Role of Antioxidants in Alleviating Bisphenol A Toxicity
Abstract
:1. Introduction
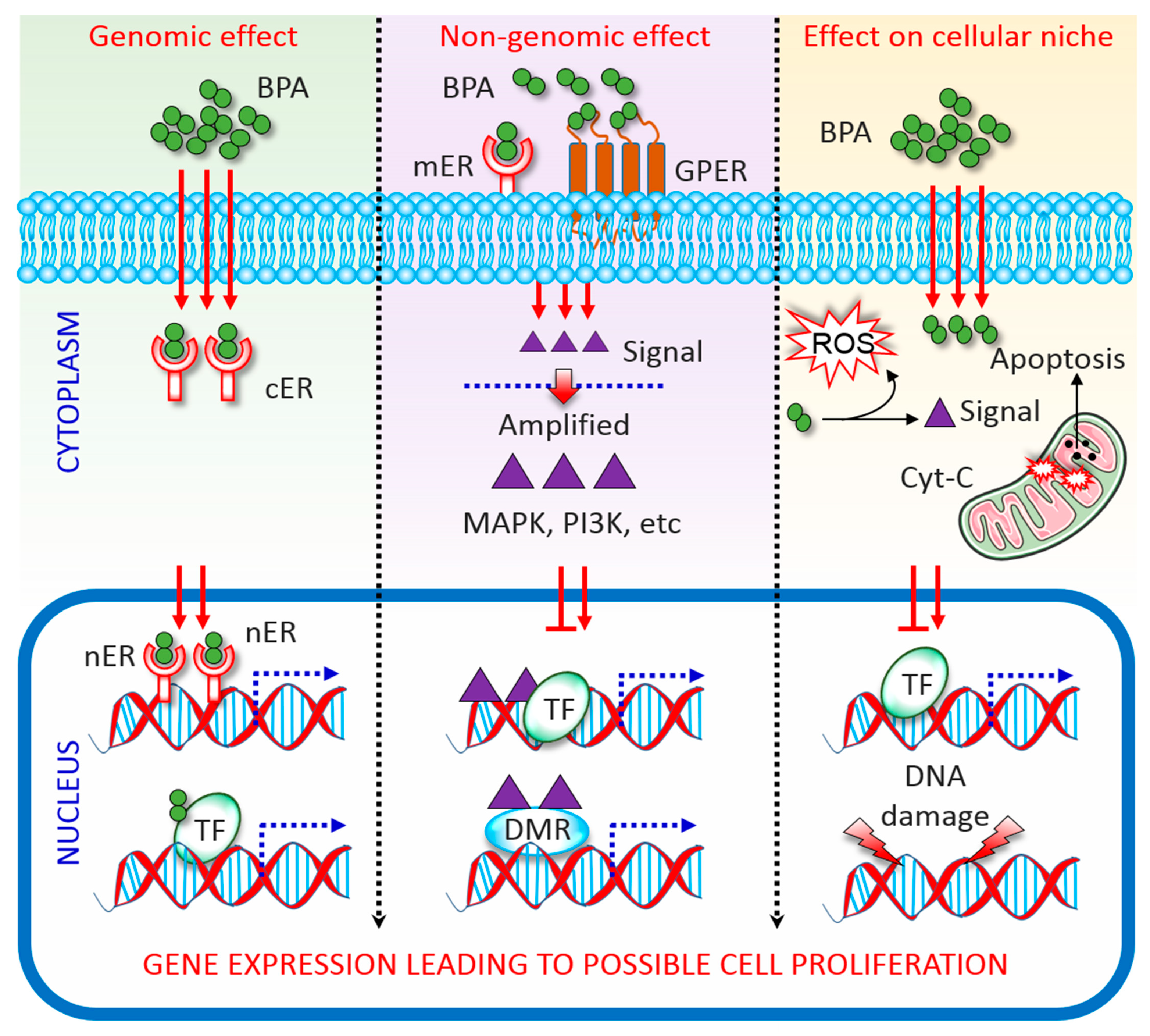
2. Overview of BPA Activity
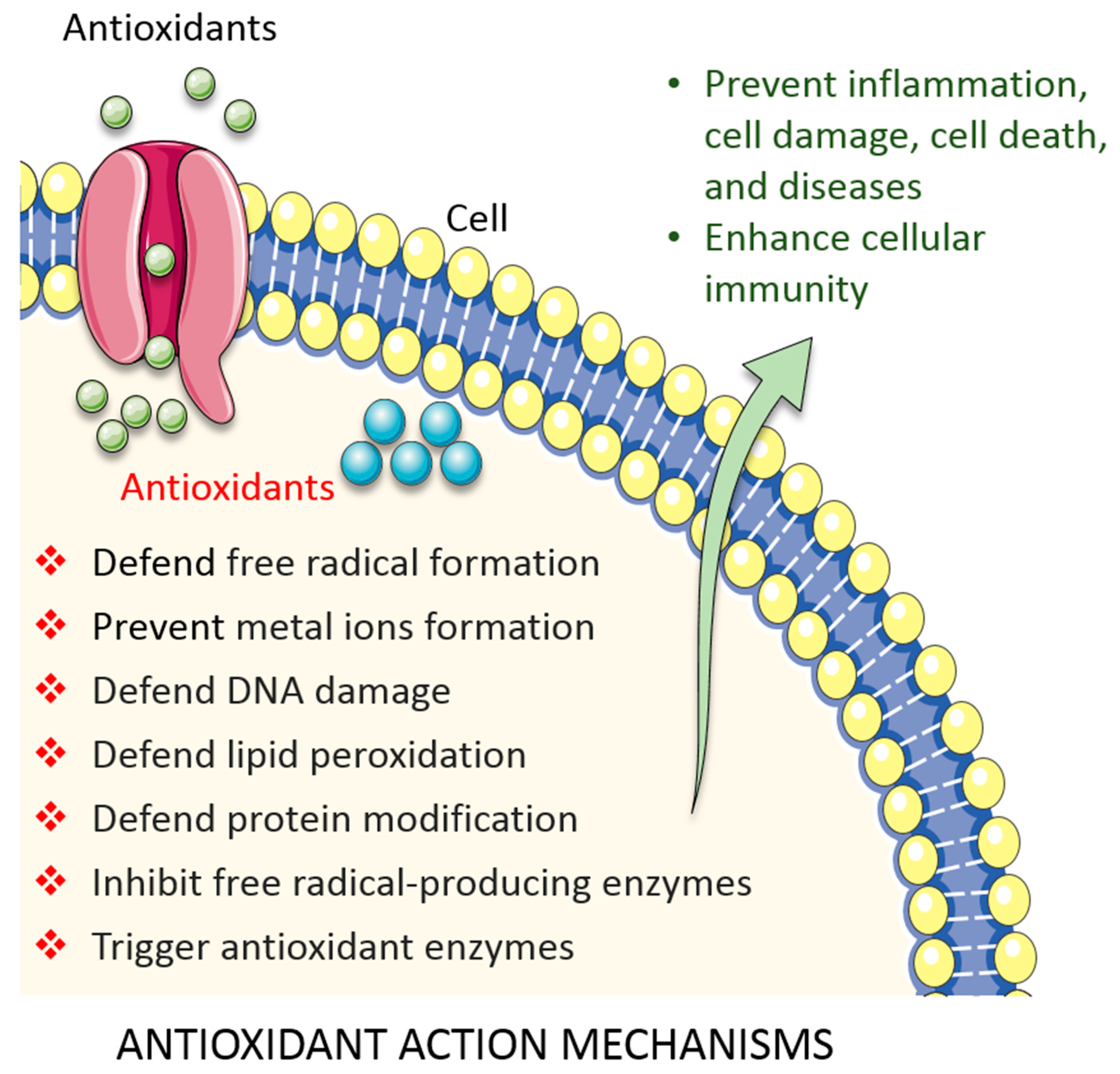
3. Overview of Antioxidant Activity
4. Role of Antioxidants in Overcoming BPA Toxicity
4.1. Enzymatic Antioxidants
4.1.1. SOD
4.1.2. CAT
4.1.3. GSH System
4.1.4. Uric Acid
4.2. Non-Enzymatic Antioxidants
4.2.1. Vitamin C
4.2.2. Vitamin E
4.2.3. Vitamin A
4.2.4. Melatonin
4.2.5. Quercetin (Flavonoid)
4.2.6. Lycopene (Carotenoid)
4.2.7. Synthetic Antioxidants
4.2.8. Other Natural Antioxidants
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2008, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Kwon, W.-S.; Lee, J.-S.; Yoon, S.-J.; Ryu, B.-Y.; Pang, M.-G. Bisphenol-A Affects Male Fertility via Fertility-related Proteins in Spermatozoa. Sci. Rep. 2015, 5, 9169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.; Kwon, W.-S.; Yoon, S.-J.; Park, Y.-J.; Ryu, B.-Y.; Pang, M.-G. A novel approach to assessing bisphenol-A hazards using an in vitro model system. BMC Genom. 2016, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Pang, W.K.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Multigenerational and transgenerational impact of paternal bisphenol A exposure on male fertility in a mouse model. Hum. Reprod. 2020, deaa13. [Google Scholar] [CrossRef]
- Rahman, S.; Kwon, W.-S.; Karmakar, P.C.; Yoon, S.-J.; Ryu, B.-Y.; Pang, M.-G. Gestational Exposure to Bisphenol A Affects the Function and Proteome Profile of F1 Spermatozoa in Adult Mice. Environ. Health Perspect. 2016, 125, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Kwon, W.-S.; Ryu, D.-Y.; Khatun, A.; Karmakar, P.C.; Ryu, B.-Y.; Pang, M.-G. Functional and Proteomic Alterations of F1 Capacitated Spermatozoa of Adult Mice Following Gestational Exposure to Bisphenol A. J. Proteome Res. 2017, 17, 524–535. [Google Scholar] [CrossRef]
- Reddivari, L.; Veeramachaneni, D.N.R.; Walters, W.A.; Lozupone, C.; Palmer, J.; Hewage, M.K.K.; Bhatnagar, R.; Amir, A.; Kennett, M.J.; Knight, R.; et al. Perinatal Bisphenol A Exposure Induces Chronic Inflammation in Rabbit Offspring via Modulation of Gut Bacteria and Their Metabolites. mSystems 2017, 2, e00093-17. [Google Scholar] [CrossRef] [Green Version]
- Lemmen, J.G.; Arends, R.J.; Van Boxtel, A.L.; Van Der Saag, P.T.; Van Der Burg, B. Tissue- and time-dependent estrogen receptor activation in estrogen reporter mice. J. Mol. Endocrinol. 2004, 32, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Saltzman, W.; Ziegler, T.E. Functional Significance of Hormonal Changes in Mammalian Fathers. J. Neuroendocr. 2014, 26, 685–696. [Google Scholar] [CrossRef] [Green Version]
- Erler, C.; Novak, J. Bisphenol A Exposure: Human Risk and Health Policy. J. Pediatr. Nurs. 2010, 25, 400–407. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Paterson, M.; Fisher, H.; Buckingham, D.W.; Van Duin, M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell Sci. 1995, 108, 2017–2025. [Google Scholar] [PubMed]
- Rahman, S.; Kang, K.-H.; Arifuzzaman, S.; Pang, W.-K.; Ryu, D.-Y.; Song, W.-H.; Park, Y.-J.; Pang, M.-G. Effect of antioxidants on BPA-induced stress on sperm function in a mouse model. Sci. Rep. 2019, 9, 10584-10. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Prete, C.; Tafuri, S.; Ciani, F.; Pasolini, M.P.; Ciotola, F.; Albarella, S.; Carotenuto, D.; Peretti, V.; Cocchia, N. Influences of dietary supplementation withLepidium meyenii(Maca) on stallion sperm production and on preservation of sperm quality during storage at 5 °C. Andrology 2018, 6, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Losano, J.D.D.A.; Angrimani, D.S.R.; Rui, B.; Bicudo, L.C.; Dalmazzo, A.; Silva, B.C.; Viana, C.H.; Mendes, C.M.; Assumpcao, M.E.; Barnabe, V.H.; et al. The addition of docosahexaenoic acid (DHA) and antioxidants (glutathione peroxidase and superoxide dismutase) in extenders to epididymal sperm cryopreservation in bulls. Zygote 2018, 26, 199–206. [Google Scholar] [CrossRef]
- Babu, S.; Uppu, S.; Claville, M.O.; Uppu, R.M. Prooxidant actions of bisphenol A (BPA) phenoxyl radicals: Implications to BPA-related oxidative stress and toxicity. Toxicol. Mech. Methods 2013, 23, 273–280. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxidative Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative Stress and BPA Toxicity: An Antioxidant Approach for Male and Female Reproductive Dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.B.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen Receptors: How Do They Signal and What Are Their Targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [Green Version]
- Nadal, A.; Ropero, A.B.; Laribi, O.; Maillet, M.; Fuentes, E.; Soria, B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc. Natl. Acad. Sci. USA 2000, 97, 11603–11608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishunina, T.; Kruijver, F.P.; Balesar, R.; Swaab, D.F. Differential Expression of Estrogen Receptor ? and ? Immunoreactivity in the Human Supraoptic Nucleus in Relation to Sex and Aging 1. J. Clin. Endocrinol. Metab. 2000, 85, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Susiarjo, M.; Bartolomei, M.S. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin. Cell Dev. Biol. 2015, 43, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhao, Z.; Ji, W. Bisphenol A induces apoptosis, oxidative stress and inflammatory response in colon and liver of mice in a mitochondria-dependent manner. Biomed. Pharmacother. 2019, 117, 109182. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Abnous, K.; Hassani, F.V.; Hosseinzadeh, H.; Birner-Gruenberger, R.; Mehri, S. Alteration of protein profile in cerebral cortex of rats exposed to bisphenol A: A proteomics study. NeuroToxicology 2020, 78, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Z.; Shi, Q.-M.; Ge, X.; Wang, H.-X.; Li, M.-X.; Chen, G.; Wang, Q.; Ju, Q.; Zhang, J.-P.; et al. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology 2017, 387, 10–16. [Google Scholar] [CrossRef]
- Koruk, M.; Taysı, S.; Savas, M.C.; Yilmaz, O.; Akçay, F.; Karakök, M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann. Clin. Lab. Sci. 2004, 34, 57–62. [Google Scholar]
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.-D.; Sindhu, R.K.; Pang, J.; Ehdaie, A.; Vaziri, N.D. Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: Effect of antioxidant-rich diet. J. Hypertens 2004, 22, 2025–2033. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Moussa, Z.; Judeh, Z.M.; Ahmed, S.A. Nonenzymatic Exogenous and Endogenous Antioxidants. In Free Radical Medicine and Biology [Working Title]; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Steckiewicz, K.P.; Zwara, J.; Jaskiewicz, M.; Kowalski, S.; Kamysz, W.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Shape-Depended Biological Properties of Ag3PO4 Microparticles: Evaluation of Antimicrobial Properties and Cytotoxicity in In Vitro Model—Safety Assessment of Potential Clinical Usage. Oxidative Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Wuerges, J.; Lee, J.-W.; Yim, Y.-I.; Yim, H.-S.; Kang, S.-O.; Carugo, K.D. Crystal structure of nickel-containing superoxide dismutase reveals another type of active site. Proc. Natl. Acad. Sci. USA 2004, 101, 8569–8574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanth, G.K.; M, D.L.; Sadasivan, C. Bisphenol-A Can Inhibit the Enzymatic Activity of Human Superoxide Dismutase. Hum. Ecol. Risk Assessment Int. J. 2012, 19, 268–277. [Google Scholar] [CrossRef]
- Parihar, M.; Javeri, T.; Hemnani, T.; Dubey, A.; Prakash, P. Responses of superoxide dismutase, glutathione peroxidase and reduced glutathione antioxidant defenses in gills of the freshwater catfish (Heteropneustes fossilis) to short-term elevated temperature. J. Therm. Biol. 1997, 22, 151–156. [Google Scholar] [CrossRef]
- Karmakar, P.C.; Kang, H.-G.; Kim, Y.-H.; Jung, S.-E.; Rahman, S.; Lee, H.-S.; Kim, Y.-H.; Pang, M.-G.; Ryu, B.-Y. Bisphenol A Affects on the Functional Properties and Proteome of Testicular Germ Cells and Spermatogonial Stem Cells in vitro Culture Model. Sci. Rep. 2017, 7, 11858. [Google Scholar] [CrossRef]
- Moghaddam, H.S.; Samarghandian, S.; Farkhondeh, T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol. Mech. Methods 2015, 25, 507–513. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Vieira, E.; Soriano, S.; Menes, L.; Burks, D.; Quesada, I.; Nadal, A. Bisphenol A Exposure during Pregnancy Disrupts Glucose Homeostasis in Mothers and Adult Male Offspring. Environ. Health Perspect. 2010, 118, 1243–1250. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; Quesada, I.; Nadal, A. Bisphenol-A: A new diabetogenic factor? Hormones 2010, 9, 118–126. [Google Scholar] [CrossRef]
- Berger, A.; Ziv-Gal, A.; Cudiamat, J.; Wang, W.; Zhou, C.; Flaws, J.A. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod. Toxicol. 2016, 60, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Wang, W.; Peretz, J.; A Flaws, J. Bisphenol A exposure inhibits germ cell nest breakdown by reducing apoptosis in cultured neonatal mouse ovaries. Reprod. Toxicol. 2015, 57, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaydın, T.; Oznurlu, Y.; Sur, E.; Celik, I.; Uluısık, D.; Dayan, M.; Uluisik, D. Effects of bisphenol A on antioxidant system and lipid profile in rats. Biotech. Histochem. 2018, 93, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Kourouma, A.; Quan, C.; Duan, P.; Qi, S.; Yu, T.; Wang, Y.; Yang, K. Bisphenol A Induces Apoptosis in Liver Cells through Induction of ROS. Adv. Toxicol. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kamel, A.H.; Foaud, M.A.; Moussa, H.M. The adverse effects of bisphenol A on male albino rats. J. Basic Appl. Zool. 2018, 79, 6. [Google Scholar] [CrossRef] [Green Version]
- Kabuto, H.; Hasuike, S.; Minagawa, N.; Shishibori, T. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ. Res. 2003, 93, 31–35. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elobeid, M.A.; Virk, P.; Omer, S.A.; Elamin, M.; Daghestani, M.H.; AlOlayan, E.M. Bisphenol A Induces Hepatotoxicity through Oxidative Stress in Rat Model. Oxidative Med. Cell. Longev. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Hu, J.; Li, J.; Yang, Y.; Zhang, L.; Zou, L.; Gao, R.; Peng, C.; Wang, Y.; Luo, T.; et al. Bisphenol A promotes hyperuricemia via activating xanthine oxidase. FASEB J. 2018, 32, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Mourad, I.M.; Khadrawy, Y.A. The sensitivity of liver, kidney and testis of rats to oxidative stress induced by different doses of Bisphenol A. Int. J. Life Sci. Pharma Res. 2012, 2, 19–28. [Google Scholar]
- Chitra, K.C.; Rao, K.R.; Mathur, P.P. Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: A histological and biochemical study. Asian J. Androl. 2003, 5, 203–208. [Google Scholar]
- Mehranjani, M.S.; Mansoori, T. Department of Biology, Faculty of Science, Arak University, Arak, Iran Stereological study on the effect of vitamin C in preventing the adverse effects of bisphenol A on rat ovary. Int. J. Reprod. Biomed. 2016, 14, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Haroun, M.R.; Zamzam, I.S.; Metwally, E.S.; El-Shafey, R.S. Effect of vitamin c on bisphenol a induced hepato& nephrotoxicity in albino rats. Egypt. J. Forensic Sci. Appl. Toxicol. 2019, 16, 57–85. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Gupta, P. Alteration in apoptotic rate of testicular cells and sperms following administration of Bisphenol A (BPA) in Wistar albino rats. Environ. Sci. Pollut. Res. 2018, 25, 21635–21643. [Google Scholar] [CrossRef]
- Nimisha, B.; Sendhilvadivu, M. Vitamin E Modulates the Oxidant-Antioxidant Imbalance of BPA induced Oxidative Stress in Albino Rats. Int. J. Sci. Res. 2018, 7, 900–906. [Google Scholar]
- Helal, E.G.E.; Taha, N.M.; Mohamed, A.M.; Abu-Taleb, H.M. Ameliorative Effect of Vitamin E on Oxidative Stress Induced by Bisphenol A in Female Albino Rats. Egypt. J. Hosp. Med. 2016, 65, 474–478. [Google Scholar] [CrossRef]
- Shmarakov, I.; Borschovetska, V.L.; Blaner, W.S. Hepatic Detoxification of Bisphenol A is Retinoid-Dependent. Toxicol. Sci. 2017, 157, 141–155. [Google Scholar] [CrossRef]
- Aikawa, H.; Koyama, S.; Matsuda, M.; Nakahashi, K.; Akazome, Y.; Mori, T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004, 315, 119–124. [Google Scholar] [CrossRef]
- Koda, T.; Morita, M.; Imai, H. Retinoic acid inhibits uterotrophic activity of bisphenol A in adult ovariectomized rats. J. Nutr. Sci. Vitaminol. 2007, 53, 432–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.; Rahman, S.; Kaur, M.; Ahmad, F.; Rashid, H.; Ansari, R.A.; Raisuddin, S. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem. Toxicol. 2011, 49, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Edrees, G.M.; El-Missiry, M.A.; A Ali, D.; Abouel-Nour, M.; Dabdoub, B.R. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health 2016, 32, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-J.; Liu, C.; Duan, W.-X.; Xu, S.-C.; He, M.-D.; Chen, C.-H.; Wang, Y.; Zhou, Z.; Yu, Z.-P.; Zhang, L.; et al. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 752, 57–67. [Google Scholar] [CrossRef]
- Jahan, S.; Ain, Q.U.; Ullah, H. Therapeutic effects of quercetin against bisphenol A induced testicular damage in male Sprague Dawley rats. Syst. Biol. Reprod. Med. 2016, 62, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elwakeel, S.H.; El-Monem, D.D.A. Ameliorative effect of Melatonin and Quercetin against Bisphenol A induced reproductive toxicity in male albino mice. Cienc. Tec. Vitivinic. 2018, 33, 31–64. [Google Scholar]
- Elgawish, R.A.; El-Beltagy, M.A.; El-Sayed, R.M.; Gaber, A.A.; Abdelrazek, H.M.A. Protective role of lycopene against metabolic disorders induced by chronic bisphenol A exposure in rats. Environ. Sci. Pollut. Res. 2020, 27, 9192–9201. [Google Scholar] [CrossRef] [PubMed]
- El Morsy, E.M.; Ahmed, M. Protective effects of lycopene on hippocampal neurotoxicity and memory impairment induced by bisphenol A in rats. Hum. Exp. Toxicol. 2020, 39, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, R.; Gong, X.; Shi, W.; Zhong, X. Lycopene reduces in utero bisphenol A exposure-induced mortality, benefits hormones, and development of reproductive organs in offspring mice. Environ. Sci. Pollut. Res. 2018, 25, 24041–24051. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, G.; Sadwal, S.; Aniqa, A. Alleviating impact of hydroethanolic Murraya koenigii leaves extract on bisphenol A instigated testicular lethality and apoptosis in mice. Andrologia 2020, 52, e13504. [Google Scholar] [CrossRef]
- Olukole, S.G.; Ola-Davies, E.O.; Lanipekun, D.O.; Oke, B.O. Chronic exposure of adult male Wistar rats to bisphenol A causes testicular oxidative stress: Role of gallic acid. Endocr. Regul. 2020, 54, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Saadeldin, I.M.; Hussein, M.M.A.; Suleiman, A.H.; Abohassan, M.G.; Ahmed, M.M.; Moustafa, A.A.; Moumen, A.F.; Swelum, S.A. Ameliorative effect of ginseng extract on phthalate and bisphenol A reprotoxicity during pregnancy in rats. Environ. Sci. Pollut. Res. 2018, 25, 21205–21215. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Kassim, N.M.; Othman, S. Tualang Honey Protects against BPA-Induced Morphological Abnormalities and Disruption of ERα, ERβ, and C3 mRNA and Protein Expressions in the Uterus of Rats. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Othman, S.; Kassim, N.M. Potential protective effect of Tualang honey on BPA-induced ovarian toxicity in prepubertal rat. BMC Complement. Altern. Med. 2014, 14, 509. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Khan, M.J. Bisphenol A analogues bisphenol B, bisphenol F, and bisphenol S induce oxidative stress, disrupt daily sperm production, and damage DNA in rat spermatozoa: A comparative in vitro and in vivo study. Toxicol. Ind. Health 2019, 35, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michałowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. Vitr. 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Sangai, N.P.; Patel, C.; Pandya, H.A. Ameliorative effects of quercetin against bisphenol A-caused oxidative stress in human erythrocytes: An in vitro and in silico study. Toxicol. Res. 2018, 7, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hao, J.; Hu, J.; Pu, J.; Lü, Z.; Zhao, L.; Wang, Q.; Yu, Q.; Wang, Y.; Li, G. Protective effects of ginsenosides against Bisphenol A-induced cytotoxicity in 15P-1 Sertoli cells via extracellular signal-regulated kinase 1/2 signalling and antioxidant mechanisms. Basic Clin. Pharmacol. Toxicol. 2012, 111, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.; Kang, J.-S.; Kim, K.M. Cultivated wild ginseng extracts upregulate the anti-apoptosis systems in cells and mice induced by bisphenol A. Mol. Cell. Toxicol. 2017, 13, 73–82. [Google Scholar] [CrossRef]
- Djelic, N.; Anderson, D. The effect of the antioxidant catalase on oestrogens, triiodothyronine, and noradrenaline in the Comet assay. Teratog. Carcinog. Mutagen. 2003, 23, 69–81. [Google Scholar] [CrossRef]
- Banerjee, O.; Singh, S.; Prasad, S.K.; Bhattacharjee, A.; Banerjee, A.; Banerjee, A.; Saha, A.; Maji, B.K.; Mukherjee, S. Inhibition of catalase activity with 3-amino-1,2,4-triazole intensifies bisphenol A (BPA)-induced toxicity in granulosa cells of female albino rats. Toxicol. Ind. Health 2018, 34, 787–797. [Google Scholar] [CrossRef]
- Morris, G.; Anderson, G.; Dean, O.; Berk, M.; Galecki, P.; Martin-Subero, M.; Maes, M. The Glutathione System: A New Drug Target in Neuroimmune Disorders. Mol. Neurobiol. 2014, 50, 1059–1084. [Google Scholar] [CrossRef]
- Meister, A.; E Anderson, M. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef]
- A El-Missiry, M. Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 124, 233–237. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Spitsin, S.; Scott, G.S.; Mikheeva, T.; Zborek, A.; Kean, R.B.; Brimer, C.M.; Koprowski, H.; Hooper, D.C. Comparison of uric acid and ascorbic acid in protection against EAE. Free Radic. Biol. Med. 2002, 33, 1363–1371. [Google Scholar] [CrossRef]
- Amaro, S.; Soy, D.; Obach, V.; Cervera, A.; Planas, A.M.; Chamorro, A. A Pilot Study of Dual Treatment with Recombinant Tissue Plasminogen Activator and Uric Acid in Acute Ischemic Stroke. Stroke 2007, 38, 2173–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culleton, B.F.; Larson, M.G.; Kannel, W.B.; Levy, D. Serum uric acid and risk for cardiovascular disease and death: The Framingham Heart Study. Ann. Intern. Med. 1999, 131, 7–13. [Google Scholar] [CrossRef]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey (NHANES). JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef] [Green Version]
- Goschorska, M.; Gutowska, I.; Baranowska-Bosiacka, I.; Barczak, K.; Chlubek, D. The Use of Antioxidants in the Treatment of Migraine. Antioxidants 2020, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Frei, B. Ascorbic acid protects lipids in human plasma and low-density lipoprotein against oxidative damage. Am. J. Clin. Nutr. 1991, 54 (Suppl. 6), 1113S–1118S. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Boersma, M.G.; De Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.; Van Zanden, J.J.; Van Der Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Aydoğan, M.; Korkmaz, A.; Barlas, N.; Kolankaya, D. Pro-oxidant effect of vitamin C coadministration with bisphenol A, nonylphenol, and octylphenol on the reproductive tract of male rats. Drug Chem. Toxicol. 2009, 33, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Aydoğan, M.; Korkmaz, A.; Barlas, N.; Kolankaya, D. The effect of vitamin C on bisphenol A, nonylphenol and octylphenol induced brain damages of male rats. Toxicology 2008, 249, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bindhumol, V.; Chitra, K.; Mathur, P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 2003, 188, 117–124. [Google Scholar] [CrossRef]
- Korkmaz, A.; Ahbab, M.A.; Kolankaya, D.; Barlas, N. Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 2010, 48, 2865–2871. [Google Scholar] [CrossRef]
- Karageorgos, N.; Patsoukis, N.; Chroni, E.; Konstantinou, D.; Assimakopoulos, S.F.; Georgiou, C. Effect of N-acetylcysteine, allopurinol and vitamin E on jaundice-induced brain oxidative stress in rats. Brain Res. 2006, 1111, 203–212. [Google Scholar] [CrossRef]
- Gartner, L.P.; Hiatt, J.L. Color Textbook of Histology e-Book, 3rd ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2006; pp. 303–326. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahsan, H.; Mukhtar, H.; Ahmad, N. Combination of vitamin E and selenium causes an induction of apoptosis of human prostate cancer cells by enhancing Bax/Bcl-2 ratio. Prostate 2008, 68, 1624–1634. [Google Scholar] [CrossRef] [Green Version]
- Theriault, A.; Chao, J.-T.; Wang, Q.; Gapor, A.; Adeli, K. Tocotrienol: A review of its therapeutic potential. Clin. Biochem. 1999, 32, 309–319. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Favier, A. Oxidative stress: Conceptual and experimental interest in comprehension mechanism of diseases and therapeutic potentiel. Biochim. Mech. L’Act. Chim. 2003, 5, 108–115. [Google Scholar]
- Amraoui, W.; Adjabi, N.; Bououza, F.; Boumendjel, M.; Taibi, F.; Boumendjel, A.; Abdennour, C.; Messarah, M. Modulatory Role of Selenium and Vitamin E, Natural Antioxidants, against Bisphenol A-Induced Oxidative Stress in Wistar Albinos Rats. Toxicol. Res. 2018, 34, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci, B.; Bahadir, A.; Tuncel, O.K.; Bilgici, B. Influence of α-tocopherol and α-lipoic acid on bisphenol-A-induced oxidative damage in liver and ovarian tissue of rats. Toxicol. Ind. Health 2016, 32, 1381–1390. [Google Scholar] [CrossRef]
- Bozkurt, A.K. Alpha-tocopherol (Vitamin E) and iloprost attenuate reperfusion injury in skeletal muscle ischemia/reperfusion injury. J. Cardiovasc. Surg. 2002, 43, 693–696. [Google Scholar]
- Omran, B.; Abdallah, E.; Abdelwahab, M. Study of Probable Toxic Effects of Bisphenol A & the Protective Role of Vitamin E on Testes and Prostate of Adult Male Albino Rats. Ain Shams J. Forensic Med. Clin. Toxicol. 2017, 29, 7–18. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, Y.; Zhong, Y.; Gao, X.; Tan, T. Effect of vitamin E on reproductive functions and anti-oxidant activity of adolescent male mice exposed to bisphenol A. Wei Sheng Yan Jiu 2013, 42, 18–22. [Google Scholar]
- Yoganathan, T.; Eskild, W.; Hansson, V. Investigation of detoxification capacity of rat testicular germ cells and sertoli cells. Free Radic. Biol. Med. 1989, 7, 355–359. [Google Scholar] [CrossRef]
- Gavazza, M.; Català, A. The effect of α-tocopherol on lipid peroxidation of microsomes and mitochondria from rat testis. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 247–254. [Google Scholar] [CrossRef]
- Malivindi, R.; Rago, V.; De Rose, D.; Gervasi, M.C.; Cione, E.; Russo, G.; Santoro, M.; Aquila, S. Influence of all-trans retinoic acid on sperm metabolism and oxidative stress: Its involvement in the physiopathology of varicocele-associated male infertility. J. Cell. Physiol. 2018, 233, 9526–9537. [Google Scholar] [CrossRef]
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic. Biol. Med. 1999, 26, 746–761. [Google Scholar] [CrossRef]
- Niwa, T.; Fujimoto, M.; Kishimoto, K.; Yabusaki, Y.; Ishibashi, F.; Katagiri, M. Metabolism and interaction of bisphenol A in human hepatic cytochrome P450 and steroidogenic CYP17. Biol. Pharm. Bull. 2001, 24, 1064–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Tezuka, Y.; Ushiyama, A.; Kawashima, C.; Kitagawara, Y.; Takahashi, K.; Ohta, S.; Mashino, T. Ipso substitution of bisphenol A catalyzed by microsomal cytochrome P450 and enhancement of estrogenic activity. Toxicol. Lett. 2011, 203, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Kotnik, P.; Trontelj, J.; Knez, Ž.; Mašič, L.P. Bioactivation of bisphenol A and its analogs (BPF, BPAF, BPZ and DMBPA) in human liver microsomes. Toxicol. Vitr. 2013, 27, 1267–1276. [Google Scholar] [CrossRef]
- Guerrero, J.; Reiter, R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Reiter, R.J. Pharmacological actions of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2002, 2, 153–165. [Google Scholar] [CrossRef]
- Blask, D.; Sauer, L.; Dauchy, R. Melatonin as a Chronobiotic / Anticancer Agent: Cellular, Biochemical, and Molecular Mechanisms of Action and their Implications for Circadian-Based Cancer Therapy. Curr. Top. Med. Chem. 2002, 2, 113–132. [Google Scholar] [CrossRef]
- Onaolapo, O.; Onaolapo, A.Y. Melatonin, adolescence, and the brain: An insight into the period-specific influences of a multifunctional signaling molecule. Birth Defects Res. 2017, 109, 1659–1671. [Google Scholar] [CrossRef]
- Kratz, E.M.; Piwowar, A. Melatonin, advanced oxidation protein products and total antioxidant capacity as seminal parameters of prooxidant-antioxidant balance and their connection with expression of metalloproteinases in context of male fertility. J. Physiol. Pharmacol. 2017, 68, 659–668. [Google Scholar]
- Swarnakar, S.; Paul, S.; Singh, L.P.; Reiter, R.J. Matrix metalloproteinases in health and disease: Regulation by melatonin. J. Pineal Res. 2010, 50, 8–20. [Google Scholar] [CrossRef]
- Loren, P.; Sánchez, R.; Arias, M.-E.; Felmer, R.; Risopatron, J.; Cheuquemán, C. Melatonin Scavenger Properties against Oxidative and Nitrosative Stress: Impact on Gamete Handling and In Vitro Embryo Production in Humans and Other Mammals. Int. J. Mol. Sci. 2017, 18, 1119. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.; González, B.; Sempere, V.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin Reduces Oxidative Stress Damage Induced by Hydrogen Peroxide in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1066. [Google Scholar] [CrossRef]
- Bisquert, R.; Muñiz-Calvo, S.; Guillamón, J.M. Protective Role of Intracellular Melatonin Against Oxidative Stress and UV Radiation in Saccharomyces cerevisiae. Front. Microbiol. 2018, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Beigh, S.; Chaudhari, B.P.; Sharma, S.; Abdi, S.A.H.; Ahmad, S.; Ahmad, F.; Parvez, S.; Raisuddin, S. Mitochondrial dysfunction induced by Bisphenol A is a factor of its hepatotoxicity in rats. Environ. Toxicol. 2015, 31, 1922–1934. [Google Scholar] [CrossRef]
- Martín, M.; Macías, M.; Escames, G.; León, J.; Acuña-Castroviejo, D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000, 14, 1677–1679. [Google Scholar] [CrossRef]
- Quiroz, Y.; Ferrebuz, A.; Romero, F.; Vaziri, N.D.; Rodriguez-Iturbe, B. Melatonin ameliorates oxidative stress, inflammation, proteinuria, and progression of renal damage in rats with renal mass reduction. Am. J. Physiol. Physiol. 2008, 294, F336–F344. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef] [Green Version]
- Dizakar, S.; Özen, A.; Erdoğan, D.; Peker, T.; Akçay, N.C.; Türkoğlu, I.; Eşmekaya, M.A.; Ömeroğlu, S. Effects of co-administered melatonin, fructose and bisphenol A (BPA) on rat epididymis and sperm characteristics. Biotech. Histochem. 2019, 95, 18–26. [Google Scholar] [CrossRef]
- Olukole, S.G.; Ajani, S.O.; Ola-Davies, E.O.; Lanipekun, D.O.; Aina, O.O.; Oyeyemi, M.O.; Oke, B.O. Melatonin ameliorates bisphenol A-induced perturbations of the prostate gland of adult Wistar rats. Biomed. Pharmacother. 2018, 105, 73–82. [Google Scholar] [CrossRef]
- Dernek, D.; Akçay, N.C.; Kartal, B.; Dizakar, S.; Özen, A.; Aydin, V.; Ömeroğlu, S.; Türkoğlu, I. Possible effects of melatonin against rat uterus exposure to bisphenol A during neonatal period. Environ. Sci. Pollut. Res. 2017, 24, 26829–26838. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Othman, A.I.; Al-Abdan, M.A.; El-Sayed, A.A. Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against bisphenol-A-induced apoptosis. J. Neurol. Sci. 2014, 347, 251–256. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Antimicrobial Activities and Phytochemical Profiles of Endemic Medicinal Plants of Mauritius. Pharm. Biol. 2005, 43, 237–242. [Google Scholar] [CrossRef]
- Pandey, A.K. Anti-staphylococcal activity of a pan-tropical aggressive and obnoxious weed Parthenium histerophorus: An in vitro study. Natl. Acad. Sci. Lett. 2007, 30, 383–386. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Baghel, S.S.; Shrivastava, N.; Baghel, R.S.; Agrawal, P.; Rajput, S. A review of quercetin: Antioxidant and anticancer properties. World J. Pharm. Pharm. Sci. 2012, 1, 146–160. [Google Scholar]
- Parasuraman, S.; David, A.V.A.; Arulmoli, R. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Nègre-Salvayre, A.; Salvayre, R. Quercetin prevents the cytotoxicity of oxidized LDL on lymphoid cell lines. Free Radic. Biol. Med. 1992, 12, 101–106. [Google Scholar] [CrossRef]
- Sangai, N.P.; Verma, R.J. Quercetin ameliorates bisphenol A-induced toxicity in mice. Acta Pol. Pharm. Drug Res. 2012, 69, 557–563. [Google Scholar]
- Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Dec, K.; Styburski, D.; Nowakowska, A.; Gutowska, I. The influence of polyphenols on metabolic disorders caused by compounds released from plastics—Review. Chemosphere 2019, 240, 124901. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. USA 1999, 96, 8867–8872. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Tayama, S. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch. Toxicol. 2000, 74, 99–105. [Google Scholar] [CrossRef]
- Samova, S.; Doctor, H.; Verma, R. Protective effect of Quercetin on Bisphenol -A induced enzymatic changes in testis of mice. Int. J. Pharm. Sci. Res. 2008, 9, 1256–1262. [Google Scholar]
- Mazroa, S.A. Effect of bisphenol A on the cauda epididymis of adult male albino rats and the possible protective role of quercetin. Egypt. J. Histol. 2011, 34, 377–390. [Google Scholar] [CrossRef]
- Kubavat, K.K.; Samova, S.; Doctor, H.; Sindhav, G.M.; Verma, R.J. Hepatoprotective Effect of Quercetin on Bisphenol A-Induced Toxicity. Acta Chem. Iasi 2018, 26, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Paiva, S.A.; Russell, R.M. Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.; Abouzed, T.; Nasr, S. Lycopene modulates cholinergic dysfunction, Bcl-2/Bax balance, and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in a rat model. Can. J. Physiol. Pharmacol. 2016, 94, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Kumar, A. Lycopene protects against memory impairment and mito-oxidative damage induced by colchicine in rats: An evidence of nitric oxide signaling. Eur. J. Pharmacol. 2013, 721, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvan, P.; Bharathiraja, K.; Vijayaprakash, S. Protective role of lycopene on bisphenol A induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Int. J. Pharma Biol. Sci. 2013, 4, 131–143. [Google Scholar]
- Simic, M.G.; Hunter, E.P.L. Chemical Changes in Food during Processing; Basic Symposium Series; Springer: Boston, MA, USA, 1985; pp. 107–119. [Google Scholar] [CrossRef]
- Kaczmarski, M.; Wójcicki, J.; Samochowiec, L.; Dutkiewicz, T.; Sych, Z. The influence of exogenous antioxidants and physical exercise on some parameters associated with production and removal of free radicals. Die Pharm. 1999, 54, 303–306. [Google Scholar]
- Porter, W.L. Autoxidation in Food and Biological Systems; Springer: Boston, MA, USA, 1980; pp. 295–365. [Google Scholar] [CrossRef]
- Witschi, H. Enhancement of tumor formation in mouse lung by dietary butylated hydroxytoluene. Toxicology 1981, 21, 95–104. [Google Scholar] [CrossRef]
- Okubo, T.; Yokoyama, Y.; Kano, K.; Kano, I. Cell death induced by the phenolic antioxidant tert-butylhydroquinone and its metabolite tert-butylquinone in human monocytic leukemia U937 cells. Food Chem. Toxicol. 2003, 41, 679–688. [Google Scholar] [CrossRef]
- Okubo, T.; Nagai, F.; Ushiyama, K.; Kano, I. Contribution of oxygen radicals to DNA cleavage by quinone compounds derived from phenolic antioxidants, tert-butylhydroquinone and 2,5-di-tert-butylhydroquinone. Toxicol. Lett. 1997, 90, 11–18. [Google Scholar] [CrossRef]
- Ramana, K.V.; Reddy, A.B.M.; Majeti, N.V.R.K.; Singhal, S.S. Therapeutic Potential of Natural Antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Aljadi, A.; Kamaruddin, M. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Mohamed, M.; Sirajudeen, K.; Swamy, M.; Yaacob, N.S.; Sulaiman, S.A. Studies on the Antioxidant Properties of Tualang Honey of Malaysia. Afr. J. Tradit. Complement. Altern. Med. 2009, 7, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic Acid Composition and Antioxidant Properties of Malaysian Honeys. J. Food Sci. 2011, 76, 921–928. [Google Scholar] [CrossRef]
- Ghashm, A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Syazana, M.S.N.; Halim, A.S.; Gan, S.H.; Shamsuddin, S. Antiproliferative effect of methanolic extraction of tualang honey on human keloid fibroblasts. BMC Complement. Altern. Med. 2011, 11, 82. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.; Sulaiman, S.A.; Jaafar, H.; Sirajudeen, K.N.S. Antioxidant Protective Effect of Honey in Cigarette Smoke-Induced Testicular Damage in Rats. Int. J. Mol. Sci. 2011, 12, 5508–5521. [Google Scholar] [CrossRef] [Green Version]
- Zaid, S.S.M.; Sulaiman, S.A.; Othman, N.H.; Soelaiman, I.-N.; Shuid, A.N.; Mohamad, N.; Muhamad, N. Protective effects of Tualang honey on bone structure in experimental postmenopausal rats. Clinics 2012, 67, 779–784. [Google Scholar] [CrossRef]
- Rubin, B.S.; Murray, M.K.; Damassa, D.A.; King, J.C.; Soto, A.M. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 2001, 109, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Markey, C.M.; Coombs, M.A.; Sonnenschein, C.; Soto, A.M. Mammalian development in a changing environment: Exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol. Dev. 2002, 5, 67–75. [Google Scholar] [CrossRef]
- Adewale, H.B.; Jefferson, W.N.; Newbold, R.R.; Patisaul, H.B. Neonatal Bisphenol-A Exposure Alters Rat Reproductive Development and Ovarian Morphology Without Impairing Activation of Gonadotropin-Releasing Hormone Neurons1. Biol. Reprod. 2009, 81, 690–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Bahrke, M.S.; Morgan, W.R. Evaluation of the ergogenic properties of ginseng: An update. Sports Med. 2000, 29, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Zhou, Y.-P.; Xie, J.-T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.-S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naval, M.; Gómez-Serranillos, M.P.; Carretero, M.; Villar, A. Neuroprotective effect of a ginseng (Panax ginseng) root extract on astrocytes primary culture. J. Ethnopharmacol. 2007, 112, 262–270. [Google Scholar] [CrossRef]
- Yang, M.; Lee, H.-S.; Hwang, M.-W.; Jin, M. Effects of Korean red ginseng (Panax Ginseng Meyer) on bisphenol A exposure and gynecologic complaints: Single blind, randomized clinical trial of efficacy and safety. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
| BPA Dose | Target Antioxidant and Dose | Experimental Design | Mechanism of Action | Reference |
|---|---|---|---|---|
| 5, 50, and 500 µg/kg bodyweight/d | Superoxide dismutase (SOD) | 8-week-old male rats were exposed to BPA for 8 weeks | SOD levels in the liver were decreased by the higher dose tested | [44] |
| 0, 2, 10, and 50 mg/kg bodyweight/d | 50-d-old male rats were treated with BPA for 30 d | SOD levels were reduced by the highest concentration tested | [45] | |
| 20 and 100 mg/kg bodyweight/d | Male albino rats were exposed to BPA for 30 d | SOD levels decreased in the liver and testis | [46] | |
| 5, 50, and 500 µg/kg bodyweight/d | Catalase (CAT) | 8-week-old male rats were exposed to BPA for 8 weeks | CAT levels in the liver were reduced upon exposure to the highest dose | [44] |
| 0, 2, 10, and 50 mg/kg bodyweight/d | 50-d-old male rats were treated with BPA for 30 d | CAT levels in the liver were reduced in a dose-dependent manner | [45] | |
| 50 and 25 mg/kg bodyweight/d | Male mice were given BPA intraperitoneally for 5 d | CAT activity was significantly reduced in the liver | [47] | |
| 20 and 100 mg/kg bodyweight/d | Glutathione (GSH) | Male rats were exposed to BPA for 30 d | GSH levels decreased in the testis and liver | [46] |
| 0.1, 1, 10, and 50 mg/kg bodyweight/d | Male rats were given BPA for 4 weeks | GSH levels decreased in the liver and reactive oxygen species (ROS) levels increased | [48] | |
| 50 and 25 mg/kg bodyweight/day | Male mice were given BPA intraperitoneally for 5 d | GSH levels decreased in the kidney but were unchanged in the liver | [47] | |
| 5, 50, and 500 µg/kg bodyweight/d | Uric acid | 6-week-old male mice were given BPA for 8 weeks | BPA decreased hepatic uric acid levels | [49] |
| 25 and 10 mg/kg bodyweight/d | Adult male rats were administered BPA for 6 and 10 weeks | Both dosages increased uric acid levels in the kidney leading to its malfunction | [50] | |
| 0.2, 2, and 20 µg/kg bodyweight/d | Vitamin C, 40 mg/kg bodyweight/d | Male rats were exposed to BPA for 60 d | Vitamin C had a protective effect on the epididymis in BPA-exposed rats | [51] |
| 60 µg/kg bodyweight/day | Vitamin C, 150 mg/kg bodyweight/d | Female rats were co-administered BPA and Vitamin C for 20 d | BPA reduced the volume of the ovary cortex and medulla, and the volume of oocyte; vitamin C treatment alleviated these effects | [52] |
| 25 mg/kg bodyweight/d | Vitamin C, 60 and 5.5 mg/kg bodyweight/d | Rats co-administered BPA and Vitamin C for 6 weeks | Vitamin C co-treatment reduced BPA hepatotoxicity and nephrotoxicity | [53] |
| 5, 50, 100 μg/100 g bodyweight /d | Vitamin E, 4 mg /100 g bodyweight/d | 3-month-old male rats were co-administered BPA and Vitamin E for 3 months | Vitamin E protected the testicular cells and epididymal sperm from apoptosis in BPA-exposed rats | [54] |
| 20 mg/kg bodyweight/d | Vitamin E, 200 mg/kg bodyweight/d | Male rats were treated for 15 d | BPA enhanced lipid peroxidation in the blood; this was alleviated by vitamin E | [55] |
| 20 mg/kg bodyweight/d | Vitamin E, 0.57 mg /100 g bodyweight/d | 6 to 8-week-old female rats were treated for 15 d | Vitamin E restored the function of hypothalamus–pituitary–gonadal axis in BPA-exposed rats | [56] |
| 50 mg/kg bodyweight/d | Vitamin A, 3000 IU | 10 to 12-week-old male mice were treated for 3 d | Vitamin A co-administration alleviated BPA toxicity in the liver | [57] |
| 50 µg/d | Vitamin A, 100 IU | Male mice were co-administered BPA and Vitamin A for 5 d from the day of birth | Treatment with vitamin A increased sperm motility | [58] |
| 100 mg/kg bodyweight/d | Vitamin A, 5 mg/kg bodyweight/d | 10 to 11-week-old female rats were co-administered BPA and Vitamin A for 3 d | Vitamin A minimized epithelium cell proliferation in BPA-exposed mice | [59] |
| 10 mg/kg bodyweight/d | Melatonin, 10 mg/kg bodyweight/d | Male mice were co-administered BPA and melatonin for 14 d | Melatonin reduced mitochondrial toxicity in the testis of BPA-exposed rats | [60] |
| 50 mg/kg bodyweight /d | Melatonin, 10 mg/kg bodyweight/d | 8-week-old male rats were co-administered BPA and melatonin for 6 weeks | Melatonin improved GSH, SOD, CAT, malondialdehyde, and H2O2 levels in mice co-treated with BPA | [61] |
| 200 mg/kg bodyweight/d | Melatonin, 10 mg/kg bodyweight/d | 8-week-old male rats were co-administered for 10 d | Melatonin repaired DNA damage in male germ cells by suppressing the oxidative stress in BPA-treated rats | [62] |
| 50 mg/kg bodyweight/d | Quercetin, 50 mg/kg bodyweight/d | Adult male rats were co-administered BPA and quercetin for 52 d | Quercetin reduced plasma total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels in BPA-treated rats | [63] |
| 50 and 100 mg/kg bodyweight/d | Quercetin, 10 mg/kg bodyweight/d | Adult male mice were co-administered BPA and quercetin for 6 weeks | Quercetin reduced abnormal testis weight, and improved sperm quality and quantity in BPA-exposed mice | [64] |
| 10 mg/kg bodyweight /day | Lycopene, 10 mg/kg bodyweight/d | Male rats were co-administered BPA and lycopene for 3 months | Lycopene enhanced LYC glucose haemostasis, fat mass, and thyroid hormone levels, and decreased oxidative stress in BPA-treated rats | [65] |
| 50 mg/kg bodyweight/d | Lycopene, 10 mg/kg bodyweight/d | 8-week-old male rats were gavaged 3 d a week for 6 weeks | BPA induced neurotoxicity in the hippocampus by eliciting oxidative stress; lycopene inhibited this effect | [66] |
| 500 mg/kg bodyweight/d | Lycopene, 20 mg/kg bodyweight/d | 8-week-old female and male mice were exposed to BPA from PD8 to PD14, and to lycopene from PD1 to PD7 | Lycopene reduced the negative effect of BPA on pregnant mice | [67] |
| 1 mg/kg bodyweight/d | Murraya koenigii, 200 mg/kg bodyweight/d | Male mice were treated for 8 weeks | M. koenigii extract recovered sperm parameters and reduced ROS, lipid peroxidation, and apoptotic protein levels in BPA-induced mice | [68] |
| 10 mg/kg bodyweight/d | Gallic acid, 20 mg/kg bodyweight/d | Male albino rats were exposed to BPA and gallic acid for 45 d | Gallic acid reduced the chronic stress caused by BPA, by increasing antioxidant enzyme levels and lowering lipid peroxidation | [69] |
| 150 mg/kg bodyweight/d | Ginseng, 200 mg/kg bodyweight/d | Adult female albino rats were given BPA and ginseng from pregnancy day 0 until day 20 | Ginseng reduced testosterone and progesterone levels in BPA-treated pregnant rats | [70] |
| 10 mg/kg bodyweight/d | Tualang honey, 200 mg/kg bodyweight/d | Female rats were treated for 6 consecutive weeks | BPA-induced uterine disturbance was lessened by Tualang honey owing to its phytochemical properties | [71] |
| 21-year-old pre-pubertal female rats were treated for 6 consecutive weeks | Tualang honey decreased ovarian toxicity by reducing morphological abnormalities and enhancing the normal oestrous cycle | [72] |
| BPA Dose | Target Antioxidant and Dose | Experimental Design | Mechanism of Action | Reference |
|---|---|---|---|---|
| 0, 1,10, and 100 µg/L | Superoxide dismutase (SOD) | Rat sperm and testicular tissues were incubated with BPA for 2 h | BPA exposure increased SOD activity by eliciting oxidative stress | [73] |
| 0.1 to 500 µg/mL | SOD | Erythrocytes were treated with BPA for 1, 4, or 24 h | SOD activity was reduced in BPA-incubated erythrocytes | [74] |
| Catalase | Catalase levels were reduced by increased number of hydrogen peroxide ions generation by BPA | |||
| Glutathione (GSH) | BPA-treated cells showed decrease GSH levels | |||
| 100 µM | GSH, 5 mM | Mouse spermatozoa were treated with BPA and glutathione for 6 h | GSH reduced oxidation and compromised acrosome integrity in BPA-exposed spermatozoa | [13] |
| 100 µM | Vitamin C, 100 µM | Mouse spermatozoa were treated with BPA and glutathione for 6 h | Motility of the spermatozoa was increased and stress reduced upon vitamin C treatment | [13] |
| 100 µM | Vitamin E, 2 mM | Mouse spermatozoa were treated with BPA and glutathione for 6 h | Vitamin E restored fertilization and embryo development capacity of BPA-treated cells | [13] |
| 125 µM | Melatonin, 0.5 µM | Kidney mitochondria were pre-incubated with melatonin for 5 min and then exposed to BPA for 15 min | Melatonin protected mitochondrial function, reduced malondialdehyde levels, and increased GSH levels in BPA-exposed cells | [18] |
| 25–150 µg/mL | Quercetin, 10–50 µg/mL | Blood samples | BPA reduced the activity of enzymatic antioxidants while quercetin ameliorated this effect | [75] |
| 50 µM | Synthetic antioxidants butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) with rifampicin, 100 µM | 100 µM rifampicin was mixed with 0.2 µM horseradish peroxidase (HRP), 100 µM H2O2, and 50 µM phenolic compounds BHA, BHT, or BPA in 1.0 ml of 0.1 M phosphate buffer, pH 5.5; oxidation of rifampicin was then monitored for 5 min | BPA had the highest pro-oxidant activity in BHA and BHT in a rifampicin solution, which had a protective effect against oxidative stress | [17] |
| 10 µM | Synthetic antioxidants BHT and BHA with NADPH, 100 µM, in 1.0 mL of phosphate buffer | 100 µM reduced NADPH was incubated with 0.2 µM HRP, 10 µM H2O2, and 10 µM BHA, BHT, or BPA in 1.0 ml of 10 mM phosphate buffer, pH 7.0, containing 50 µM DTPA; oxidation of NADPH was then monitored continuously for 5 min | BPA showed higher oxidation of NADPH compared to synthetic antioxidants | |
| 10, 20 µM | Ginseng, 75 µg/mL | Sertoli cells were cultured for 6, 24, or 48 h | Ginseng increased the activity of antioxidative enzymes, and reduced cell apoptosis and lipid peroxidation in BPA-treated cells | [76] |
| 100 µM | Ginseng, 10, 25, and 50 µg/mL | Leydig and Sertoli cells were pre-treated with ginseng for 1 h and then with BPA for 24 h | Ginseng prevented apoptotic cell death and its anti-apoptotic ability could be useful for cellular defence | [77] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amjad, S.; Rahman, M.S.; Pang, M.-G. Role of Antioxidants in Alleviating Bisphenol A Toxicity. Biomolecules 2020, 10, 1105. https://doi.org/10.3390/biom10081105
Amjad S, Rahman MS, Pang M-G. Role of Antioxidants in Alleviating Bisphenol A Toxicity. Biomolecules. 2020; 10(8):1105. https://doi.org/10.3390/biom10081105
Chicago/Turabian StyleAmjad, Shehreen, Md Saidur Rahman, and Myung-Geol Pang. 2020. "Role of Antioxidants in Alleviating Bisphenol A Toxicity" Biomolecules 10, no. 8: 1105. https://doi.org/10.3390/biom10081105








