Preclinical Efficacy and Safety of an Anti-Human VEGFA and Anti-Human NRP1 Dual-Targeting Bispecific Antibody (IDB0076)
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Protein Expression and Purification
2.3. Affinity Measurement by Surface Plasmon Resonance Spectroscopy
2.4. A Wound Healing Assay
2.5. A Permeability Assay
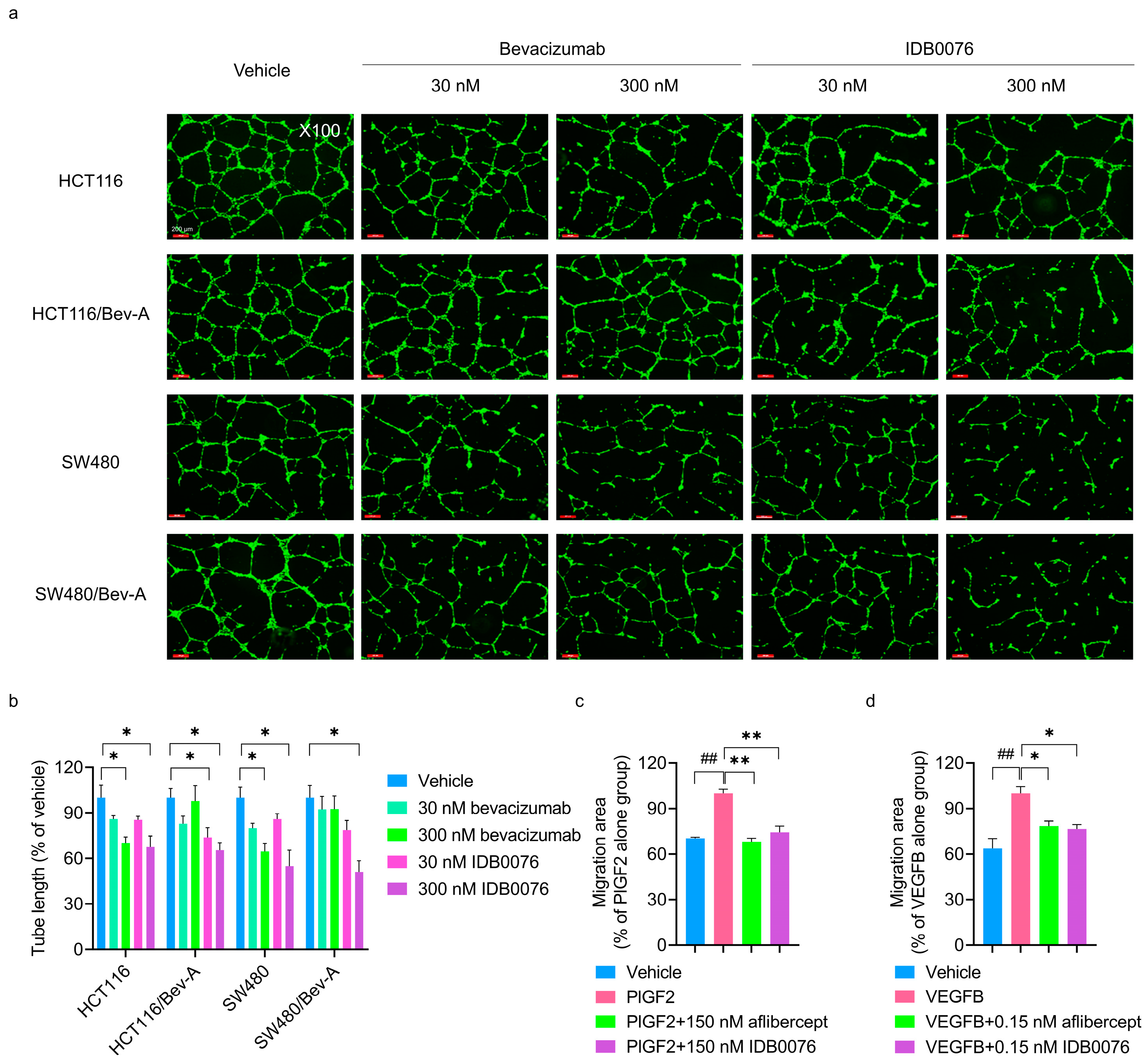
2.6. A Tube Formation Assay
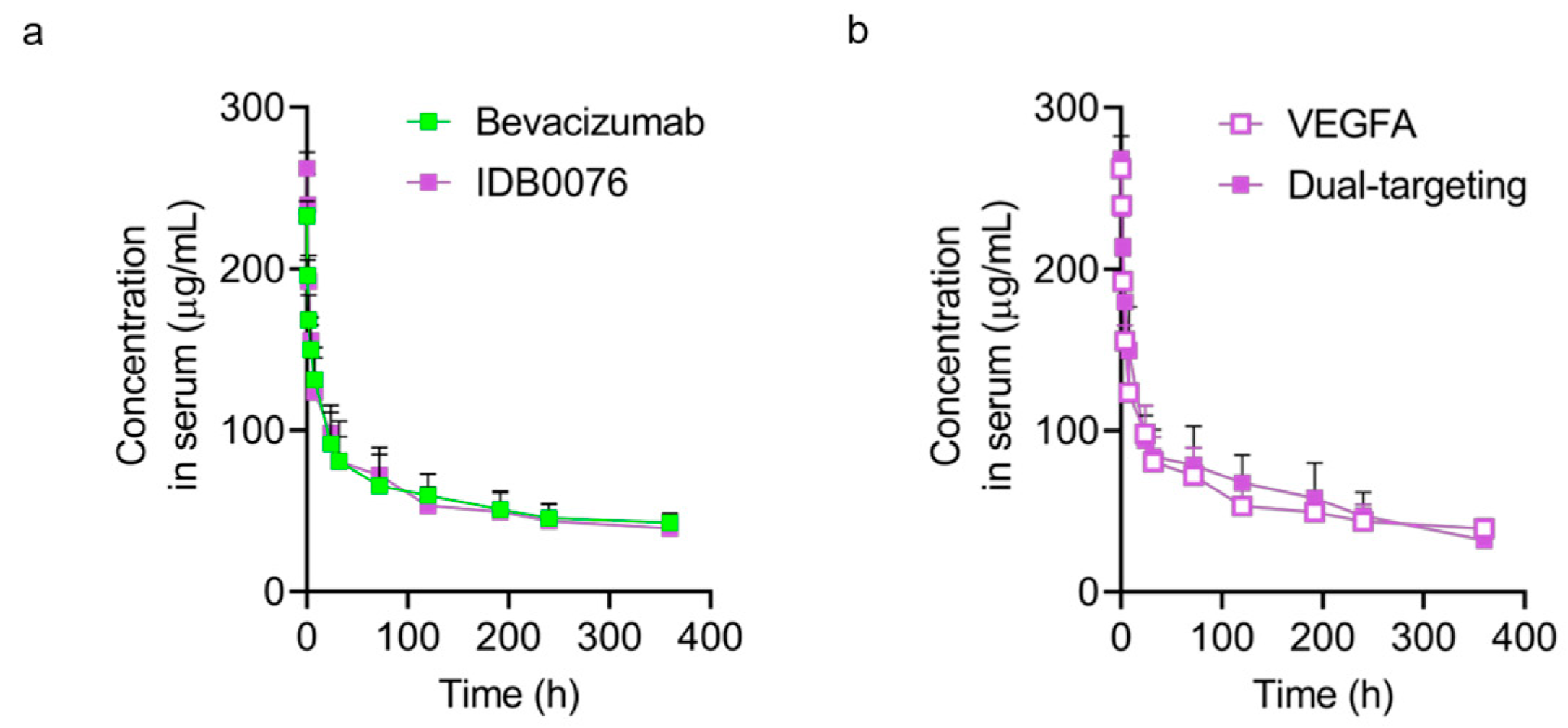
2.7. A Pharmacokinetic (PK) Assay
2.8. In Vivo Antitumor Activity of IDB0076
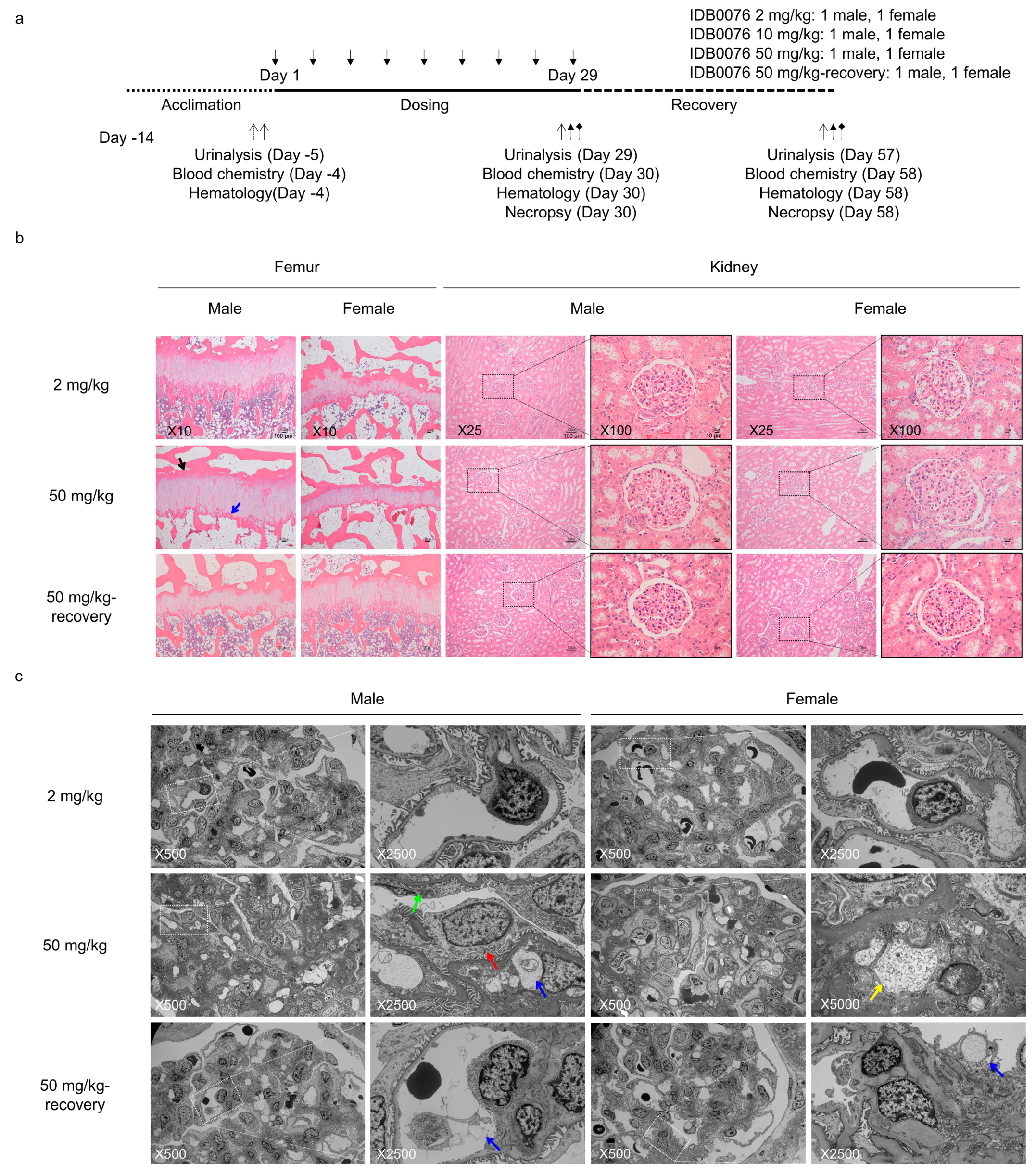
2.9. Toxicity Evaluation
2.10. Statistical Analysis
3. Results
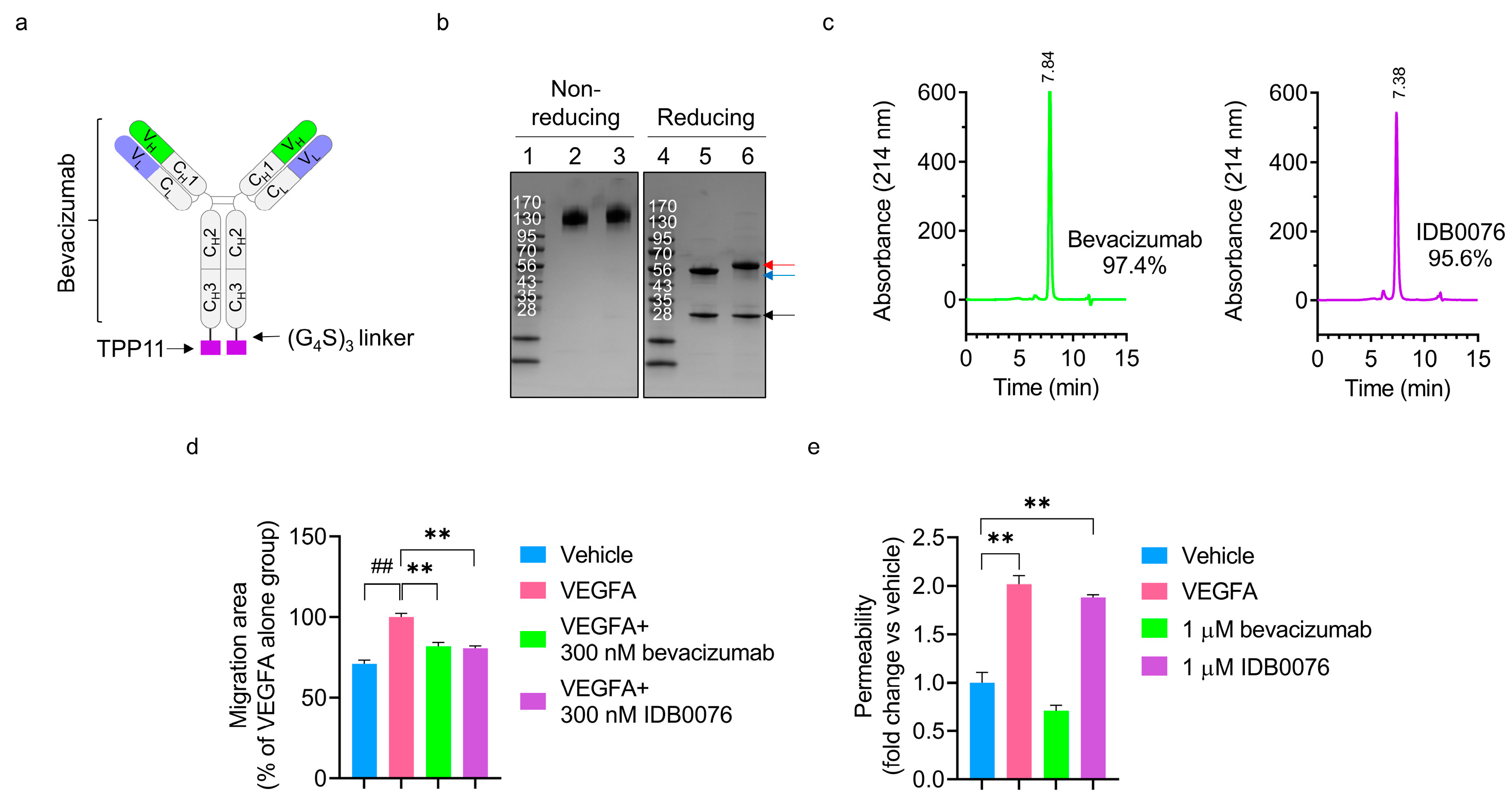
3.1. Preparation and Characterization of IDB0076
3.2. Assays of IDB0076 Binding
3.3. Inhibition of Multiple Signaling Pathways of Proangiogenic Growth Factors by IDB0076
3.4. Pharmacokinetics of IDB0076
3.5. Antitumor Activity of IDB0076 in a Pancreatic Cancer Xenograft Model
3.6. Toxicity of IDB0076 in Cynomolgus Monkeys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef]
- Sherwood, L.M.; Parris, E.E.; Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Goldberg, R.M. Update on Anti-Angiogenesis Therapy in Colorectal Cancer. Curr. Color. Cancer Rep. 2015, 11, 378–387. [Google Scholar] [CrossRef]
- Mulcahy, M.F. Bevacizumab in the therapy for refractory metastatic colorectal cancer. Boil. Targets Ther. 2008, 2, 53–59. [Google Scholar] [CrossRef]
- Sanz-Garcia, E.; Grasselli, J.; Argilés, G.; Elez, M.E.; Tabernero, J. Current and advancing treatments for metastatic colorectal cancer. Expert Opin. Boil. Ther. 2015, 16, 93–110. [Google Scholar] [CrossRef]
- Gacche, R. Compensatory angiogenesis and tumor refractoriness. Oncogenesis 2015, 4, e153. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B.; Iii, A.B.B. Bevacizumab in Combination With Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results From the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Zirlik, K.; Duyster, J. Anti-Angiogenics: Current Situation and Future Perspectives. Oncol. Res. Treat. 2018, 41, 166–171. [Google Scholar] [CrossRef]
- Liang, W.; Wu, X.; Hong, S.; Zhang, Y.; Kang, S.; Fang, W.; Qin, T.; Huang, Y.; Zhao, H.; Zhang, L. Multi-Targeted Antiangiogenic Tyrosine Kinase Inhibitors in Advanced Non-Small Cell Lung Cancer: Meta-Analyses of 20 Randomized Controlled Trials and Subgroup Analyses. PLOS ONE 2014, 9, e109757. [Google Scholar] [CrossRef]
- Maj, E.; Papiernik, D.; Wietrzyk, J. Antiangiogenic cancer treatment: The great discovery and greater complexity (Review). Int. J. Oncol. 2016, 49, 1773–1784. [Google Scholar] [CrossRef]
- Kong, D.-H.; Kim, M.R.; Jang, J.H.; Na, H.-J.; Lee, S. A Review of Anti-Angiogenic Targets for Monoclonal Antibody Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1786. [Google Scholar] [CrossRef]
- Garcia, V.M.; Basu, B.; Molife, L.R.; Kaye, S. Combining Antiangiogenics to Overcome Resistance: Rationale and Clinical Experience. Clin. Cancer Res. 2012, 18, 3750–3761. [Google Scholar] [CrossRef]
- Prud’Homme, G.J.; Glinka, Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2012, 3, 921–939. [Google Scholar] [CrossRef]
- Chaudhary, B.; Khaled, Y.; Ammori, B.J.; Elkord, E. Neuropilin 1: Function and therapeutic potential in cancer. Cancer Immunol. Immunother. 2013, 63, 81–99. [Google Scholar] [CrossRef]
- Pan, Q.; Chanthery, Y.; Liang, W.-C.; Stawicki, S.; Mak, J.; Rathore, N.; Tong, R.K.; Kowalski, J.; Yee, S.F.; Pacheco, G.; et al. Blocking Neuropilin-1 Function Has an Additive Effect with Anti-VEGF to Inhibit Tumor Growth. Cancer Cell 2007, 11, 53–67. [Google Scholar] [CrossRef]
- Patnaik, A.; Lorusso, P.M.; Messersmith, W.A.; Papadopoulos, K.P.; Gore, L.; Beeram, M.; Ramakrishnan, V.; Kim, A.H.; Beyer, J.C.; Shih, L.M.; et al. A Phase Ib study evaluating MNRP1685A, a fully human anti-NRP1 monoclonal antibody, in combination with bevacizumab and paclitaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 951–960. [Google Scholar] [CrossRef]
- Shin, T.-H.; Sung, E.-S.; Kim, Y.-J.; Kim, K.-S.; Kim, S.-H.; Kim, S.-K.; Lee, Y.-D.; Kim, Y.S. Enhancement of the Tumor Penetration of Monoclonal Antibody by Fusion of a Neuropilin-Targeting Peptide Improves the Antitumor Efficacy. Mol. Cancer Ther. 2014, 13, 651–661. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2016, 3–12. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Bae, J.; Shin, T.-H.; Kang, S.H.; Jeong, M.; Han, Y.; Park, J.-H.; Kim, S.-K.; Kim, Y.S. Immunoglobulin Fc-fused, neuropilin-1-specific peptide shows efficient tumor tissue penetration and inhibits tumor growth via anti-angiogenesis. J. Control. Release 2015, 216, 56–68. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Jung, K.; Baek, D.-S.; Hong, S.-S.; Kim, Y.-S. Co-targeting of EGF receptor and neuropilin-1 overcomes cetuximab resistance in pancreatic ductal adenocarcinoma with integrin β1-driven Src-Akt bypass signaling. Oncogene 2017, 36, 2543–2552. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Baek, D.-S.; Lee, S.; Park, D.; Na Kang, H.; Cho, B.C.; Kim, Y.S. Dual-targeting of EGFR and Neuropilin-1 attenuates resistance to EGFR-targeted antibody therapy in KRAS-mutant non-small cell lung cancer. Cancer Lett. 2019, 466, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-C.; Wu, X.; Peale, F.V.; Lee, C.V.; Meng, Y.G.; Gutierrez, J.; Fu, L.; Malik, A.K.; Gerber, H.-P.; Ferrara, N.; et al. Cross-species Vascular Endothelial Growth Factor (VEGF)-blocking Antibodies Completely Inhibit the Growth of Human Tumor Xenografts and Measure the Contribution of Stromal VEGF. J. Boil. Chem. 2005, 281, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.-P.; Wu, X.; Yu, L.; Wiesmann, C.; Liang, X.H.; Lee, C.V.; Fuh, G.; Olsson, C.; Damico, L.; Xie, D.; et al. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc. Natl. Acad. Sci. USA 2007, 104, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Samuel, S.; Gaur, P.; Lü, J.; A Dallas, N.; Xia, L.; Bose, D.; Ramachandran, V.; Ellis, L.M. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. Br. J. Cancer 2011, 104, 1270–1277. [Google Scholar] [CrossRef]
- Seo, N.; Polozova, A.; Zhang, M.; Yates, Z.; Cao, S.; Li, H.; Kuhns, S.; Maher, G.; McBride, H.J.; Liu, J. Analytical and functional similarity of Amgen biosimilar ABP 215 to bevacizumab. mAbs 2018, 10, 678–691. [Google Scholar] [CrossRef]
- Lin, Y.S.; Nguyen, C.; Mendoza, J.L.; Escandon, E.; Fei, D.; Meng, Y.G.; Modi, N.B. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J. Pharmacol. Exp. Ther. 1999, 288, 371–378. [Google Scholar]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 141. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, S.; Zhang, B.; Liu, J.; Qin, Y.; Xu, J.; Yu, X. Role of angiogenesis in pancreatic cancer biology and therapy. Biomed. Pharmacother. 2018, 108, 1135–1140. [Google Scholar] [CrossRef]
- Korc, M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol. Cancer 2003, 2, 8. [Google Scholar] [CrossRef]
- Chen, C.-T.; Hung, M.-C. Beyond anti-VEGF: Dual-targeting antiangiogenic and antiproliferative therapy. Am. J. Transl. Res. 2013, 5, 393–403. [Google Scholar]
- Tufro, A.; Veron, D. VEGF and podocytes in diabetic nephropathy. Semin. Nephrol. 2012, 32, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-C.; Dennis, M.S.; Stawicki, S.; Chanthery, Y.; Pan, Q.; Chen, Y.; Eigenbrot, C.; Yin, J.; Koch, A.W.; Wu, X.; et al. Function Blocking Antibodies to Neuropilin-1 Generated from a Designed Human Synthetic Antibody Phage Library. J. Mol. Boil. 2007, 366, 815–829. [Google Scholar] [CrossRef]
- Ryan, A.M.; Eppler, D.B.; Hagler, K.E.; Bruner, R.H.; Thomford, P.J.; Hall, R.L.; Shopp, G.M.; O’Neill, C.A. Preclinical Safety Evaluation of rhuMAbVEGF, an Antiangiogenic Humanized Monoclonal Antibody. Toxicol. Pathol. 1999, 27, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Peraza, M.A.; Rule, K.E.; Shiue, M.H.; Finch, G.L.; Thibault, S.; Brown, P.R.; Clarke, D.W.; Leach, M.W. Nonclinical assessments of the potential biosimilar PF-06439535 and bevacizumab. Regul. Toxicol. Pharmacol. 2018, 95, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.-B.; Braun, G.B.; Ruoslahti, E. Neuropilin-1 and heparan sulfate proteoglycans cooperate in cellular uptake of nanoparticles functionalized by cationic cell-penetrating peptides. Sci. Adv. 2015, 1, e1500821. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, U.; Cao, S.; Shergill, U.; Jagavelu, K.; Geng, Z.; Yin, M.; De Assuncao, T.M.; Cao, Y.; Szabolcs, A.; Thorgeirsson, S.; et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res. 2012, 72, 4047–4059. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Zhang, Q.; Dong, X.; Gao, Y.; He, Y.; Qiao, H.; Xie, F.; Xie, X.-J.; Sun, X. Neuropilin-1 is associated with clinicopathology of gastric cancer and contributes to cell proliferation and migration as multifunctional co-receptors. J. Exp. Clin. Cancer Res. 2016, 35, 16. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef]
- Jung, K.; Kim, J.-A.; Kim, Y.-J.; Lee, H.W.; Kim, C.-H.; Haam, S.; Kim, Y.S. A Neuropilin-1 Antagonist Exerts Antitumor Immunity by Inhibiting the Suppressive Function of Intratumoral Regulatory T Cells. Cancer Immunol. Res. 2019, 8, 46–56. [Google Scholar] [CrossRef]

| Immobilized Antigen | Analyte | ka (M−1s−1) | kd (s−1) | KD (M) | |
|---|---|---|---|---|---|
| Protein | Species | ||||
| NRP1 ECD | Human | IDB0076 | 1.221 × 105 | 1.921 × 10−3 | 1. 574 × 10−8 |
| NRP1 ECD | Mouse | IDB0076 | 1.885 × 105 | 4.142 × 10−3 | 2.197 × 10−8 |
| VEGFA | Human | IDB0076 | 2.904 × 104 | 3.491 × 10−5 | 1.202 × 10−9 |
| VEGFA | Human | Bevacizumab | 3.099 × 104 | 4.457 × 10−5 | 1.438 × 10−9 |
| Method | Drug | AUCall (μg/mL*day) | AUCinf (μg/mL*day) | t1/2 (days) | C0 (μg/mL) | CL (mL/day/kg) | Vss (mL/kg) |
|---|---|---|---|---|---|---|---|
| VEGFA binding | Bevacizumab | 886.5 ± 71.8 | 1769.5 ± 110.4 | 14.7 ± 1.8 | 235.8 ± 4.4 | 5.7 ± 0.3 | 122.1 ± 13.0 |
| IDB0076 | 868.1 ± 61.6 | 1722.5 ± 173.1 | 14.7 ± 2.0 | 264.1 ± 4.6 | 6.1 ± 0.7 | 122.2 ± 11.8 | |
| Dual-targeting | IDB0076 | 947.9 ± 104.4 | 1384.2 ± 100.7 | 9.5 ± 0.6 | 270.8 ± 5.7 | 7.4 ± 0.5 | 99.4 ± 13.9 |
| Sex | Dose | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | GGT (IU/L) | ||||||||
| Pre | End ofdosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | ||
| Males | 2 mg/kg | 66 | 53 | N/A | 54 | 43 | N/A | 1912 | 2038 | N/A | 70 | 80 | N/A |
| 10 mg/kg | 30 | 55 | N/A | 34 | 47 | N/A | 1223 | 1102 | N/A | 58 | 59 | N/A | |
| 50 mg/kg | 27 | 44 | N/A | 21 | 21 | N/A | 1166 | 1265 | N/A | 67 | 97 | N/A | |
| 50 mg/kg-recovery | 34 | 34 | 27 | 33 | 24 | 21 | 1178 | 1107 | 1412 | 48 | 57 | 52 | |
| Females | 2 mg/kg | 43 | 50 | N/A | 83 | 76 | N/A | 694 | 613 | N/A | 56 | 54 | N/A |
| 10 mg/kg | 44 | 35 | N/A | 108 | 44 | N/A | 554 | 466 | N/A | 52 | 52 | N/A | |
| 50 mg/kg | 30 | 46 | N/A | 40 | 45 | N/A | 677 | 882 | N/A | 45 | 54 | N/A | |
| 50 mg/kg-recovery | 42 | 104 | 34 | 71 | 53 | 35 | 822 | 1124 | 674 | 43 | 53 | 38 | |
| Sex | Dose | Creatinine (mg/dL) | CK (IU/L) | Total bilirubin (mg/dL) | Urea nitrogen (mg/dL) | ||||||||
| Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | ||
| Males | 2 mg/kg | 0.66 | 0.60 | N/A | 377 | 198 | N/A | 0.07 | 0.10 | N/A | 34.3 | 30.3 | N/A |
| 10 mg/kg | 0.69 | 0.66 | N/A | 254 | 452 | N/A | 0.17 | 0.25 | N/A | 19.4 | 18.4 | N/A | |
| 50 mg/kg | 0.85 | 0.82 | N/A | 188 | 156 | N/A | 0.11 | 0.08 | N/A | 22.0 | 22.6 | N/A | |
| 50 mg/kg-recovery | 0.58 | 0.58 | 0.58 | 174 | 111 | 118 | 0.12 | 0.13 | 0.08 | 17.8 | 18.2 | 21.1 | |
| Females | 2 mg/kg | 0.53 | 0.49 | N/A | 199 | 254 | N/A | 0.21 | 0.12 | N/A | 16.3 | 22.0 | N/A |
| 10 mg/kg | 0.65 | 0.59 | N/A | 177 | 160 | N/A | 0.14 | 0.13 | N/A | 18.2 | 17.4 | N/A | |
| 50 mg/kg | 0.71 | 0.78 | N/A | 378 | 133 | N/A | 0.17 | 0.18 | N/A | 23.7 | 22.5 | N/A | |
| 50 mg/kg-recovery | 0.60 | 0.63 | 0.64 | 187 | 529 | 364 | 0.15 | 0.12 | 0.13 | 16.8 | 16.7 | 15.4 | |
| Sex | Dose | Glucose (mg/dL) | IP (mg/dL) | TP (g/dL) | Albumin (g/dL) | ||||||||
| Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | ||
| Males | 2 mg/kg | 78 | 83 | N/A | 4.98 | 4.80 | N/A | 7.6 | 7.0 | N/A | 3.8 | 3.5 | N/A |
| 10 mg/kg | 75 | 72 | N/A | 6.50 | 4.81 | N/A | 7.6 | 7.2 | N/A | 4.1 | 3.7 | N/A | |
| 50 mg/kg | 85 | 78 | N/A | 5.67 | 3.12 | N/A | 6.8 | 7.8 | N/A | 3.6 | 3.5 | N/A | |
| 50 mg/kg-recovery | 85 | 83 | 79 | 5.53 | 3.79 | 4.36 | 7.9 | 8.2 | 7.5 | 4.1 | 3.8 | 3.7 | |
| Females | 2 mg/kg | 78 | 73 | N/A | 4.99 | 3.46 | N/A | 7.4 | 7.0 | N/A | 4.4 | 4.0 | N/A |
| 10 mg/kg | 59 | 63 | N/A | 6.00 | 4.19 | N/A | 7.6 | 7.1 | N/A | 3.6 | 3.5 | N/A | |
| 50 mg/kg | 66 | 61 | N/A | 5.36 | 3.46 | N/A | 7.6 | 7.6 | N/A | 4.0 | 3.5 | N/A | |
| 50 mg/kg-recovery | 80 | 76 | 71 | 4.65 | 2.61 | 4.33 | 7.8 | 8.0 | 7.8 | 4.3 | 3.5 | 4.1 | |
| Sex | Dose | Globulin (g/dL) | Albumin/globulin ratio | TG (mg/dL) | Total cholesterol (mg/dL) | ||||||||
| Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of Dosing | End of recovery | Pre | End of dosing | End of Recovery | ||
| Males | 2 mg/kg | 3.8 | 3.5 | N/A | 1.00 | 1.00 | N/A | 103 | 67 | N/A | 123 | 120 | N/A |
| 10 mg/kg | 3.5 | 3.5 | N/A | 1.17 | 1.06 | N/A | 29 | 24 | N/A | 158 | 172 | N/A | |
| 50 mg/kg | 3.2 | 4.3 | N/A | 1.13 | 0.81 | N/A | 37 | 46 | N/A | 168 | 177 | N/A | |
| 50 mg/kg-recovery | 3.8 | 4.4 | 3.8 | 1.08 | 0.86 | 0.97 | 32 | 18 | 38 | 135 | 147 | 128 | |
| Females | 2 mg/kg | 3.0 | 3.0 | N/A | 1.47 | 1.33 | N/A | 39 | 96 | N/A | 92 | 87 | N/A |
| 10 mg/kg | 4.0 | 3.6 | N/A | 0.90 | 0.97 | N/A | 63 | 35 | N/A | 156 | 162 | N/A | |
| 50 mg/kg | 3.6 | 4.1 | N/A | 1.11 | 0.85 | N/A | 40 | 57 | N/A | 127 | 223 | N/A | |
| 50 mg/kg-recovery | 3.5 | 4.5 | 3.7 | 1.23 | 0.78 | 1.11 | 47 | 31 | 26 | 148 | 196 | 148 | |
| Sex | Dose | Organ/Tissue | Findings | Severity |
|---|---|---|---|---|
| Light-microscopic findings | ||||
| Males | 2 mg/kg | N/A | - | - |
| 10 mg/kg | Femur | Decreased number of primary trabecula bone Increased thickness of physis | Slight Slight | |
| 50 mg/kg | Femur | Decreased number of primary trabecula bone Increased thickness of physis | Slight Slight | |
| Kidney | Enlargement of glomeruli | Very slight | ||
| 50 mg/kg-recovery | Femur | - | - | |
| Females | 2 mg/kg | N/A | - | - |
| 10 mg/kg | N/A | - | - | |
| 50 mg/kg | Kidney | Enlargement of glomeruli | Very slight | |
| 50 mg/kg-recovery | N/A | - | - | |
| Electron-microscopic findings | ||||
| Males | 2 mg/kg | Kidney (Glomerulus) | - | - |
| 50 mg/kg | Hypertrophy of podocytes Shortening of foot process in podocytes Retention of small/large vesicles in capillaries | Slight Very slight Slight | ||
| 50 mg/kg-recovery | Retention of vesicles in capillaries | Very slight | ||
| Females | 2 mg/kg | - | - | |
| 50 mg/kg | Hypertrophy of podocytes Shortening of foot process in podocytes Retention of small/large vesicles in capillaries Granular/vesicular material in mesangial cells | Slight Very slight Slight Very slight | ||
| 50 mg/kg-recovery | Retention of vesicles in capillaries | Very slight | ||
| Sex | Dose | Color | pH | Ketones (mg/dL) | Bilirubin (mg/dL) | Occult blood (mg/dL) | ||||||||||
| Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | ||
| Males | 2 mg/kg | 0 | 0 | N/A | 8.5 | 8.5 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | 0 | 0 | N/A |
| 10 mg/kg | 0 | 0 | N/A | 8.5 | 8.5 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | |
| 50 mg/kg | 0 | 0 | N/A | 8.5 | 8.5 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | |
| 50 mg/kg-recovery | 0 | 0 | 0 | 8.5 | 8.5 | 8.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Females | 2 mg/kg | 0 | 0 | N/A | 8.5 | 8.5 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | 0 | 0 | N/A |
| 10 mg/kg | 0 | 0 | N/A | 8.5 | 8.5 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | |
| 50 mg/kg | 0 | 0 | N/A | 8.5 | 8.5 | N/A | 0 | 0 | N/A | 0 | 0 | N/A | 0 | 0.015 | N/A | |
| 50 mg/kg-recovery | 0 | 0 | 0 | 8.5 | 8.5 | 8.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sex | Dose | Urobilinogen (Ehrlich units/dL) | Urine volume (mL) | Specific gravity | Protein (mg/dL) | Glucose (mg/dL) | ||||||||||
| Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | ||
| Males | 2 mg/kg | 0 | 0 | N/A | 78 | 75 | N/A | 1.024 | 1.025 | N/A | 0.2 | 2.4 | N/A | 0 | 2 | N/A |
| 10 mg/kg | 0 | 0 | N/A | 52 | 88 | N/A | 1.021 | 1.016 | N/A | 6.7 | 8.0 | N/A | 4 | 5 | N/A | |
| 50 mg/kg | 0 | 0 | N/A | 80 | 122 | N/A | 1.021 | 1.017 | N/A | 3.8 | 13.0 | N/A | 3 | 8 | N/A | |
| 50 mg/kg-recovery | 0 | 0 | 0 | 96 | 106 | 116 | 1.016 | 1.015 | 1.018 | 1.6 | 4.2 | 0.5 | 4 | 6 | 1 | |
| Females | 2 mg/kg | 0 | 0 | N/A | 126 | 190 | N/A | 1.014 | 1.013 | N/A | 3.1 | 3.6 | N/A | 2 | 6 | N/A |
| 10 mg/kg | 0 | 0 | N/A | 108 | 164 | N/A | 1.012 | 1.009 | N/A | 4.9 | 14.4 | N/A | 1 | 5 | N/A | |
| 50 mg/kg | 0 | 0 | N/A | 108 | 78 | N/A | 1.012 | 1.015 | N/A | 1.7 | 2.7 | N/A | 3 | 4 | N/A | |
| 50 mg/kg-recovery | 0 | 0 | 0 | 88 | 182 | 96 | 1.018 | 1.010 | 1.014 | 8.7 | 36.8 | 14.0 | 5 | 8 | 6 | |
| Sex | Dose | Na (mEq/L) | K (mEq/L) | Cl (mEq/L) | ||||||||||||
| Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | Pre | End of dosing | End of recovery | ||||||||
| Males | 2 mg/kg | 15 | 20 | N/A | 100.7 | 112.0 | N/A | 78 | 78 | N/A | ||||||
| 10 mg/kg | 23 | 27 | N/A | 75.7 | 39.3 | N/A | 66 | 56 | N/A | |||||||
| 50 mg/kg | 23 | 21 | N/A | 75.0 | 47.1 | N/A | 63 | 40 | N/A | |||||||
| 50 mg/kg-recovery | 16 | 11 | 13 | 53.5 | 52.3 | 74.4 | 41 | 29 | 47 | |||||||
| Females | 2 mg/kg | 20 | 15 | N/A | 21.0 | 39.7 | N/A | 47 | 33 | N/A | ||||||
| 10 mg/kg | 42 | 19 | N/A | 30.0 | 22.8 | N/A | 46 | 22 | N/A | |||||||
| 50 mg/kg | 24 | 30 | N/A | 38.8 | 45.0 | N/A | 31 | 20 | N/A | |||||||
| 50 mg/kg-recovery | 36 | 25 | 26 | 37.0 | 21.6 | 27.0 | 42 | 31 | 27 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.-H.; Kwon, H.-S.; Kim, B.; Min, G.; Shin, C.; Yang, S.-W.; Lee, S.W.; Lee, Y.; Hong, D.; Kim, Y.-S. Preclinical Efficacy and Safety of an Anti-Human VEGFA and Anti-Human NRP1 Dual-Targeting Bispecific Antibody (IDB0076). Biomolecules 2020, 10, 919. https://doi.org/10.3390/biom10060919
Ko J-H, Kwon H-S, Kim B, Min G, Shin C, Yang S-W, Lee SW, Lee Y, Hong D, Kim Y-S. Preclinical Efficacy and Safety of an Anti-Human VEGFA and Anti-Human NRP1 Dual-Targeting Bispecific Antibody (IDB0076). Biomolecules. 2020; 10(6):919. https://doi.org/10.3390/biom10060919
Chicago/Turabian StyleKo, Jong-Hee, Hyuk-Sang Kwon, Bomin Kim, Gihong Min, Chorong Shin, Seok-Woo Yang, Seong Wook Lee, Youngmin Lee, Dahae Hong, and Yong-Sung Kim. 2020. "Preclinical Efficacy and Safety of an Anti-Human VEGFA and Anti-Human NRP1 Dual-Targeting Bispecific Antibody (IDB0076)" Biomolecules 10, no. 6: 919. https://doi.org/10.3390/biom10060919
APA StyleKo, J.-H., Kwon, H.-S., Kim, B., Min, G., Shin, C., Yang, S.-W., Lee, S. W., Lee, Y., Hong, D., & Kim, Y.-S. (2020). Preclinical Efficacy and Safety of an Anti-Human VEGFA and Anti-Human NRP1 Dual-Targeting Bispecific Antibody (IDB0076). Biomolecules, 10(6), 919. https://doi.org/10.3390/biom10060919






