Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction
Abstract
1. Formation and Metabolism of Homocysteine
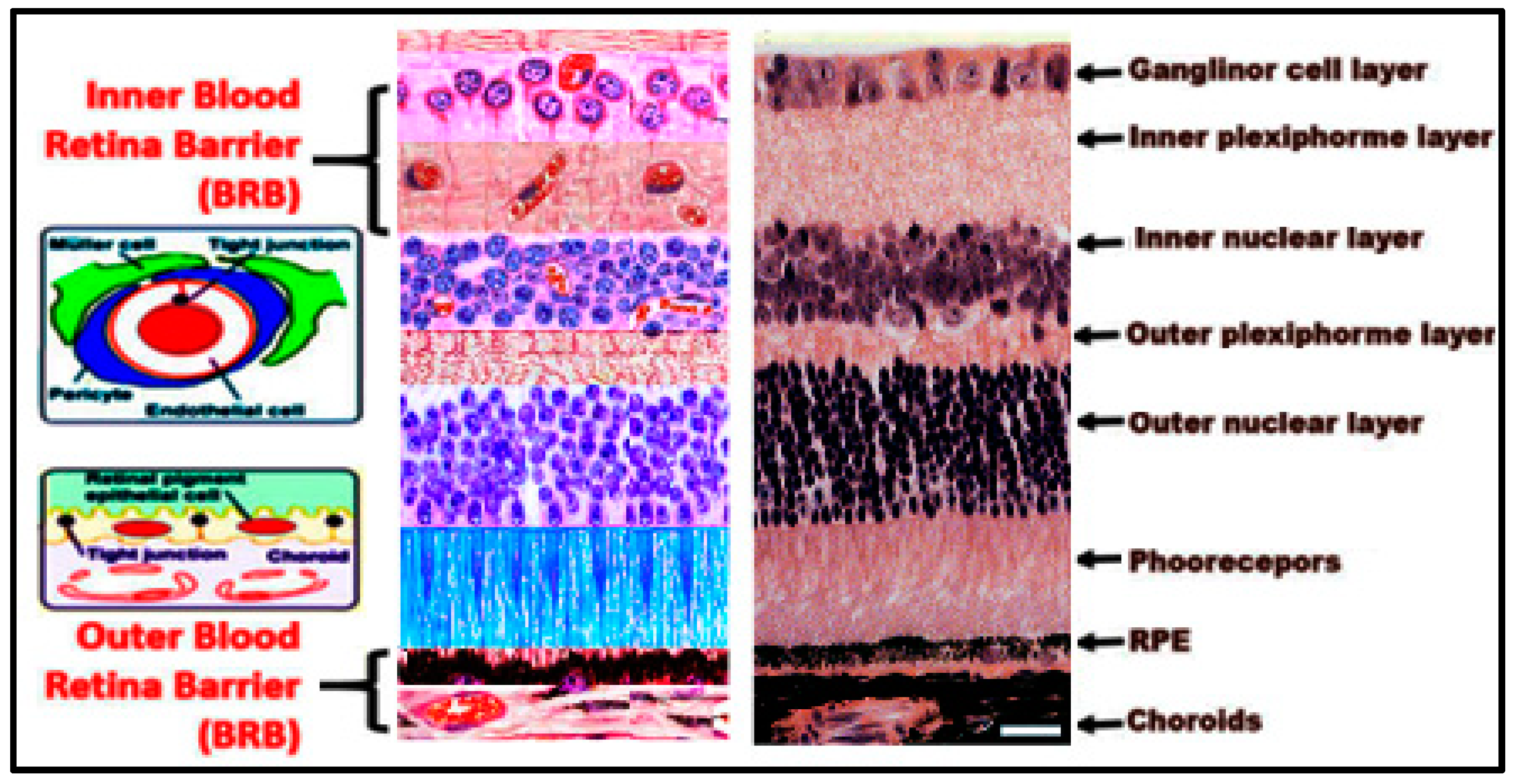
2. Structure of the Retina
3. Effect of Homocysteine on Retinal Vasculature and Blood Retinal Barrier (BRB)
4. Hyperhomocysteinemia and Retinal Diseases
5. Hyperhomocysteinemia and Age-Related Macular Degeneration
6. Hyperhomocysteinemia and Diabetic Retinopathy
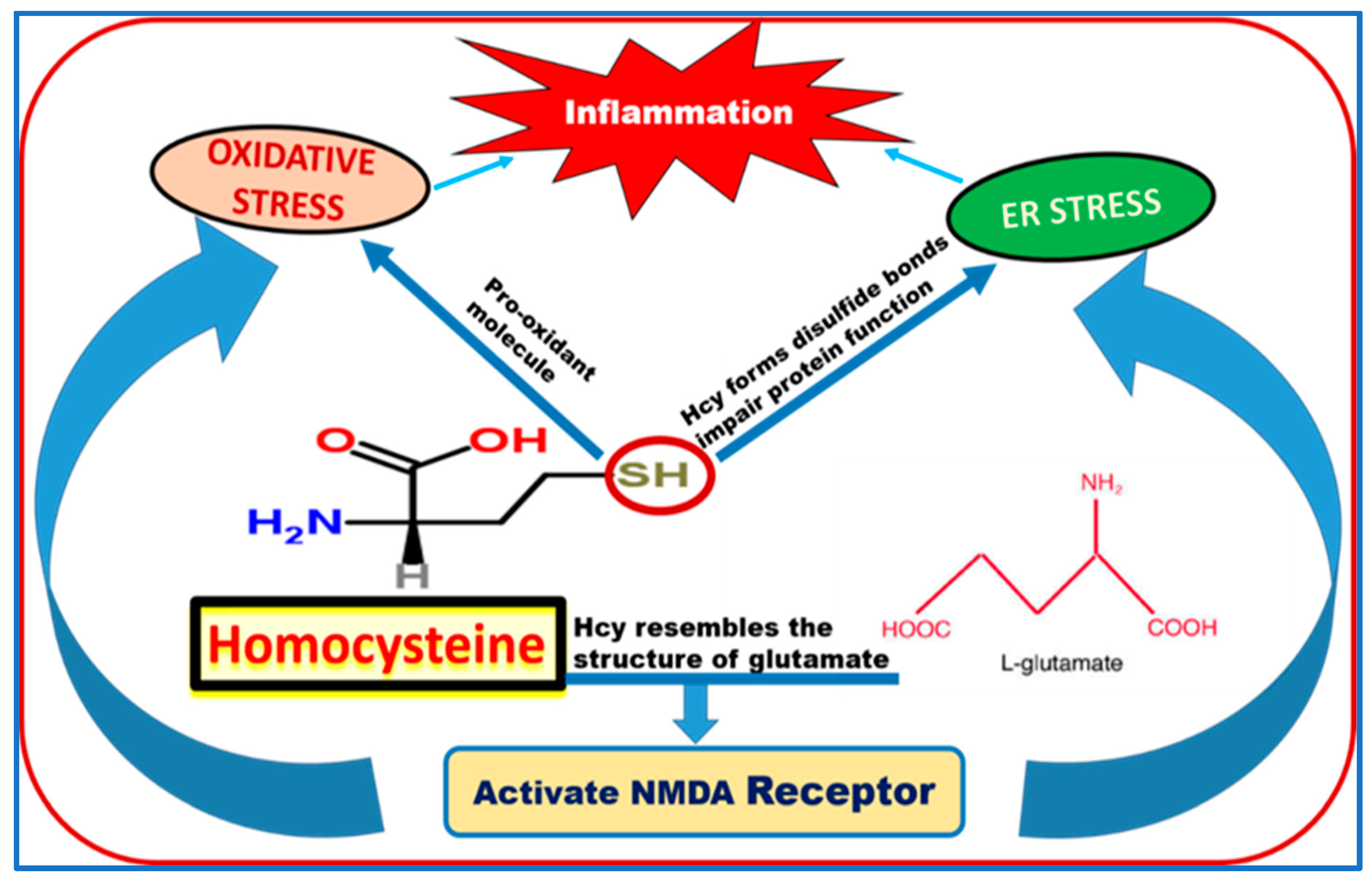
7. Possible Mechanisms of Homocysteine-Induced BRB Dysfunction
8. Hyperhomocysteinemia Is a Risk Factor for Neurological Diseases
Author Contributions
Funding
Conflicts of Interest
References
- Frantzen, F.; Faaren, A.L.; Alfheim, I.; Nordhei, A.K. Enzyme conversion immunoassay for determining total homocysteine in plasma or serum. Clin. Chem. 1998, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Manaster, B.J.; Ensign, M.F. The role of imaging in musculoskeletal tumors. Semin Ultrasound CT MR 1989, 10, 498–517. [Google Scholar] [PubMed]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, A.S. Homocysteine and cardiovascular disease: A review of the evidence. Diabetes Vasc. Dis. Res. 2007, 4, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Debreceni, B.; Debreceni, L. The role of homocysteine-lowering B-vitamins in the primary prevention of cardiovascular disease. Cardiovasc. Ther. 2014, 32, 130–138. [Google Scholar] [CrossRef]
- Shevchuk, S.V.; Postovitenko, K.P.; Iliuk, I.A.; Bezsmertna, H.V.; Bezsmertnyi, Y.O.; Kurylenko, I.V.; Biloshytska, A.V.; Baranova, I.V. The relationship between homocysteine level and vitamins B12, B9 and B6 status in patients with chronic kidney disease. Wiad. Lek. 2019, 72, 532–538. [Google Scholar]
- Shen, L.; Ji, H.F. Associations between Homocysteine, Folic Acid, Vitamin B12 and Alzheimer′s Disease: Insights from Meta-Analyses. J. Alzheimers Dis. 2015, 46, 777–790. [Google Scholar] [CrossRef]
- Shu, X.J.; Li, Z.F.; Chang, Y.W.; Liu, S.Y.; Wang, W.H. Effects of folic acid combined with vitamin B12 on DVT in patients with homocysteine cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2538–2544. [Google Scholar]
- Satyanarayana, A.; Balakrishna, N.; Pitla, S.; Reddy, P.Y.; Mudili, S.; Lopamudra, P.; Suryanarayana, P.; Viswanath, K.; Ayyagari, R.; Reddy, G.B. Status of B-vitamins and homocysteine in diabetic retinopathy: Association with vitamin-B12 deficiency and hyperhomocysteinemia. PLoS ONE 2011, 6, e26747. [Google Scholar] [CrossRef]
- House, A.A.; Eliasziw, M.; Cattran, D.C.; Churchill, D.N.; Oliver, M.J.; Fine, A.; Dresser, G.K.; Spence, J.D. Effect of B-vitamin therapy on progression of diabetic nephropathy: A randomized controlled trial. JAMA 2010, 303, 1603–1609. [Google Scholar] [CrossRef]
- Fratoni, V.; Brandi, M.L. B vitamins, homocysteine and bone health. Nutrients 2015, 7, 2176–2192. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Lagana, A.S. The Link between Homocysteine and Omega-3 Polyunsaturated Fatty Acid: Critical Appraisal and Future Directions. Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zheng, J.; Chen, Y.; Yang, B.; Wahlqvist, M.L.; Li, D. High consumption of Omega-3 polyunsaturated fatty acids decrease plasma homocysteine: A meta-analysis of randomized, placebo-controlled trials. Nutrition 2011, 27, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Valayannopoulos, V.; Schiff, M.; Guffon, N.; Nadjar, Y.; Garcia-Cazorla, A.; Martinez-Pardo Casanova, M.; Cano, A.; Couce, M.L.; Dalmau, J.; Pena-Quintana, L.; et al. Betaine anhydrous in homocystinuria: Results from the RoCH registry. Orphanet J. Rare Dis. 2019, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H.; Skovby, F.; Levy, H.L.; Pettigrew, K.D.; Wilcken, B.; Pyeritz, R.E.; Andria, G.; Boers, G.H.; Bromberg, I.L.; Cerone, R.; et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am. J. Hum. Genet. 1985, 37, 1–31. [Google Scholar]
- Grieco, A.J. Homocystinuria: Pathogenetic mechanisms. Am. J. Med. Sci. 1977, 273, 120–132. [Google Scholar] [CrossRef]
- Rozen, R. Molecular genetics of methylenetetrahydrofolate reductase deficiency. J. Inherit. Metab. Dis. 1996, 19, 589–594. [Google Scholar] [CrossRef]
- Schiff, M.; Blom, H.J. Treatment of inherited homocystinurias. Neuropediatrics 2012, 43, 295–304. [Google Scholar] [CrossRef]
- Kolb, H. Simple Anatomy of the Retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Shakib, M.; Cunha-Vaz, J.G. Studies on the permeability of the blood-retinal barrier. IV. Junctional complexes of the retinal vessels and their role in the permeability of the blood-retinal barrier. Exp. Eye Res. 1966, 5, 229–234. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. The blood-retinal barriers. Doc. Ophthalmol. 1976, 41, 287–327. [Google Scholar] [CrossRef]
- Golestaneh, N.; Chu, Y.; Xiao, Y.Y.; Stoleru, G.L.; Theos, A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017, 8, e2537. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Lee, J.E.; Kim, H.J.; Song, J.W.; Choi, S.H. Immediate break-down of blood retinal barrier by infusion of triolein emulsion observed by fluorescein angiography. Curr. Eye Res. 2011, 36, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Toda, R.; Kawazu, K.; Oyabu, M.; Miyazaki, T.; Kiuchi, Y. Comparison of drug permeabilities across the blood-retinal barrier, blood-aqueous humor barrier, and blood-brain barrier. J. Pharm. Sci. 2011, 100, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G. Studies on the permeability of the blood-retinal barrier. 3. Breakdown of the blood-retinal barrier by circulatory disturbances. Br. J. Ophthalmol. 1966, 50, 505–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosoya, K.; Tomi, M. Advances in the cell biology of transport via the inner blood-retinal barrier: Establishment of cell lines and transport functions. Biol. Pharm. Bull. 2005, 28, 1–8. [Google Scholar] [CrossRef]
- Tawfik, A.; Al-Shabrawey, M.; Roon, P.; Sonne, S.; Covar, J.A.; Matragoon, S.; Ganapathy, P.S.; Atherton, S.S.; El-Remessy, A.; Ganapathy, V.; et al. Alterations of retinal vasculature in cystathionine-Beta-synthase mutant mice, a model of hyperhomocysteinemia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 939–949. [Google Scholar] [CrossRef]
- Tawfik, A.; Markand, S.; Al-Shabrawey, M.; Mayo, J.N.; Reynolds, J.; Bearden, S.E.; Ganapathy, V.; Smith, S.B. Alterations of retinal vasculature in cystathionine-beta-synthase heterozygous mice: A model of mild to moderate hyperhomocysteinemia. Am. J. Pathol 2014, 184, 2573–2585. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Mander, S.; Hussein, K.A.; Elsherbiny, N.M.; Smith, S.B.; Al-Shabrawey, M.; Tawfik, A. Hyperhomocysteinemia disrupts retinal pigment epithelial structure and function with features of age-related macular degeneration. Oncotarget 2016, 7, 8532–8545. [Google Scholar] [CrossRef]
- Mohamed, R.; Sharma, I.; Ibrahim, A.S.; Saleh, H.; Elsherbiny, N.M.; Fulzele, S.; Elmasry, K.; Smith, S.B.; Al-Shabrawey, M.; Tawfik, A. Hyperhomocysteinemia Alters Retinal Endothelial Cells Barrier Function and Angiogenic Potential via Activation of Oxidative Stress. Sci. Rep. 2017, 7, 11952. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, X.; Jiang, W.; Bi, H. Central retinal venous occlusion in a child with hyperhomocysteinemia: A case report. Medicine 2019, 98, e15813. [Google Scholar] [CrossRef]
- Koylu, M.T.; Kucukevcilioglu, M.; Erdurman, F.C.; Durukan, A.H.; Sobaci, G.; Torun, D.; Tunca, Y.; Ayyildiz, O. Association of retinal vein occlusion, homocysteine, and the thrombophilic mutations in a Turkish population: A case-control study. Ophthalmic. Genet. 2017, 38, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Shute, C. A case report of branch retinal artery occlusion in a teenager due to hyperhomocysteinaemia; the interplay of genetic and nutritional defects. BMC Ophthalmol. 2018, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Y.; Duan, Y.; Kuang, Y.Q.; Lin, D. Homocysteine in retinal artery occlusive disease: A meta-analysis of cohort studies. Sci. Rep. 2017, 7, 15708. [Google Scholar] [CrossRef] [PubMed]
- Junemann, A.; Rejdak, R.; Hohberger, B. Significance of Homocysteine in Glaucoma. Klin Monbl Augenheilkd 2018, 235, 163–174. [Google Scholar] [CrossRef]
- Ghorbanihaghjo, A.; Javadzadeh, A.; Argani, H.; Nezami, N.; Rashtchizadeh, N.; Rafeey, M.; Rohbaninoubar, M.; Rahimi-Ardabili, B. Lipoprotein(a), homocysteine, and retinal arteriosclerosis. Mol. Vis. 2008, 14, 1692–1697. [Google Scholar]
- Mudd, S.H. Hypermethioninemias of genetic and non-genetic origin: A review. Am. J. Med. Genet. C Semin Med. Genet. 2011, 157, 3–32. [Google Scholar] [CrossRef]
- Ajith, T.A.; Ranimenon. Homocysteine in ocular diseases. Clin. Chim. Acta 2015, 450, 316–321. [Google Scholar] [CrossRef]
- Gupta, P.; John, D.; Rebekah, G.; John, S.S. Role of hyperhomocysteinemia in proliferative diabetic retinopathy: A case-control study. Indian J. Ophthalmol. 2018, 66, 1435–1440. [Google Scholar] [CrossRef]
- Dong, N.; Shi, H.; Tang, X. Plasma homocysteine levels are associated with macular thickness in type 2 diabetes without diabetic macular edema. Int. Ophthalmol. 2018, 38, 737–746. [Google Scholar] [CrossRef]
- Lei, X.; Zeng, G.; Zhang, Y.; Li, Q.; Zhang, J.; Bai, Z.; Yang, K. Association between homocysteine level and the risk of diabetic retinopathy: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 61. [Google Scholar] [CrossRef]
- Simo-Servat, O.; Simo, R.; Hernandez, C. Circulating Biomarkers of Diabetic Retinopathy: An Overview Based on Physiopathology. J. Diabetes Res. 2016, 2016, 5263798. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, Y.; Liu, G.; Liu, X.; Wang, F.; Yu, J. Relationship between homocysteine level and diabetic retinopathy: A systematic review and meta-analysis. Diagn. Pathol. 2014, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, K.; Saxena, S.; Mahdi, A.A.; Shukla, R.K.; Meyer, C.H.; Akduman, L.; Khanna, V.K. Increased serum level of homocysteine correlates with retinal nerve fiber layer thinning in diabetic retinopathy. Mol. Vis. 2016, 22, 1352–1360. [Google Scholar] [PubMed]
- Rodrigo, F.; Ruiz-Moreno, J.M.; Garcia, J.B.; Torregrosa, M.E.; Segura, J.V.; Pinero, D.P. Color Doppler imaging of the retrobulbar circulation and plasmatic biomarkers of vascular risk in age-related macular degeneration: A pilot study. Indian J. Ophthalmol. 2018, 66, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Cook, N.R.; Chiuve, S.E.; Ridker, P.M.; Gaziano, J.M. Prospective study of plasma homocysteine, its dietary determinants, and risk of age-related macular degeneration in men. Ophthalmic. Epidemiol. 2018, 25, 79–88. [Google Scholar] [CrossRef]
- Pinna, A.; Zaccheddu, F.; Boscia, F.; Carru, C.; Solinas, G. Homocysteine and risk of age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2018, 96, e269–e276. [Google Scholar] [CrossRef]
- Huang, P.; Wang, F.; Sah, B.K.; Jiang, J.; Ni, Z.; Wang, J.; Sun, X. Homocysteine and the risk of age-related macular degeneration: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 10585. [Google Scholar] [CrossRef]
- Arnold, J.J.; Heriot, W. Age related macular degeneration. BMJ Clin. Evid. 2007, 4, 701. [Google Scholar]
- Klein, R.; Peto, T.; Bird, A.; Vannewkirk, M.R. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef]
- Nowak, J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 1984, 102, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.; Moss, S.E.; Klein, R. Is menarche associated with diabetic retinopathy? Diabetes Care 1990, 13, 1034–1038. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998, 317, 703–713. [Google Scholar] [CrossRef]
- Klein, B.E.; Moss, S.E.; Klein, R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care 1990, 13, 34–40. [Google Scholar] [CrossRef]
- Goldstein, M.; Leibovitch, I.; Yeffimov, I.; Gavendo, S.; Sela, B.A.; Loewenstein, A. Hyperhomocysteinemia in patients with diabetes mellitus with and without diabetic retinopathy. Eye 2004, 18, 460–465. [Google Scholar] [CrossRef]
- Wotherspoon, F.; Laight, D.W.; Browne, D.L.; Turner, C.; Meeking, D.R.; Allard, S.E.; Munday, L.J.; Shaw, K.M.; Cummings, M.H. Plasma homocysteine, oxidative stress and endothelial function in patients with Type 1 diabetes mellitus and microalbuminuria. Diabet. Med. 2006, 23, 1350–1356. [Google Scholar] [CrossRef]
- Van Leeuwen-Wintjens, H.R.; Muls, E.E. The implications of hyperhomocysteinemia in a patient with type 1 diabetes. Acta Clin. Belg. 1998, 53, 349–352. [Google Scholar] [CrossRef]
- Feng, Y.; Shan, M.Q.; Bo, L.; Zhang, X.Y.; Hu, J. Association of homocysteine with type 1 diabetes mellitus: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 12529–12538. [Google Scholar]
- Li, J.; Zhang, H.; Yan, L.; Xie, M.; Chen, J. Fracture is additionally attributed to hyperhomocysteinemia in men and premenopausal women with type 2 diabetes. J. Diabetes Investig. 2014, 5, 236–241. [Google Scholar] [CrossRef]
- Aydin, E.; Demir, H.D.; Ozyurt, H.; Etikan, I. Association of plasma homocysteine and macular edema in type 2 diabetes mellitus. Eur. J. Ophthalmol. 2008, 18, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ukinc, K.; Ersoz, H.O.; Karahan, C.; Erem, C.; Eminagaoglu, S.; Hacihasanoglu, A.B.; Yilmaz, M.; Kocak, M. Methyltetrahydrofolate reductase C677T gene mutation and hyperhomocysteinemia as a novel risk factor for diabetic nephropathy. Endocrine 2009, 36, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, O.; Perna, A.F.; Mancini, F.P.; Iovine, C.; Cuomo, V.; Sacco, M.; Tufano, A.; Rivellese, A.A.; Ingrosso, D.; Riccardi, G. Plasma homocysteine and microvascular complications in type 1 diabetes. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 297–304. [Google Scholar]
- Kowluru, R.A.; Mohammad, G.; Sahajpal, N. Correction to: Faulty homocysteine recycling in diabetic retinopathy. Eye Vis. 2020, 7, 11. [Google Scholar] [CrossRef]
- Tawfik, A.; Mohamed, R.; Elsherbiny, N.M.; DeAngelis, M.M.; Bartoli, M.; Al-Shabrawey, M. Homocysteine: A Potential Biomarker for Diabetic Retinopathy. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef]
- Selhub, J.; Jacques, P.F.; Wilson, P.W.; Rush, D.; Rosenberg, I.H. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 1993, 270, 2693–2698. [Google Scholar] [CrossRef]
- Dardiotis, E.; Arseniou, S.; Sokratous, M.; Tsouris, Z.; Siokas, V.; Mentis, A.A.; Michalopoulou, A.; Andravizou, A.; Dastamani, M.; Paterakis, K.; et al. Vitamin B12, folate, and homocysteine levels and multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2017, 17, 190–197. [Google Scholar] [CrossRef]
- Ratnam, S.; Maclean, K.N.; Jacobs, R.L.; Brosnan, M.E.; Kraus, J.P.; Brosnan, J.T. Hormonal regulation of cystathionine beta-synthase expression in liver. J. Biol. Chem. 2002, 277, 42912–42918. [Google Scholar] [CrossRef]
- Beard, R.S., Jr.; Reynolds, J.J.; Bearden, S.E. Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood 2011, 118, 2007–2014. [Google Scholar] [CrossRef]
- Tawfik, A.; Smith, S.B. Increased ER stress as a mechanism of retinal neurovasculopathy in mice with severe hyperhomocysteinemia. Austin J. Clin. Ophthalmol. 2014, 1, 1023. [Google Scholar]
- Moore, P.; El-sherbeny, A.; Roon, P.; Schoenlein, P.V.; Ganapathy, V.; Smith, S.B. Apoptotic cell death in the mouse retinal ganglion cell layer is induced in vivo by the excitatory amino acid homocysteine. Exp. Eye Res. 2001, 73, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, P.S.; Moister, B.; Roon, P.; Mysona, B.A.; Duplantier, J.; Dun, Y.; Moister, T.K.; Farley, M.J.; Prasad, P.D.; Liu, K.; et al. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4460–4470. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Du, Y.; Miller, C.M.; Hatala, D.A.; Levin, L.A. Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am. J. Pathol. 2010, 176, 2550–2558. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef] [PubMed]
- Diederen, R.M.; La Heij, E.C.; Deutz, N.E.; Kijlstra, A.; Kessels, A.G.; Van Eijk, H.M.; Liem, A.T.; Dieudonne, S.; Hendrikse, F. Increased glutamate levels in the vitreous of patients with retinal detachment. Exp. Eye Res. 2006, 83, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, P.S.; White, R.E.; Ha, Y.; Bozard, B.R.; McNeil, P.L.; Caldwell, R.W.; Kumar, S.; Black, S.M.; Smith, S.B. The role of N-methyl-D-aspartate receptor activation in homocysteine-induced death of retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5515–5524. [Google Scholar] [CrossRef]
- Lipton, S.A.; Kim, W.K.; Choi, Y.B.; Kumar, S.; D′Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Jiang, C.; Xu, M.; Pang, Y.; Feng, J.; Xiang, X.; Kong, W.; Xu, G.; Li, Y.; et al. Hyperhomocysteinemia promotes insulin resistance by inducing endoplasmic reticulum stress in adipose tissue. J. Biol. Chem. 2013, 288, 9583–9592. [Google Scholar] [CrossRef]
- Lentz, S.R. Mechanisms of homocysteine-induced atherothrombosis. J. Thromb. Haemost. 2005, 3, 1646–1654. [Google Scholar] [CrossRef]
- Kuo, H.K.; Sorond, F.A.; Chen, J.H.; Hashmi, A.; Milberg, W.P.; Lipsitz, L.A. The role of homocysteine in multisystem age-related problems: A systematic review. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1190–1201. [Google Scholar] [CrossRef]
- Harker, L.A.; Slichter, S.J.; Scott, C.R.; Ross, R. Homocystinemia. Vascular injury and arterial thrombosis. N. Engl. J. Med. 1974, 291, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Lalla, E.; Lu, Y.; Gleason, M.R.; Wolf, B.M.; Tanji, N.; Ferran, L.J., Jr.; Kohl, B.; Rao, V.; Kisiel, W.; et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J. Clin. Invest. 2001, 107, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Biochemical evidence for a link between elevated levels of homocysteine and lipid peroxidation in vivo. Curr. Atheroscler. Rep. 1999, 1, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, P.; Kok, F.J.; Kruyssen, D.A.; Schouten, E.G.; Witteman, J.C.; Grobbee, D.E.; Ueland, P.M.; Refsum, H. Plasma total homocysteine, B vitamins, and risk of coronary atherosclerosis. Arter. Thromb. Vasc. Biol. 1997, 17, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Elmasry, K.; Mohamed, R.; Sharma, I.; Elsherbiny, N.M.; Liu, Y.; Al-Shabrawey, M.; Tawfik, A. Epigenetic modifications in hyperhomocysteinemia: Potential role in diabetic retinopathy and age-related macular degeneration. Oncotarget 2018, 9, 12562–12590. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Sharma, I.; Kira, D.; Alhusban, S.; Samra, Y.A.; Jadeja, R.; Martin, P.; Al-Shabrawey, M.; Tawfik, A. Homocysteine Induces Inflammation in Retina and Brain. Biomolecules 2020, 10, 393. [Google Scholar] [CrossRef]
- Oshitari, T.; Hata, N.; Yamamoto, S. Endoplasmic reticulum stress and diabetic retinopathy. Vasc. Health Risk Manag. 2008, 4, 115–122. [Google Scholar] [CrossRef]
- Yoshida, H. ER stress and diseases. FEBS J. 2007, 274, 630–658. [Google Scholar] [CrossRef]
- Ozcan, U.; Yilmaz, E.; Ozcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Gorgun, C.Z.; Hotamisligil, G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Yu, Q.; Wang, M.; Zhang, S.X. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009, 583, 1521–1527. [Google Scholar] [CrossRef]
- Adachi, T.; Teramachi, M.; Yasuda, H.; Kamiya, T.; Hara, H. Contribution of p38 MAPK, NF-kappaB and glucocorticoid signaling pathways to ER stress-induced increase in retinal endothelial permeability. Arch. Biochem. Biophys. 2012, 520, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Arnal, E.; Miranda, M.; Johnsen-Soriano, S.; Alvarez-Nolting, R.; Diaz-Llopis, M.; Araiz, J.; Cervera, E.; Bosch-Morell, F.; Romero, F.J. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr. Eye Res. 2009, 34, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, J.; Chen, Y.; Wang, J.J.; Ratan, R.; Zhang, S.X. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Muller cell-derived inflammatory cytokine production in diabetes. Diabetes 2012, 61, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Sturgill-Short, G.; Ganapathy, P.; Tawfik, A.; Peachey, N.S.; Smith, S.B. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp. Eye Res. 2012, 96, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Lu, S.C.; Lee, C.M.; Chen, Y.J.; Dugan, T.A.; Huang, W.H.; Chang, S.F.; Liao, W.S.; Chen, C.H.; Lee, Y.T. Homocysteine inhibits arterial endothelial cell growth through transcriptional downregulation of fibroblast growth factor-2 involving G protein and DNA methylation. Circ. Res. 2008, 102, 933–941. [Google Scholar] [CrossRef]
- Ingrosso, D.; Cimmino, A.; Perna, A.F.; Masella, L.; De Santo, N.G.; De Bonis, M.L.; Vacca, M.; D′Esposito, M.; D′Urso, M.; Galletti, P.; et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 2003, 361, 1693–1699. [Google Scholar] [CrossRef]
- Castro, R.; Rivera, I.; Struys, E.A.; Jansen, E.E.; Ravasco, P.; Camilo, M.E.; Blom, H.J.; Jakobs, C.; Tavares de Almeida, I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003, 49, 1292–1296. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, J.; Garg, G.; Kumar, A.; Patowary, A.; Karthikeyan, G.; Ramakrishnan, L.; Brahmachari, V.; Sengupta, S. Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol. 2008, 27, 357–365. [Google Scholar] [CrossRef]
- Kalani, A.; Kamat, P.K.; Tyagi, S.C.; Tyagi, N. Synergy of homocysteine, microRNA, and epigenetics: A novel therapeutic approach for stroke. Mol. Neurobiol. 2013, 48, 157–168. [Google Scholar] [CrossRef]
- Li, J.; McRoberts, J.A.; Ennes, H.S.; Trevisani, M.; Nicoletti, P.; Mittal, Y.; Mayer, E.A. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G219–G228. [Google Scholar] [CrossRef]
- Patton, A.J.; Genever, P.G.; Birch, M.A.; Suva, L.J.; Skerry, T.M. Expression of an N-methyl-D-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone 1998, 22, 645–649. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, G. Transcriptional regulation of PSA-NCAM expression by NMDA receptor activation in RA-differentiated C6 glioma cultures. Brain Res. Bull. 2009, 79, 157–168. [Google Scholar] [CrossRef]
- LeMaistre, J.L.; Sanders, S.A.; Stobart, M.J.; Lu, L.; Knox, J.D.; Anderson, H.D.; Anderson, C.M. Coactivation of NMDA receptors by glutamate and D-serine induces dilation of isolated middle cerebral arteries. J. Cereb. Blood Flow Metab. 2012, 32, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Reijerkerk, A.; Kooij, G.; Van der Pol, S.M.; Leyen, T.; Lakeman, K.; Van Het Hof, B.; Vivien, D.; De Vries, H.E. The NR1 subunit of NMDA receptor regulates monocyte transmigration through the brain endothelial cell barrier. J. Neurochem. 2010, 113, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Krizbai, I.A.; Deli, M.A.; Pestenacz, A.; Siklos, L.; Szabo, C.A.; Andras, I.; Joo, F. Expression of glutamate receptors on cultured cerebral endothelial cells. J. Neurosci. Res. 1998, 54, 814–819. [Google Scholar] [CrossRef]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Sharp, C.D.; Hines, I.; Houghton, J.; Warren, A.; Jackson, T.H.t.; Jawahar, A.; Nanda, A.; Elrod, J.W.; Long, A.; Chi, A.; et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2592–H2598. [Google Scholar] [CrossRef]
- Liu, X.; Hunter, C.; Weiss, H.R.; Chi, O.Z. Effects of blockade of ionotropic glutamate receptors on blood-brain barrier disruption in focal cerebral ischemia. Neurol. Sci. 2010, 31, 699–703. [Google Scholar] [CrossRef]
- Paul, C.; Bolton, C. Modulation of blood-brain barrier dysfunction and neurological deficits during acute experimental allergic encephalomyelitis by the N-methyl-D-aspartate receptor antagonist memantine. J. Pharmacol. Exp. Ther. 2002, 302, 50–57. [Google Scholar] [CrossRef]
- Gunasekar, P.G.; Kanthasamy, A.G.; Borowitz, J.L.; Isom, G.E. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: Implication for cell death. J. Neurochem. 1995, 65, 2016–2021. [Google Scholar] [CrossRef]
- McCully, K.S. Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation. Ann. Clin. Lab. Sci. 2009, 39, 219–232. [Google Scholar]
- Domagala, T.B.; Undas, A.; Libura, M.; Szczeklik, A. Pathogenesis of vascular disease in hyperhomocysteinaemia. J. Cardiovasc. Risk 1998, 5, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, M.; Furusawa, K.; Kamijo, M.; Kimura, T.; Matsunaga, M.; Baba, M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Brain Res. Mol. Brain Res. 2005, 136, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Mahta, A.; Mallack, E.; Luo, J.J. Hyperhomocysteinemia and neurologic disorders: A review. J. Clin. Neurol. 2014, 10, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Lee, Y.S.; Bae, H.J.; Kang, D.W. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke 2014, 45, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Hewedi, D.H.; Eissa, A.M.; Myers, C.E.; Sadek, H.A. The relationship between associative learning, transfer generalization, and homocysteine levels in mild cognitive impairment. PLoS ONE 2012, 7, e46496. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; De Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Bialecka, M.; Robowski, P.; Honczarenko, K.; Roszmann, A.; Slawek, J. Genetic and environmental factors for hyperhomocysteinaemia and its clinical implications in Parkinson′s disease. Neurol. Neurochir. Pol. 2009, 43, 272–285. [Google Scholar]
- Licking, N.; Murchison, C.; Cholerton, B.; Zabetian, C.P.; Hu, S.C.; Montine, T.J.; Peterson-Hiller, A.L.; Chung, K.A.; Edwards, K.; Leverenz, J.B.; et al. Homocysteine and cognitive function in Parkinson′s disease. Parkinsonism Relat. Disord. 2017, 44, 1–5. [Google Scholar] [CrossRef]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Beard, R.S., Jr.; Bearden, S.E. Vascular complications of cystathionine beta-synthase deficiency: Future directions for homocysteine-to-hydrogen sulfide research. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H13–H26. [Google Scholar] [CrossRef] [PubMed]
- Dayal, S.; Lentz, S.R. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arter. Thromb. Vasc. Biol. 2008, 28, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Doubal, F.; Armitage, P.; Chappell, F.; Carpenter, T.; Munoz Maniega, S.; Farrall, A.; Sudlow, C.; Dennis, M.; Dhillon, B. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann. Neurol. 2009, 65, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Topakian, R.; Barrick, T.R.; Howe, F.A.; Markus, H.S. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J. Neurol. Neurosurg. Psychiatry 2010, 81, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta 2009, 1788, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Andras, I.E.; Deli, M.A.; Veszelka, S.; Hayashi, K.; Hennig, B.; Toborek, M. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J. Cereb. Blood Flow Metab. 2007, 27, 1431–1443. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawfik, A.; Samra, Y.A.; Elsherbiny, N.M.; Al-Shabrawey, M. Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction. Biomolecules 2020, 10, 1119. https://doi.org/10.3390/biom10081119
Tawfik A, Samra YA, Elsherbiny NM, Al-Shabrawey M. Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction. Biomolecules. 2020; 10(8):1119. https://doi.org/10.3390/biom10081119
Chicago/Turabian StyleTawfik, Amany, Yara A. Samra, Nehal M. Elsherbiny, and Mohamed Al-Shabrawey. 2020. "Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction" Biomolecules 10, no. 8: 1119. https://doi.org/10.3390/biom10081119
APA StyleTawfik, A., Samra, Y. A., Elsherbiny, N. M., & Al-Shabrawey, M. (2020). Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction. Biomolecules, 10(8), 1119. https://doi.org/10.3390/biom10081119







