Attachable Hydrogel Containing Indocyanine Green for Selective Photothermal Therapy against Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Poly(Acrylamide-co-Diallyldimethylammonium Chloride) Hydrogels
2.3. Determination of Indocyanine Green Oncentration in Poly (Acrylamide-co-Diallyldimethylammonium Chloride) Hydrogels
2.4. Characterization
2.5. Apoptosis Assay
2.6. Near-Infrared Photothermal Heating
2.7. Mice
2.8. Melanoma Photothermal Therapy
2.9. Hematoxylin and Eosin Staining
2.10. Melanoma Photothermal Therapy
3. Results
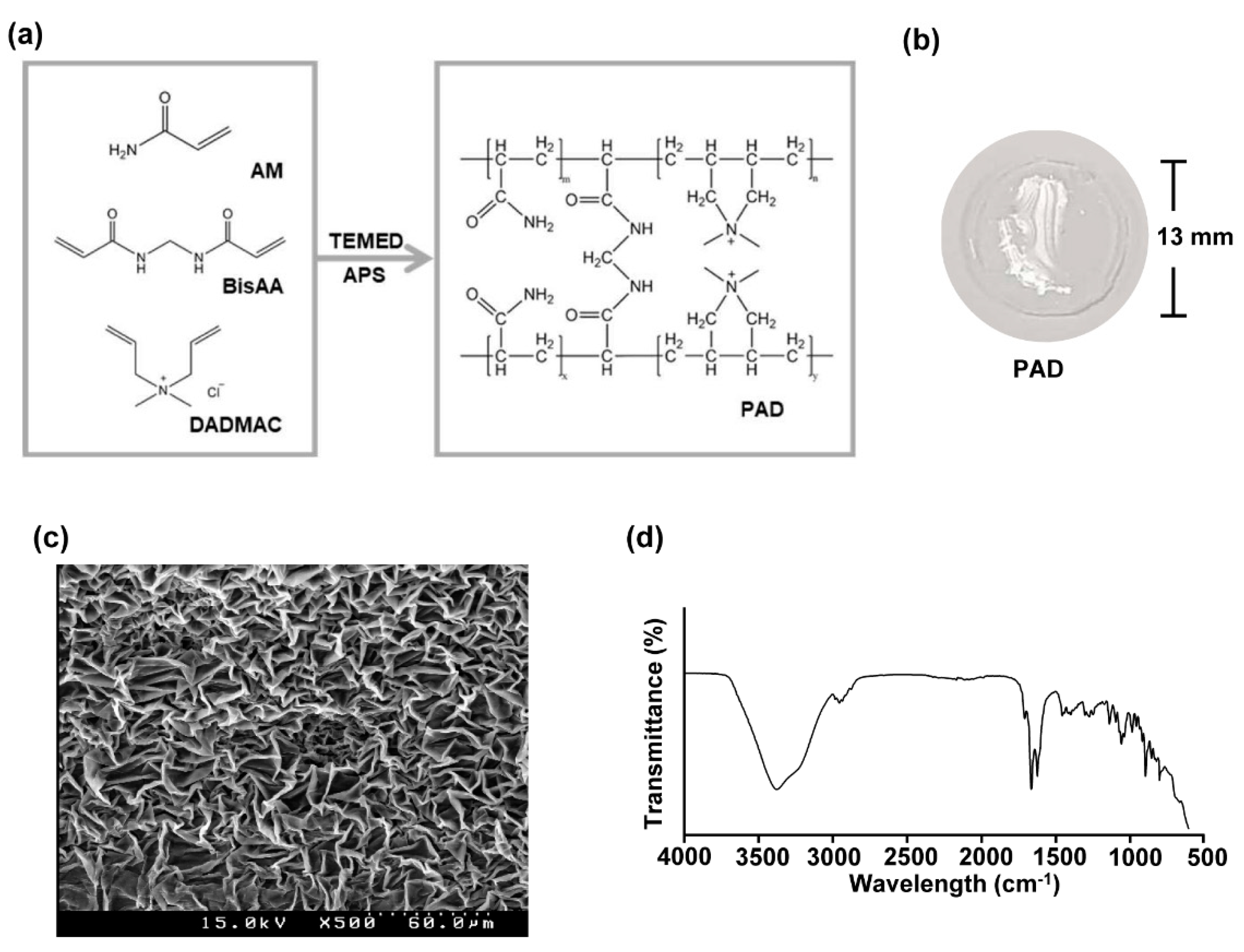
3.1. Preparation and Characterization of Poly (Acrylamide-co-Diallyldimethylammonium Chloride) Hydrogels
3.2. Preparation and Characterization of Poly (Acrylamide-co-Diallyldimethylammonium Chloride) Hydrogels
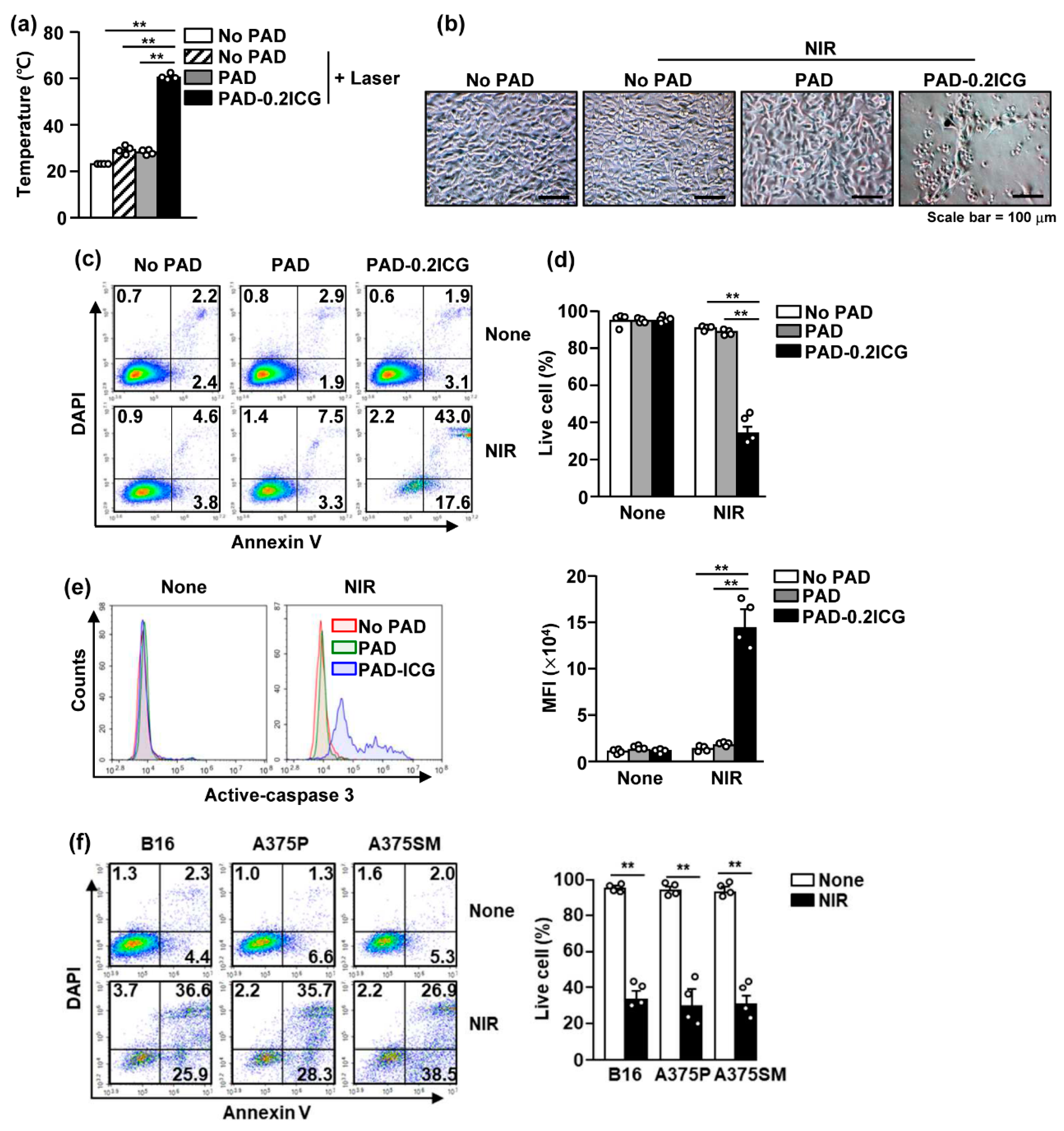
3.3. Poly (Acrylamide-co-Diallyldimethylammonium Chloride) and Laser Irradiation Induced Cell Death of Melanoma Cell Lines
3.4. Poly (Acrylamide-co-Diallyldimethylammonium Chloride) Hydrogels and Laser Irradiation Eliminated Melanoma by Photothermal Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luís, R.; Brito, C.; Pojo, M. Melanoma Metabolism: Cell Survival and Resistance to Therapy. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: Cham, Switzerland, 2020; Volume 1219, pp. 203–223. [Google Scholar]
- Abbas, O.; Miller, D.D.; Bhawan, J. Cutaneous Malignant Melanoma. Am. J. Dermatopathol. 2014, 36, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.S. Current systemic therapy for metastatic melanoma. Expert Rev. Anticancer. Ther. 2009, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Atkins, M.B.; Sober, A.J. Management of Cutaneous Melanoma. N. Engl. J. Med. 2004, 351, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Bajetta, E.; Del Vecchio, M.; Bernard-Marty, C.; Vitali, M.; Buzzoni, R.; Rixe, O.; Nova, P.; Aglione, S.; Taillibert, S.; Khayat, D. Metastatic melanoma: Chemotherapy. Semin. Oncol. 2002, 29, 427–445. [Google Scholar] [CrossRef]
- Espenel, S.; Vallard, A.; Rancoule, C.; Garcia, M.-A.; Guy, J.-B.; Chargari, C.; Deutsch, É.; Magné, N. Melanoma: Last call for radiotherapy. Crit. Rev. Oncol. 2017, 110, 13–19. [Google Scholar] [CrossRef]
- Hauschild, A.; Garbe, C. Immunotherapy: Combined immunotherapy—A new standard in metastatic melanoma? Nat. Rev. Clin. Oncol. 2015, 12, 439–440. [Google Scholar] [CrossRef]
- Yue, C.; Liu, P.; Zheng, M.; Zhao, P.; Wang, Y.; Ma, Y.; Cai, L. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials 2013, 34, 6853–6861. [Google Scholar] [CrossRef]
- Dong, X.; Liang, J.; Yang, A.; Qian, Z.; Kong, D.; Lv, F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials 2019, 209, 111–125. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Q.; Chang, J.; Wu, C. Grape Seed-Inspired Smart Hydrogel Scaffolds for Melanoma Therapy and Wound Healing. ACS Nano 2019, 13, 4302–4311. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, D.-S. Fabrication, characterization, and biological evaluation of anti-HER2 indocyanine green-doxorubicin-encapsulated PEG-b-PLGA copolymeric nanoparticles for targeted photochemotherapy of breast cancer cells. Sci. Rep. 2017, 7, 46688. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.; Park, H.-B.; Kwak, M.; Oh, J.; Lee, P.C.W.; Jin, J.-O. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J. Immunother. Cancer 2019, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, F.; Wu, B.; Chen, W.R.; Xing, D. Enhanced Tumor Treatment Using Biofunctional Indocyanine Green-Containing Nanostructure by Intratumoral or Intravenous Injection. Mol. Pharm. 2012, 9, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, T.; Masuda, K.; Urano, Y.; Kawaguchi, Y.; Satou, S.; Kaneko, J.; Hasegawa, K.; Shibahara, J.; Fukayama, M.; Tsuji, S.; et al. Mechanistic Background and Clinical Applications of Indocyanine Green Fluorescence Imaging of Hepatocellular Carcinoma. Ann. Surg. Oncol. 2013, 21, 440–448. [Google Scholar] [CrossRef]
- Chen, W.; Achazi, K.; Schade, B.; Haag, R. Charge-conversional and reduction-sensitive poly(vinyl alcohol) nanogels for enhanced cell uptake and efficient intracellular doxorubicin release. J. Control. Release 2015, 205, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; He, C.; Quan, F.; Yu, S.; Chen, X. DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018, 3, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, Y.-S.; Wu, S.-J.; Mi, F.-L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172. [Google Scholar] [CrossRef]
- Ledezma, D.; Balakrishnan, P.B.; Cano-Mejia, J.; Sweeney, E.E.; Hadley, M.; Bollard, C.M.; Villagra, A.; Fernandes, R. Indocyanine Green-Nexturastat A-PLGA Nanoparticles Combine Photothermal and Epigenetic Therapy for Melanoma. Nanomaterials 2020, 10, 161. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.-R.; Wu, C. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef]
- Komatsu, S.; Asoh, T.-A.; Ishihara, R.; Kikuchi, A. Fabrication of thermoresponsive degradable hydrogel made by radical polymerization of 2-methylene-1,3-dioxepane: Unique thermal coacervation in hydrogel. Polymer 2019, 179, 121633. [Google Scholar] [CrossRef]
- Song, C.; Phuengkham, H.; Kim, Y.S.; Dinh, V.V.; Lee, I.; Shin, I.W.; Shin, H.S.; Jin, S.M.; Um, S.H.; Lee, H.; et al. Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nat. Commun. 2019, 10, 3745. [Google Scholar] [CrossRef]
- Park, C.G.; Hartl, C.A.; Schmid, D.; Carmona, E.M.; Kim, H.-J.; Goldberg, M.S. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci. Transl. Med. 2018, 10, eaar1916. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Chao, Y.; Zhou, X.; Liang, C.; Liu, J.; Zhang, R.; Cheng, L.; Yang, K.; Pan, W.; Zhu, M.; et al. Near-Infrared-Triggered in Situ Gelation System for Repeatedly Enhanced Photothermal Brachytherapy with a Single Dose. ACS Nano 2018, 12, 9412–9422. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.-I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef]
- Kafedjiiski, K.; Jetti, R.K.; Föger, F.; Hoyer, H.; Werle, M.; Hoffer, M.; Bernkop-Schnürch, A. Synthesis and in vitro evaluation of thiolated hyaluronic acid for mucoadhesive drug delivery. Int. J. Pharm. 2007, 343, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, P.; Murali, M.R.; Samuel, S.; Raghavendran, H.R.B.; Abbas, A.A.; Kamarul, T.; Raman, M.M.; Rao, B.R.H. Three dimensional alginate-fucoidan composite hydrogel augments the chondrogenic differentiation of mesenchymal stromal cells. Carbohydr. Polym. 2016, 147, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Heal. 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Cevher, E.; Hatipoğlu, F.; Oğurtan, Z.; Baş, A.L.; Akbuğa, J. Preparation of Fucoidan-Chitosan Hydrogel and Its Application as Burn Healing Accelerator on Rabbits. Boil. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Zhao, J.; Wu, C.; Ye, C.; Huang, M.; Wang, S. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr. Polym. 2019, 222, 115039. [Google Scholar] [CrossRef]
- Girgin, B.; Korkmaz, O.; Yavaşer, R.; Karagözler, A.A. Production and drug release assesment of melatonin loaded alginate/gum arabic beads. J. Turk. Chem. Soc. Sect. A Chem. 2016, 3, 205–216. [Google Scholar] [CrossRef][Green Version]
- Griesser, J.; Hetényi, G.; Bernkop-Schnürch, A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry behind to Product Developments—What Are the Capabilities? Polymers 2018, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Fraser, I.P.; Yeo, Y.; Highley, C.B.; Bellas, E.; Kohane, D.S. Anti-inflammatory function of an in situ cross-linkable conjugate hydrogel of hyaluronic acid and dexamethasone. Biomaterials 2007, 28, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, X.; Liang, X.; Ma, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Boil. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef]
- Qi, X.; Wu, L.; Su, T.; Zhang, J.; Dong, W. Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surfaces B Biointerfaces 2018, 170, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, S.; Zou, Z.; Hai, L.; Yang, X.; Jia, X.; Zhang, A.; He, D.; He, X.; Wang, K. A zeolitic imidazolate framework-8-based indocyanine green theranostic agent for infrared fluorescence imaging and photothermal therapy. J. Mater. Chem. B 2018, 6, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lee, S.Y.; Kim, S.; Koo, J.S.; Seo, J.-H.; Jeong, D.I.; Hwang, C.; Lee, J.; Cho, H.-J. Selenium and dopamine-crosslinked hyaluronic acid hydrogel for chemophotothermal cancer therapy. J. Control. Release 2020. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, C.; Wang, T.; Chen, J.; Sun, S.-K. In Situ Fabrication of Intelligent Photothermal Indocyanine Green–Alginate Hydrogel for Localized Tumor Ablation. ACS Appl. Mater. Interfaces 2018, 11, 2782–2789. [Google Scholar] [CrossRef]
- Kirchherr, A.-K.; Briel, A.; Mäder, K. Stabilization of Indocyanine Green by Encapsulation within Micellar Systems. Mol. Pharm. 2009, 6, 480–491. [Google Scholar] [CrossRef]


| Compositions | Volume (mL) |
|---|---|
| Deionized water (DW) | 0.700 |
| Acrylamide (AM, 30%) | 1.500 |
| Diallyldimethylammonium chloride (DADMAC, 60 w/w%) | 0.750 |
| N,N’-methylenebisacrylamide (BisAA, 1.4 w/v%) | 0.750 |
| Ammonium persulfate (APS, 10 w/v%) | 0.038 |
| N,N,N’,N’-tetramethylethylenediamine (TEMED) | 0.038 |
| ICG conc. in PAD (mg/mL) | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 0 m | 1 m | 2 m | 3 m | 4 m | 5 m | ΔT | |
| 0 | 23.0 | 27.2 | 27.5 | 27.8 | 28 | 28.1 | 5.1 |
| 0.1 | 23.0 | 39.1 | 41.2 | 41.6 | 42 | 42.1 | 19.1 |
| 0.2 | 23.1 | 48.4 | 54.5 | 55.4 | 55.7 | 55.4 | 32.3 |
| 0.5 | 24.4 | 52.9 | 58.1 | 60.1 | 60.5 | 61.3 | 36.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Jin, J.-O. Attachable Hydrogel Containing Indocyanine Green for Selective Photothermal Therapy against Melanoma. Biomolecules 2020, 10, 1124. https://doi.org/10.3390/biom10081124
Hwang J, Jin J-O. Attachable Hydrogel Containing Indocyanine Green for Selective Photothermal Therapy against Melanoma. Biomolecules. 2020; 10(8):1124. https://doi.org/10.3390/biom10081124
Chicago/Turabian StyleHwang, Juyoung, and Jun-O Jin. 2020. "Attachable Hydrogel Containing Indocyanine Green for Selective Photothermal Therapy against Melanoma" Biomolecules 10, no. 8: 1124. https://doi.org/10.3390/biom10081124
APA StyleHwang, J., & Jin, J.-O. (2020). Attachable Hydrogel Containing Indocyanine Green for Selective Photothermal Therapy against Melanoma. Biomolecules, 10(8), 1124. https://doi.org/10.3390/biom10081124





