Y.; Zhang, X.-X.; Wang, P.; Liu, J.; Liu, Q.; Wang, Z. Different network pharmacology mechanisms of Danshen-based Fangjis in the treatment of stable angina. Acta Pharmacol. Sin. 2018, 39, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Huang, J.; Wang, N.; Tan, H.-Y.; Cheung, F.; Chen, F.; Feng, Y. Integrating Network Pharmacology and Pharmacological Evaluation for Deciphering the Action Mechanism of Herbal Formula Zuojin Pill in Suppressing Hepatocellular Carcinoma. Front. Pharmacol. 2019, 10, 1185. [Google Scholar] [CrossRef]
- Shawky, E. Prediction of potential cancer-related molecular targets of North African plants constituents using network pharmacology-based analysis. J. Ethnopharmacol. 2019, 238, 111826. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Z.; Bao, H.; Zhang, G.; Liu, Z. Systematic Analysis of the Pharmacological Effects of Alcoholic Components in Maotai. J. Food Sci. 2019, 84, 1949–1956. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Yan, Y.; Yang, M.; Li, C.; Li, J.; Zhong, L.; Gong, Q.; Yu, H. Network Pharmacology-Based Strategy to Investigate the Pharmacologic Mechanisms of Atractylodes macrocephala Koidz. for the Treatment of Chronic Gastritis. Front. Pharmacol. 2020, 10, 1629. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, L.; Siu, W.; Jin, Y.; Cao, M.; Li, W.; Chen, J.; Cong, W.; Ma, M.; Chen, K.-J.; et al. A combined molecular biology and network pharmacology approach to investigate the multi-target mechanisms of Chaihu Shugan San on Alzheimer’s disease. Biomed. Pharmacother. 2019, 120, 109370. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2015, 44, D380–D384. [Google Scholar] [CrossRef]
- Zhu, N.; Hou, J.; Ma, G.; Liu, J. Network Pharmacology Identifies the Mechanisms of Action of Shaoyao Gancao Decoction in the Treatment of Osteoarthritis. Med. Sci. Monit. 2019, 25, 6051–6073. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Barabasi, A.-L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2010, 12, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, J.K.; Uddin, R.; Xu, H.; Park, W.T.; Tuan, P.A.; Li, X.; Chung, E.; Lee, J.-H.; Park, S.U. Metabolomics Analysis and Biosynthesis of Rosmarinic Acid in Agastache rugosa Kuntze Treated with Methyl Jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Tan, H.-Y.; Cheung, F.; Wang, N.; Huang, J.-H.; Feng, Y. A Network-Based Pharmacology Study of the Herb-Induced Liver Injury Potential of Traditional Hepatoprotective Chinese Herbal Medicines. Molecules 2017, 22, 632. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Mao, X.; Guo, Q.-Y.; Lin, N.; Li, S. Network Pharmacology-based Approaches Capture Essence of Chinese Herbal Medicines. Chin. Herb. Med. 2016, 8, 107–116. [Google Scholar] [CrossRef]
- Choudhary, N.; Singh, V. Insights about multi-targeting and synergistic neuromodulators in Ayurvedic herbs against epilepsy: Integrated computational studies on drug-target and protein-protein interaction networks. Sci. Rep. 2019, 9, 10565. [Google Scholar] [CrossRef]
- Sharman, J.L.; Harding, S.D.; Southan, C.; Faccenda, E.; Pawson, A.J.; Davies, J.A.; NC-IUPHAR. Accessing Expert-Curated Pharmacological Data in the IUPHAR/BPS Guide to PHARMACOLOGY. Curr. Protoc. Bioinform. 2018, 61, 1.34.1–1.34.46. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Zhu, Z.; Xueying, L.; Chunlin, L.; Wen, X.; Rongrong, Z.; Jing, H.; Meilan, J.; Yuwei, X.; Zili, W. Effect of berberine on LPS-induced expression of NF-κB/MAPK signalling pathway and related inflammatory cytokines in porcine intestinal epithelial cells. Innate Immun. 2020. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, E.R.D.; Guerra, G.; Araújo, D.F.D.S.; De Araujo, A.A.; Fernandes, J.M.; Junior, R.D.A.; Da Silva, V.C.; Carvalho, T.; Ferreira, L.D.S.; Zucolotto, S.M. Gastroprotective and Antioxidant Activity of Kalanchoe brasiliensis and Kalanchoe pinnata Leaf Juices against Indomethacin and Ethanol-Induced Gastric Lesions in Rats. Int. J. Mol. Sci. 2018, 19, 1265. [Google Scholar] [CrossRef] [PubMed]
- Alqasoumi, S.; Al-Yahya, M.; Al-Howiriny, T.; Rafatullah, S.Y.E.D. Gastroprotective effect of radishRaphanus sativus’’L. on experimental gastric ulcer models in rats. Farmacia Bucuresti 2008, 56, 204. [Google Scholar]
- Tuan, P.A.; Park, W.T.; Xu, H.; Park, N.I.; Park, S.U. Accumulation of Tilianin and Rosmarinic Acid and Expression of Phenylpropanoid Biosynthetic Genes inAgastache rugosa. J. Agric. Food Chem. 2012, 60, 5945–5951. [Google Scholar] [CrossRef]
- Zhang, S.; Mo, F.; Luo, Z.; Huang, J.; Sun, C.; Zhang, R. Flavonoid Glycosides of Polygonum capitatum Protect against Inflammation Associated with Helicobacter pylori Infection. PLoS ONE 2015, 10, e0126584. [Google Scholar] [CrossRef]
- Choi, E.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory activities of Canarium subulatum Guillaumin methanol extract operate by targeting Src and Syk in the NF-κB pathway. J. Ethnopharmacol. 2019, 238, 111848. [Google Scholar] [CrossRef]
- Min, Y.S.; Bai, K.L.; Yim, S.H.; Lee, Y.J.; Song, H.J.; Kim, J.H.; Ham, I.; Whang, W.K.; Sohn, U.D. The effect of luteolin-7-O-β-d-glucuronopyranoside on gastritis and esophagitis in rats. Arch. Pharmacal. Res. 2006, 29, 484–489. [Google Scholar] [CrossRef]
- Aubert, P.; Guinobert, I.; Blondeau, C.; Bardot, V.; Ripoche, I.; Chalard, P.; Neunlist, M. Basal and Spasmolytic Effects of a Hydroethanolic Leaf Extract of Melissa officinalis L. on Intestinal Motility: An Ex Vivo Study. J. Med. Food 2019, 22, 653–662. [Google Scholar] [CrossRef]
- Kangwan, N.; Pintha, K.; Lekawanvijit, S.; Suttajit, M. Rosmarinic Acid Enriched Fraction from Perilla frutescens Leaves Strongly Protects Indomethacin-Induced Gastric Ulcer in Rats. BioMed. Res. Int. 2019, 2019, 9514703. [Google Scholar] [CrossRef]
- Chen, L.; Shao, J.; Luo, Y.; Zhao, L.; Zhao, K.; Gao, Y.; Wang, S.; Liu, Y. An integrated metabolism in vivo analysis and network pharmacology in UC rats reveal anti-ulcerative colitis effects from Sophora flavescens EtOAc extract. J. Pharm. Biomed. Anal. 2020, 186, 113306. [Google Scholar] [CrossRef]
- Piao, Y.; Li, Y.; Xu, Q.; Liu, J.-W.; Xing, C.-Z.; Xie, X.-D.; Yuan, Y. Association of MTOR and AKT Gene Polymorphisms with Susceptibility and Survival of Gastric Cancer. PLoS ONE 2015, 10, e0136447. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Vieth, M.; Gnad, T.; Bozko, P.M.; Naumann, M. Helicobacter pylori promotes eukaryotic protein translation by activating phosphatidylinositol 3 kinase/mTOR. Int. J. Biochem. Cell Biol. 2014, 55, 157–163. [Google Scholar] [CrossRef]
- Matak, P.; Heinis, M.; Mathieu, J.R.R.; Corriden, R.; Cuvellier, S.; Delga, S.; Mounier, R.; Rouquette, A.; Raymond, J.; Lamarque, M.; et al. Myeloid HIF-1 Is Protective in Helicobacter pylori–Mediated Gastritis. J. Immunol. 2015, 194, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Ansari, M.A.; Alkharfy, K.M.; Aljenoobi, F.I.; Jan, B.L.; Al-Mohizea, A.M.; Khan, A.; Ali, N.; Aljenobi, F.I. Momordica charantia polysaccharides ameliorate oxidative stress, inflammation, and apoptosis in ethanol-induced gastritis in mucosa through NF-kB signaling pathway inhibition. Int. J. Biol. Macromol. 2018, 111, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Moorchung, N.; Srivastava, A.; Sharma, A.; Achyut, B.; Mittal, B. Nuclear factor kappa-B and histopathology of chronic gastritis. Indian J. Pathol. Microbiol. 2010, 53, 424. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.L.; Aveé, P.; Ndiaye, D.; Bambou, J.-C.; Huerre, M.R.; Philpott, D.J.; Mémet, S. NF-κB Activation during Acute Helicobacter pylori Infection in Mice. Infect. Immun. 2007, 76, 551–561. [Google Scholar] [CrossRef]
- Yeo, D.; Hwang, S.J.; Kim, W.J.; Youn, H.-J.; Lee, H.-J. The aqueous extract from Artemisia capillaris inhibits acute gastric mucosal injury by inhibition of ROS and NF-kB. Biomed. Pharmacother. 2018, 99, 681–687. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, J.; Niu, C.; Yu, N.; Chen, Z.; Cong, W.; Geng, F. Mechanistic evaluation of gastroprotective effects of Kangfuxin on ethanol-induced gastric ulcer in mice. Chem. Biol. Interact. 2017, 273, 115–124. [Google Scholar] [CrossRef]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; A Peters, J.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176, S21–S141. [Google Scholar] [CrossRef]
- Alexander, S.P.; Kelly, E.; Mathie, A.; A Peters, J.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Introduction and Other Protein Targets. Br. J. Pharmacol. 2019, 176, S1–S20. [Google Scholar] [CrossRef]
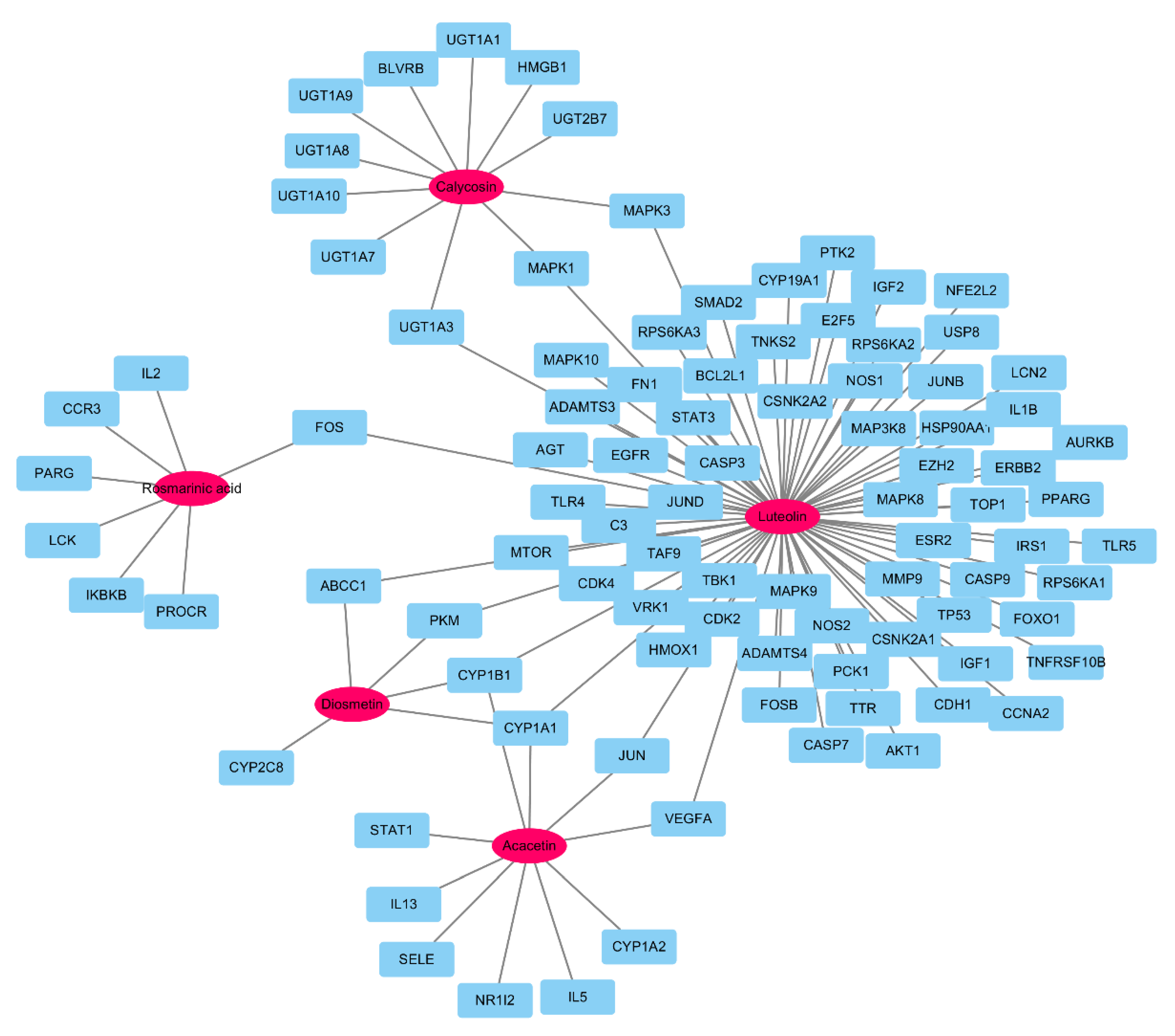
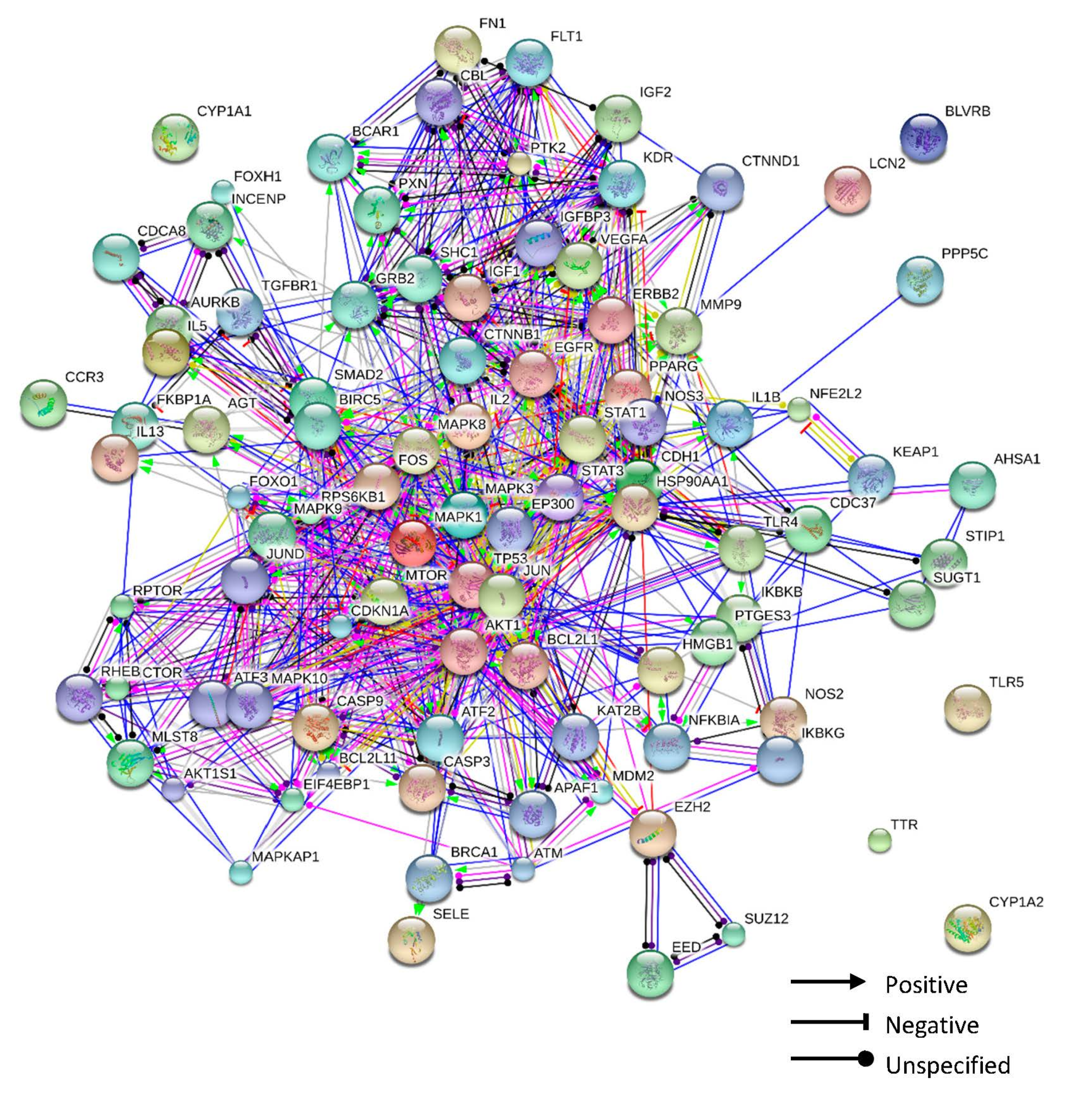
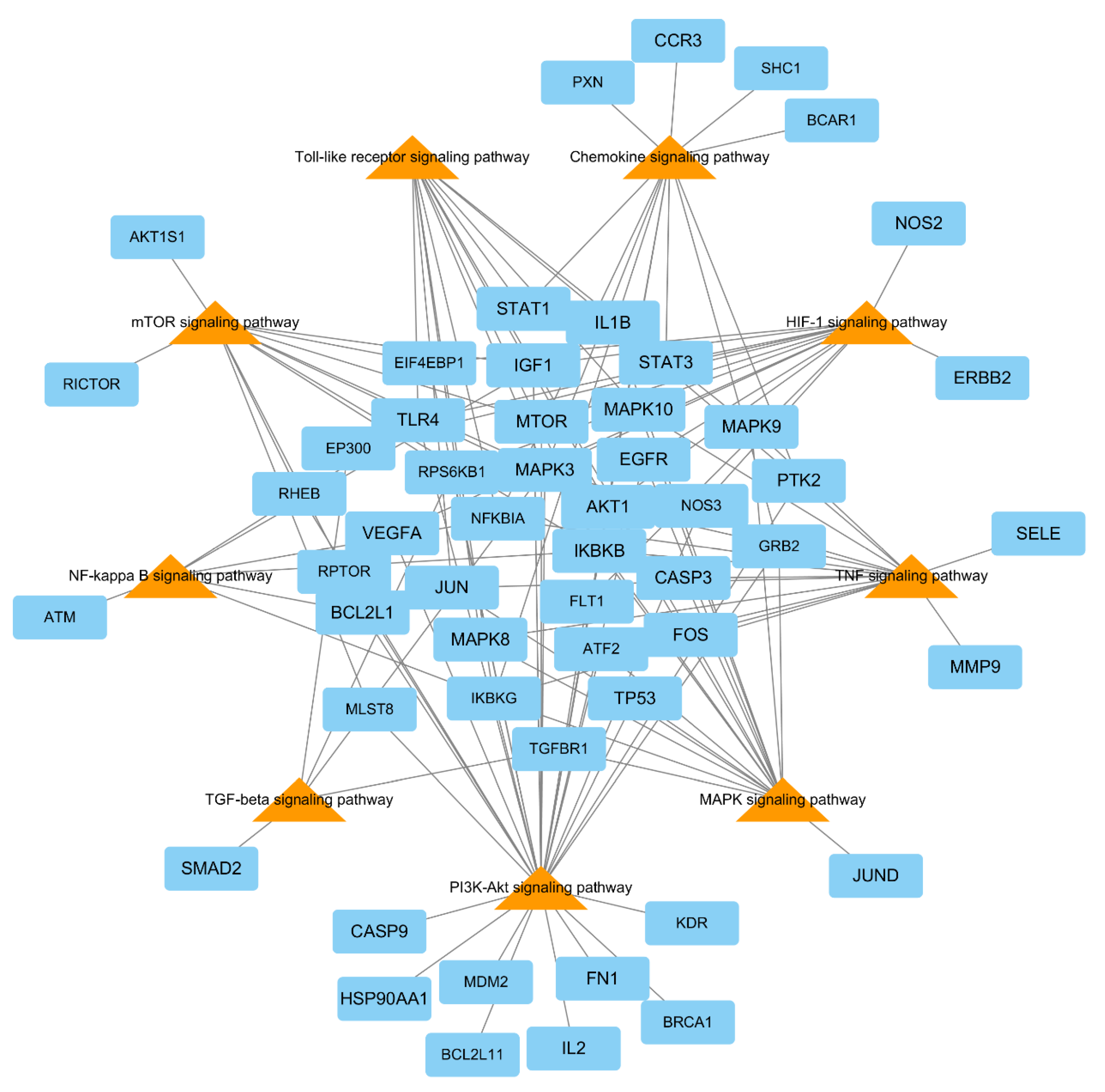
| No. | Components | Genes | Degree | Closeness Centrality | Betweenness Centrality |
|---|---|---|---|---|---|
| 1 | Acacetin | CYP1A1, CYP1A2, IL5, IL13, JUN, SELE, STAT1, VEGFA | 8 | 0.34751773 | 0.19770408 |
| 2 | Calycosin | BLVRB, HMGB1, MAPK1, MAPK3 | 4 | 0.31210191 | 0.08120748 |
| 3 | Diosmetin | CYP1A1 | 1 | 0.31612903 | 0 |
| 4 | Luteolin | AGT, AKT1, AURKB, BCL2L1, CASP3, CASP9, CDH1, CYP1A1, EGFR, ERBB2, EZH2, FN1, FOS, HSP90AA1, IGF1. IGF2, IL1B, JUN, LCN2, MAPK1, MAPK3, MAPK8, MMP9, MTOR, NFE2L2, NOS2, PPARG, PTK2, SMAD2, STAT3, TLR4, TLR5, TP53, TTR, VEGFA | 35 | 0.67123288 | 0.94727891 |
| 5 | Rosmarinic acid | CCR3, FOS, IKBKB, IL2 | 4 | 0.31612903 | 0.11989796 |
| Effects of A. rugosa Extract. | Test Type | Models | Ref. |
|---|---|---|---|
| Tyrosinase and melanogenesis inhibition | In vitro | CCD-986sk, B16F10 | [8] |
| PGE2 inhibition | In vitro | RAW264.7 | [9] |
| Anti-photoaging effect | In vitro | HS68 | [10] |
| Coagulation effect | In vitro | Blood in rabbit | [12] |
| Antioxidant and antimicrobial effect | In vitro | Six bacterial strains | [13] |
| Anti-photoaging effect | In vitro | HaCaT keratinocyte | [14] |
| NO, iNOS inhibition Gastro-protective effect | In vitro In vivo | RAW264.7 Mice | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.-H.; Kim, J.S.; Lee, J.; Seo, Y.H.; Kim, H.S.; Ryu, S.M.; Choi, G.; Moon, B.C.; Lee, A.Y. Pharmacological Effects of Agastache rugosa against Gastritis Using a Network Pharmacology Approach. Biomolecules 2020, 10, 1298. https://doi.org/10.3390/biom10091298
Nam H-H, Kim JS, Lee J, Seo YH, Kim HS, Ryu SM, Choi G, Moon BC, Lee AY. Pharmacological Effects of Agastache rugosa against Gastritis Using a Network Pharmacology Approach. Biomolecules. 2020; 10(9):1298. https://doi.org/10.3390/biom10091298
Chicago/Turabian StyleNam, Hyeon-Hwa, Joong Sun Kim, Jun Lee, Young Hye Seo, Hyo Seon Kim, Seung Mok Ryu, Goya Choi, Byeong Cheol Moon, and A Yeong Lee. 2020. "Pharmacological Effects of Agastache rugosa against Gastritis Using a Network Pharmacology Approach" Biomolecules 10, no. 9: 1298. https://doi.org/10.3390/biom10091298
APA StyleNam, H.-H., Kim, J. S., Lee, J., Seo, Y. H., Kim, H. S., Ryu, S. M., Choi, G., Moon, B. C., & Lee, A. Y. (2020). Pharmacological Effects of Agastache rugosa against Gastritis Using a Network Pharmacology Approach. Biomolecules, 10(9), 1298. https://doi.org/10.3390/biom10091298






