1H Nuclear Magnetic Resonance of Pig Seminal Plasma Reveals Intra-Ejaculate Variation in Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Boars and Ejaculates
2.2. Processing and Storage of SP
2.3. 1H RMN Analysis
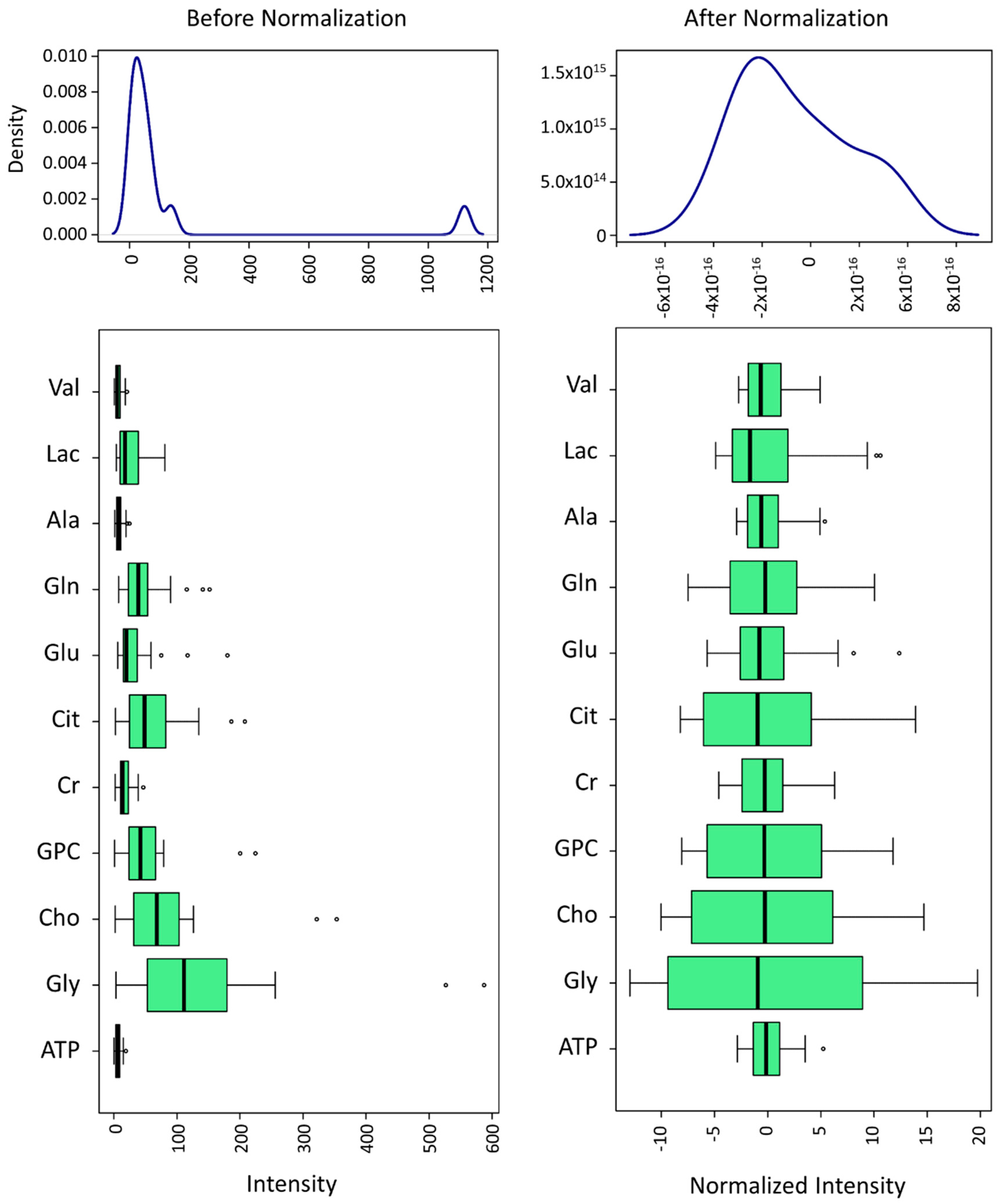
2.4. 1H RMN Data Statistical Analysis
3. Results
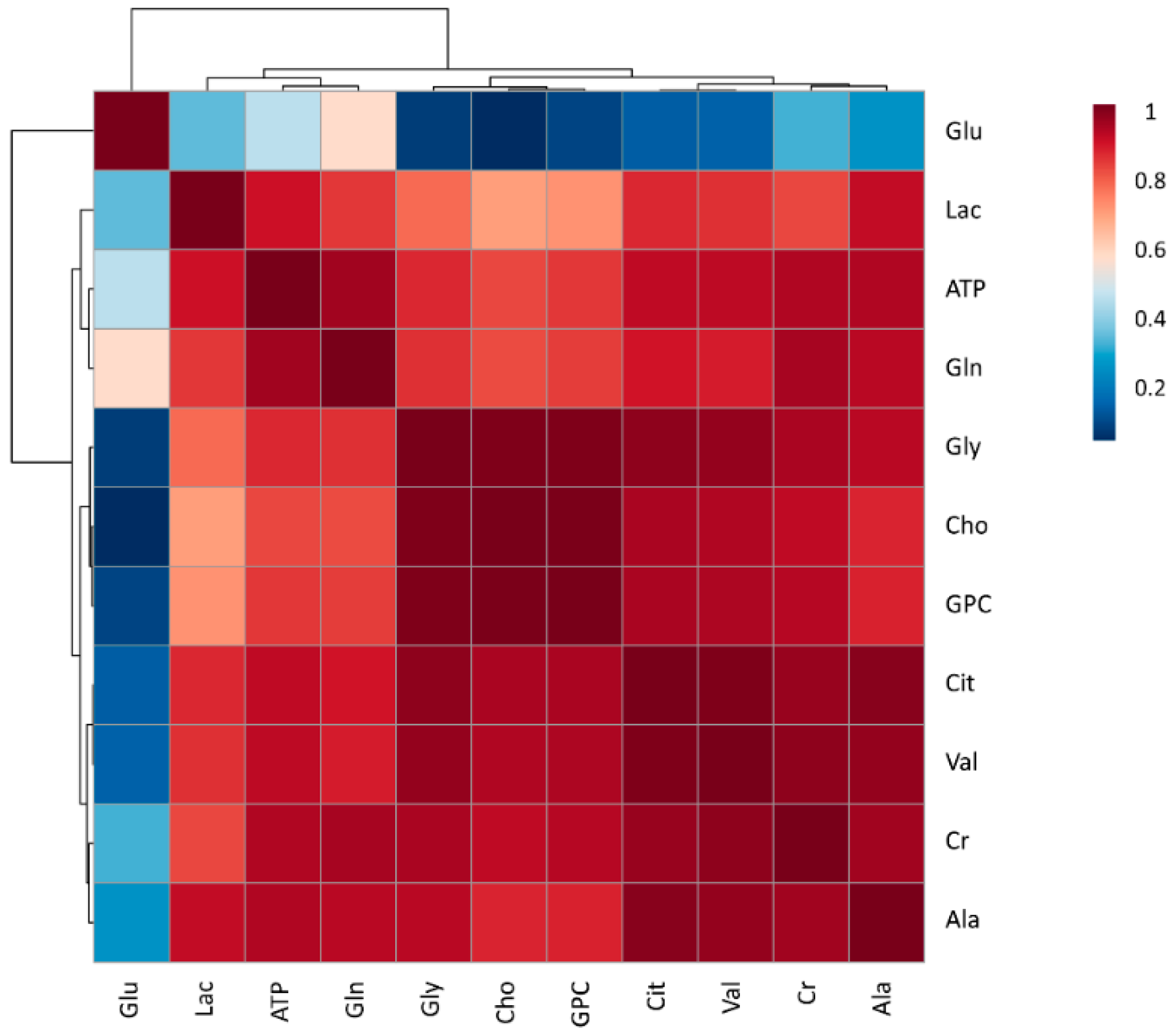
3.1. Metabolomic Composition of Pig SP
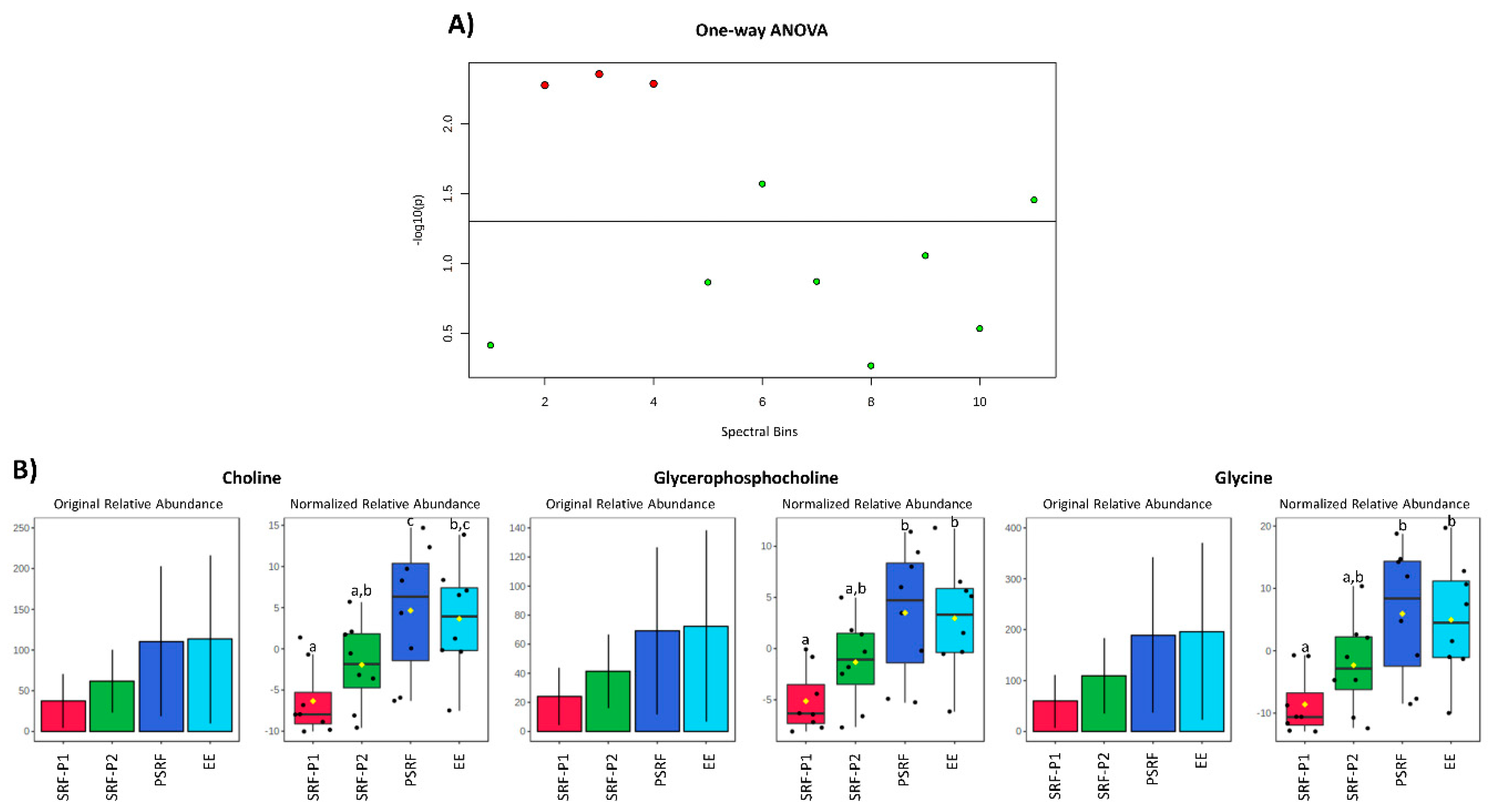
3.2. Comparison of SP Metabolites between Ejaculate-Portions and the EE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maes, D.; Nauwynck, H.; Rijsselaere, T.; Mateusen, B.; Vyt, P.; De Kruif, A.; Van Soom, A. Diseases in swine transmitted by artificial insemination: An overview. Theriogenology 2008, 70, 1337–1345. [Google Scholar] [CrossRef]
- Parrilla, I.; Del Olmo, D.; Sijses, L.; Martinez-Alborcia, M.J.; Cuello, C.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Differences in the ability of spermatozoa from individual boar ejaculates to withstand different Semen-Processing techniques. Anim. Reprod. Sci. 2012, 132, 66–73. [Google Scholar] [CrossRef]
- Roca, J.; Broekhuijse, M.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.A.; Bolarin, A. Boar differences in artificial insemination outcomes: Can they be minimized? Reprod. Domest. Anim. 2015, 50, 48–55. [Google Scholar] [CrossRef]
- Broekhuijse, M.; Feitsma, H.; Gadella, B. Artificial insemination in pigs: Predicting male fertility. Vet. Quart. 2012, 32, 151–157. [Google Scholar] [CrossRef][Green Version]
- Druart, X.; Rickard, J.P.; Tsikis, G.; De Graaf, S.P. Seminal plasma proteins as markers of sperm fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 6. [Google Scholar] [CrossRef]
- Caballero, I.; Vazquez, J.M.; Mayor, G.M.; Alminana, C.; Calvete, J.J.; Sanz, L.; Roca, J.; Martinez, E.A. PSP-I/PSP-II spermadhesin exert a decapacitation effect on highly extended boar spermatozoa. Int. J. Androl. 2009, 32, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A. Seminal fluid signaling in the female reproductive tract: Lessons from rodents and pigs1. J. Anim. Sci. 2007, 85, 36–44. [Google Scholar] [CrossRef]
- Schjenken, J.; Robertson, S.; Schjenken, J.E.; Robertson, S.A. Seminal fluid and immune adaptation for Pregnancy–Comparative biology in mammalian species. Reprod. Domest. Anim. 2014, 49, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Carrillo, A.V.; Parrilla, I.; Cerón, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Glutathione peroxidase 5 is expressed by the entire pig male genital tract and once in the seminal plasma contributes to sperm survival and in vivo fertility. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Parrilla, I.; Cerón, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. High total antioxidant capacity of the porcine seminal plasma (SP-TAC) relates to sperm survival and fertility. Sci. Rep. 2015, 5, 18538. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hebles, M.; Dorado, M.; Gallardo, M.; González-Martínez, M.; Sánchez-Martín, P. Seminal quality in the first fraction of ejaculate. Syst. Biol. Reprod. Med. 2014, 61, 113–116. [Google Scholar] [CrossRef]
- De La Torre, J.; Sánchez-Martín, P.; Gosalvez, J.; Crespo, F. Equivalent seminal characteristics in human and stallion at first and second ejaculated fractions. Andrologia 2016, 49, e12708. [Google Scholar] [CrossRef]
- Saravia, F.; Wallgren, M.; Johannisson, A.; Calvete, J.J.; Sanz, L.; Peña, F.J.; Roca, J.; Rodriguez-Martinez, H. Exposure to the seminal plasma of different portions of the boar ejaculate modulates the survival of spermatozoa cryopreserved in MiniFlatPacks. Theriogenology 2009, 71, 662–675. [Google Scholar] [CrossRef]
- Sancho, S.; Vilagran, I. The boar ejaculate: Sperm function and seminal plasma analyses. In Boar Reproduction; Bonet, S., Casas, I., Holt, W., Yeste, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 471–516. [Google Scholar]
- Einarsson, S. Studies on the composition of epididymal content and semen in the boar. Acta Veter. Scand. Suppl. 1971, 36, 1–80. [Google Scholar]
- Barranco, I.; Perez-Patiño, C.; Tvarijonaviciute, A.; Parrilla, I.; Carrillo, A.V.; Alvarez-Rodriguez, M.; Cerón, J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Active paraoxonase 1 is synthesised throughout the internal boar genital organs. Reproduction 2017, 154, 237–243. [Google Scholar] [CrossRef]
- Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Valero, M.L.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Characterization of the porcine seminal plasma proteome comparing ejaculate portions. J. Proteom. 2016, 142, 15–23. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Saravia, F.; Wallgren, M.; Tienthai, P.; Johannisson, A.; Vazquez, J.M.; Martinez, E.A.; Roca, J.; Sanz, L.; Calvete, J.J. Boar spermatozoa in the oviduct. Theriogenology 2005, 63, 514–535. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H.; Kvist, U.; Saravia, F.; Wallgren, M.; Johannisson, A.; Sanz, L.; Peña, F.J.; Martínez, E.A.; Roca, J.; Vázquez, J.M.; et al. The physiological roles of the boar ejaculate. Soc. Reprod. Fertil. Suppl. 2009, 66, 1–21. [Google Scholar]
- Sellés, E.; Wallgren, M.; Gadea, J.; Rodriguez-Martinez, H. Sperm viability and Capacitation-Like changes in fractions of boar semen after storage and freezing. In Proceedings of the 6th International Conference on Pig Reproduction, Columbia, MO, USA, 3–6 June 2001; Volume 1, p. 51. [Google Scholar]
- Peña, F.J.; Johannisson, A.; Wallgren, M.; Rodriguez-Martinez, H. Antioxidant supplementation in vitro improves boar sperm motility and mitochondrial membrane potential after cryopreservation of different fractions of the ejaculate. Anim. Reprod. Sci. 2003, 78, 85–98. [Google Scholar] [CrossRef]
- Alkmin, D.V.; Perez-Patiño, C.; Barranco, I.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Boar sperm cryosurvival is better after exposure to seminal plasma from selected fractions than to those from entire ejaculate. Cryobiology 2014, 69, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Alkmin, D.V.; Parrilla, I.; Tarantini, T.; Del Olmo, D.; Vázquez, J.M.; Martinez, E.A.; Roca, J. Seminal plasma affects sperm sex sorting in boars. Reprod. Fertil. Dev. 2016, 28, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Aneas, S.B.; Gary, B.; Bouvier, B. Collectis® automated boar collection technology. Theriogenology 2008, 70, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, R.; Vaidyanathan, S.; Dunn, W.B.; Harrigan, G.G.; Kell, D.B. Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends Biotechnol. 2004, 22, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Pastuszak, A.W.; Lamb, D.J. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil. Steril. 2013, 99, 998–1007. [Google Scholar] [CrossRef]
- Deepinder, F.; Chowdary, H.T.; Agarwal, A. Role of metabolomic analysis of biomarkers in the management of male infertility. Expert Rev. Mol. Diagn. 2007, 7, 351–358. [Google Scholar] [CrossRef]
- Moura, A.A.; Memili, E.; Portela, A.M.R.; Viana, A.G.; Velho, A.L.C.; Bezerra, M.J.B.; Vasconselos, F.R. Seminal plasma proteins and metabolites: Effects on sperm function and potential as fertility markers. Anim. Reprod. 2018, 15, 691–702. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, H.; Wang, Y.; Zhang, Z.; Wu, Q.; Wu, Q. Comprehensive metabolic profiles of seminal plasma with different forms of male infertility and their correlation with sperm parameters. J. Pharm. Biomed. Anal. 2019, 177, 112888. [Google Scholar] [CrossRef]
- Bonechi, C.; Collodel, G.; Donati, A.; Martini, S.; Moretti, E.; Rossi, C. Discrimination of human semen specimens by NMR data, sperm parameters, and statistical analysis. Syst. Biol. Reprod. Med. 2015, 61, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; Von Bergen, M.; Grunewald, S. Metabolomic profiling reveals correlations between spermiogram parameters and the metabolites present in human spermatozoa and seminal plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef] [PubMed]
- Rhemrev, J.P.; Van Overveld, F.W.; Haenen, G.R.; Teerlink, T.; Bast, A.; Vermeiden, J.P. Quantification of the nonenzymatic fast and slow TRAP in a postaddition assay in human seminal plasma and the antioxidant contributions of various seminal compounds. J. Androl. 2000, 21, 913–920. [Google Scholar] [PubMed]
- Qiao, S.; Wu, W.; Chen, M.; Tang, Q.; Xia, Y.; Jia, W.; Wang, X. Seminal plasma metabolomics approach for the diagnosis of unexplained male infertility. PLoS ONE 2017, 12, e0181115. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Seguin, F.; Barthelemy, C.; Akoka, S.; Le Pape, A.; Lansac, J.; Royere, D. 1H nuclear magnetic resonance studies of seminal plasma from fertile and infertile men. J. Reprod. Fertil. 1993, 97, 51–55. [Google Scholar] [CrossRef]
- Mumcu, A.; Karaer, A.; Dogan, B.; Tuncay, G. Metabolomics analysis of seminal plasma in patients with idiopathic Oligoasthenoteratozoospermia using high-resolution NMR spectroscopy. Andrology 2020, 8, 450–456. [Google Scholar] [CrossRef]
- Jayaraman, V.; Ghosh, S.; Sengupta, A.; Srivastava, S.; Sonawat, H.M.; Narayan, P.K. Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J. Assist. Reprod. Genet. 2014, 31, 1195–1204. [Google Scholar] [CrossRef]
- Kumar, A.; Kroetsch, T.; Blondin, P.; Anzar, M. Fertility-Associated metabolites in bull seminal plasma and blood serum: 1H nuclear magnetic resonance analysis. Mol. Reprod. Dev. 2015, 82, 123–131. [Google Scholar] [CrossRef]
- Velho, A.L.C.; Menezes, E.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Metabolomic markers of fertility in bull seminal plasma. PLoS ONE 2018, 13, e0195279. [Google Scholar] [CrossRef]
- Menezes, E.B.; Velho, A.L.C.; Santos, F.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Uncovering sperm metabolome to discover biomarkers for bull fertility. BMC Genom. 2019, 20, 714–716. [Google Scholar] [CrossRef]
- Minai-Tehrani, A.; Jafarzadeh, N.; Gilany, K. Metabolomics: A State-Of-The-Art technology for better understanding of male infertility. Andrologia 2015, 48, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Chiba, L. Pig Nutrition and feeding. In Animal Nutrition Handbook; Chiba, L., Ed.; Auburn University: Auburn, AL, USA, 2009; pp. 285–315. [Google Scholar]
- Kuster, C.; Althouse, G. The fecundity of porcine semen stored for 2 to 6 days in androhep® and X-cell™ extenders. Theriogenology 1999, 52, 365–376. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The human metabolome database. Nucleic Acids Res. 2007, 35, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res. 2007, 36, 402–408. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef]
- Robertson, S.A. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005, 322, 43–52. [Google Scholar] [CrossRef]
- Bromfield, J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014, 31, 627–636. [Google Scholar] [CrossRef]
- Morgan, H.L.; Watkins, A.J. The influence of seminal plasma on offspring development and health. Semin. Cell Dev. Biol. 2019, 97, 131–137. [Google Scholar] [CrossRef]
- Roca, J.; Parrilla, I.; Bolarin, A.; Martinez, E.A.; Rodriguez-Martinez, H. Will AI in pigs become more efficient? Theriogenology 2016, 86, 187–193. [Google Scholar] [CrossRef]
- Popwell, J.M.; Flowers, W.L. Variability in relationships between semen quality and estimates of in vivo and in vitro fertility in boars. Anim. Reprod. Sci. 2004, 81, 97–113. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.; Mactier, S.; Kohnke, P.; Kershaw-Young, C.; Bathgate, R.; Gibb, Z.; Crossett, B.; Tsikis, G.; Labas, V.; et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteom. 2013, 91, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Tenori, L.; Cascante, M.; De Atauri Carulla, P.R.; Martins dos Santos, V.A.P.; Saccenti, E. From correlation to causation: Analysis of metabolomics data using systems biology approaches. Metabolomics 2018, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Diez, M.; Adam, J.; Adamski, J.; Chasapi, S.A.; Luchinat, C.; Peters, A.; Prehn, C.; Santucci, C.; Spyridonidis, A.; Spyroulias, G.A.; et al. Plasma and serum metabolite association networks: Comparability within and between studies using NMR and MS profiling. J. Proteome Res. 2017, 16, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New aspects of amino acid metabolism in cancer. Br. J. Cancer 2019, 122, 150–156. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Sonkar, K.; Ayyappan, V.; Tressler, C.M.; Adelaja, O.; Cai, R.; Cheng, M.; Glunde, K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2019, 32, e4112. [Google Scholar] [CrossRef]
- Lynch, M.; Masters, J.; Pryor, J.; Lindon, J.; Spraul, M.; Foxall, P.; Nicholson, J.K. Ultra high field NMR spectroscopic studies on human seminal fluid, seminal vesicle and prostatic secretions. J. Pharm. Biomed. Anal. 1994, 12, 5–19. [Google Scholar] [CrossRef]
- Bonet, S.; Garcia, E.; Sepúlveda, L.; Muela, L.S. The boar reproductive system. In Boar Reproduction; Springer Science and Business Media LLC: Berlin, Germany, 2013; pp. 65–107. [Google Scholar]
- Lindholmer, C. Survival of human spermatozoa in different fractions of split ejaculate. Fertil. Steril. 1973, 24, 521–526. [Google Scholar] [CrossRef]
- Amelar, R.D.; Hotchkiss, R.S. The split ejaculate: Its use in the management of male infertility. Fertil. Steril. 1965, 16, 46–60. [Google Scholar] [CrossRef]
- Mortimer, D. Biochemistry of spermatozoa and seminal plasma. In Practical Laboratory Andrology; Oxford University Press: New York, NY, USA, 1994; pp. 89–109. [Google Scholar]
- Cross, N.L.; Mahasreshti, P. Proteasome fraction of human seminal plasma prevents sperm from becoming acrosomally responsive to the agonist progesterone. Arch. Androl. 1997, 39, 39–44. [Google Scholar] [CrossRef]
- Melendrez, C.S.; Meizel, S. Studies of Porcine and Human Sperm Suggesting a Role for a Sperm Glycine Receptor/CIChannel in the Zona Pellucida-Initiated Acrosome Reaction1. Biol. Reprod. 1995, 53, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, S.R.; Monclus, M.D.L.Á.; Rensetti, D.E.; Fornés, M.W.; Aveldaño, M.I. Differential involvement of rat sperm choline glycerophospholipids and sphingomyelin in capacitation and the acrosomal reaction. Enferm. Infecc. Microbiol. Clin. 2010, 28, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Craciunescu, C.N.; Guo, Z.; Teng, Y.-W.; Thresher, R.J.; Blusztajn, J.K.; Zeisel, S.H. Deletion of murine choline dehydrogenase results in diminished sperm motility. FASEB J. 2010, 24, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Son, J.H.; Meizel, S. The mouse sperm glycine receptor/chloride channel: Cellular localization and involvement in the acrosome reaction initiated by glycine. J. Androl. 2000, 21, 99–106. [Google Scholar] [PubMed]
- Llanos, M.N.; Ronco, A.M.; Meizel, S.; Aguirre, M.C. Hamster sperm glycine receptor: Evidence for its presence and involvement in the acrosome reaction. Mol. Reprod. Dev. 2000, 58, 205–215. [Google Scholar] [CrossRef]
- Bray, C.; Son, J.-H.; Kumar, P.; Harris, J.D.; Meizel, S. A Role for the human sperm glycine receptor/Cl−Channel in the acrosome reaction initiated by recombinant ZP31. Biol. Reprod. 2002, 66, 91–97. [Google Scholar] [CrossRef]
- Mieusset, R.; Bujan, L.; Mansat, A.; Pontonnier, F.; Grandjean, H.; Chap, H. Glycerophosphocholine in seminal plasma of fertile and infertile men. Int. J. Androl. 1988, 11, 405–413. [Google Scholar] [CrossRef]
- Geer, B. Dietary Choline Requirements for Sperm Motility and Normal Mating Activity in Drosophila melanogaster. Biol. Bull. 1967, 133, 548–566. [Google Scholar] [CrossRef]
- chikawa, T.; Oeda, T.; Ohmori, H.; Schill, W.B. Reactive oxygen species influence the acrosome reaction but not acrosin activity in human spermatozoa. Int. J. Androl. 1999, 22, 37–42. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm cells. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Griveau, J.F.; Le Lannou, D. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int. J. Androl. 1997, 20, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 1–7. [Google Scholar] [CrossRef]
- Gupta, A.; Mahdi, A.A.; Ahmad, M.K.; Shukla, K.K.; Jaiswer, S.P.; Shankhwar, S.N. 1H NMR spectroscopic studies on human seminal plasma: A probative discriminant function analysis classification model. J. Pharm. Biomed. Anal. 2011, 54, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Seguin, F.; Bujan, L.; Barthelemy, C.; Mieusset, R.; Lansac, J. Quantification by magnetic resonance spectroscopy of metabolites in seminal plasma able to differentiate different forms of azoospermia. Hum. Reprod. 1998, 13, 132–135. [Google Scholar] [CrossRef]
- Gupta, A.; Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Bansal, N.; Sankhwar, P.; Sankhwar, S.N. Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: A proton NMR study at 800 MHz. J. Ethnopharmacol. 2013, 149, 208–214. [Google Scholar] [CrossRef]
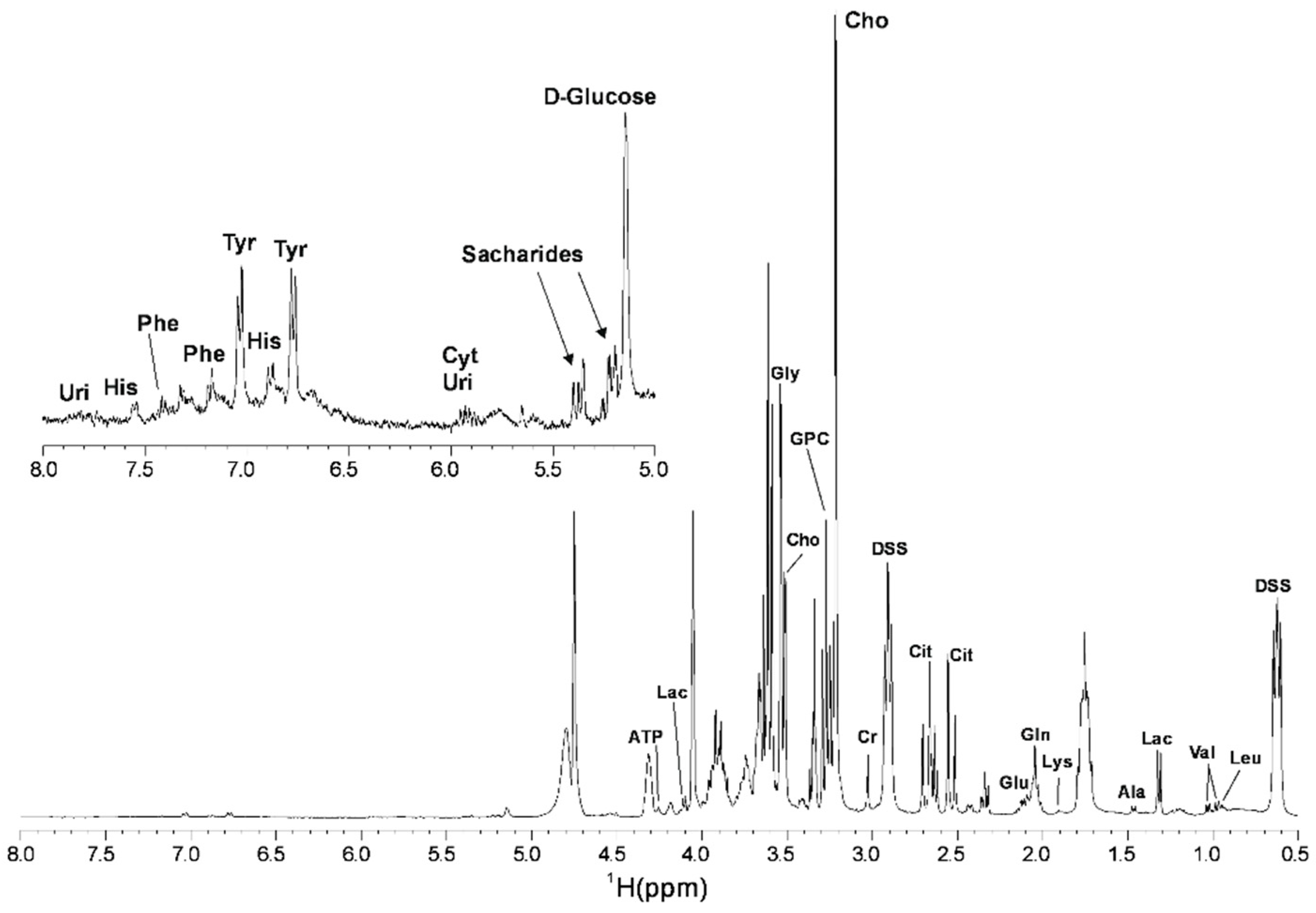

| Metabolite | Chemical Shift (ppm) |
|---|---|
| Uridine | 7.83 |
| Histidine | 7.55 |
| Phenylalanine | 7.41 |
| Phenylalanine | 7.18 |
| Tyrosine | 7.04 |
| Histidine | 6.89 |
| Tyrosine | 6.77 |
| Cytidine | 5.93 |
| Uridine | 5.84 |
| D-Glucose | 5.14 |
| ATP * | 4.25 |
| Lactate | 4.10 |
| Glycine * | 3.54 |
| Choline * | 3.51 |
| GPC * | 3.27 |
| Choline | 3.22 |
| Creatinine * | 3.03 |
| Citrate * | 2.53 |
| Glutamic acid * | 2.11 |
| Glutamine * | 2.05 |
| Lysine | 1.91 |
| Alanine * | 1.47 |
| Lactate * | 1.32 |
| Valine * | 1.03 |
| Valine/Leucine | 0.98 |
| Leucine | 0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo-Otero, Y.; Fernández-López, P.; Gil-Caballero, S.; Fernandez-Fuertes, B.; Bonet, S.; Barranco, I.; Yeste, M. 1H Nuclear Magnetic Resonance of Pig Seminal Plasma Reveals Intra-Ejaculate Variation in Metabolites. Biomolecules 2020, 10, 906. https://doi.org/10.3390/biom10060906
Mateo-Otero Y, Fernández-López P, Gil-Caballero S, Fernandez-Fuertes B, Bonet S, Barranco I, Yeste M. 1H Nuclear Magnetic Resonance of Pig Seminal Plasma Reveals Intra-Ejaculate Variation in Metabolites. Biomolecules. 2020; 10(6):906. https://doi.org/10.3390/biom10060906
Chicago/Turabian StyleMateo-Otero, Yentel, Pol Fernández-López, Sergi Gil-Caballero, Beatriz Fernandez-Fuertes, Sergi Bonet, Isabel Barranco, and Marc Yeste. 2020. "1H Nuclear Magnetic Resonance of Pig Seminal Plasma Reveals Intra-Ejaculate Variation in Metabolites" Biomolecules 10, no. 6: 906. https://doi.org/10.3390/biom10060906
APA StyleMateo-Otero, Y., Fernández-López, P., Gil-Caballero, S., Fernandez-Fuertes, B., Bonet, S., Barranco, I., & Yeste, M. (2020). 1H Nuclear Magnetic Resonance of Pig Seminal Plasma Reveals Intra-Ejaculate Variation in Metabolites. Biomolecules, 10(6), 906. https://doi.org/10.3390/biom10060906






