Cigarette Smoking during Pregnancy: Effects on Antioxidant Enzymes, Metallothionein and Trace Elements in Mother-Newborn Pairs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Sample Collection
2.3. Superoxide Dismutase Analyses
2.4. Glutathione Peroxidase Analyses
2.5. Metallothionein Analyses
2.6. Protein Analysis
2.7. Trace Element Analyses
2.8. Cotinine Analysis
2.9. Statistical Analyses
3. Results
3.1. Maternal and Neonatal General Characteristics
3.2. Comparison of Measured Parameters Between Maternal and Neonatal Compartments
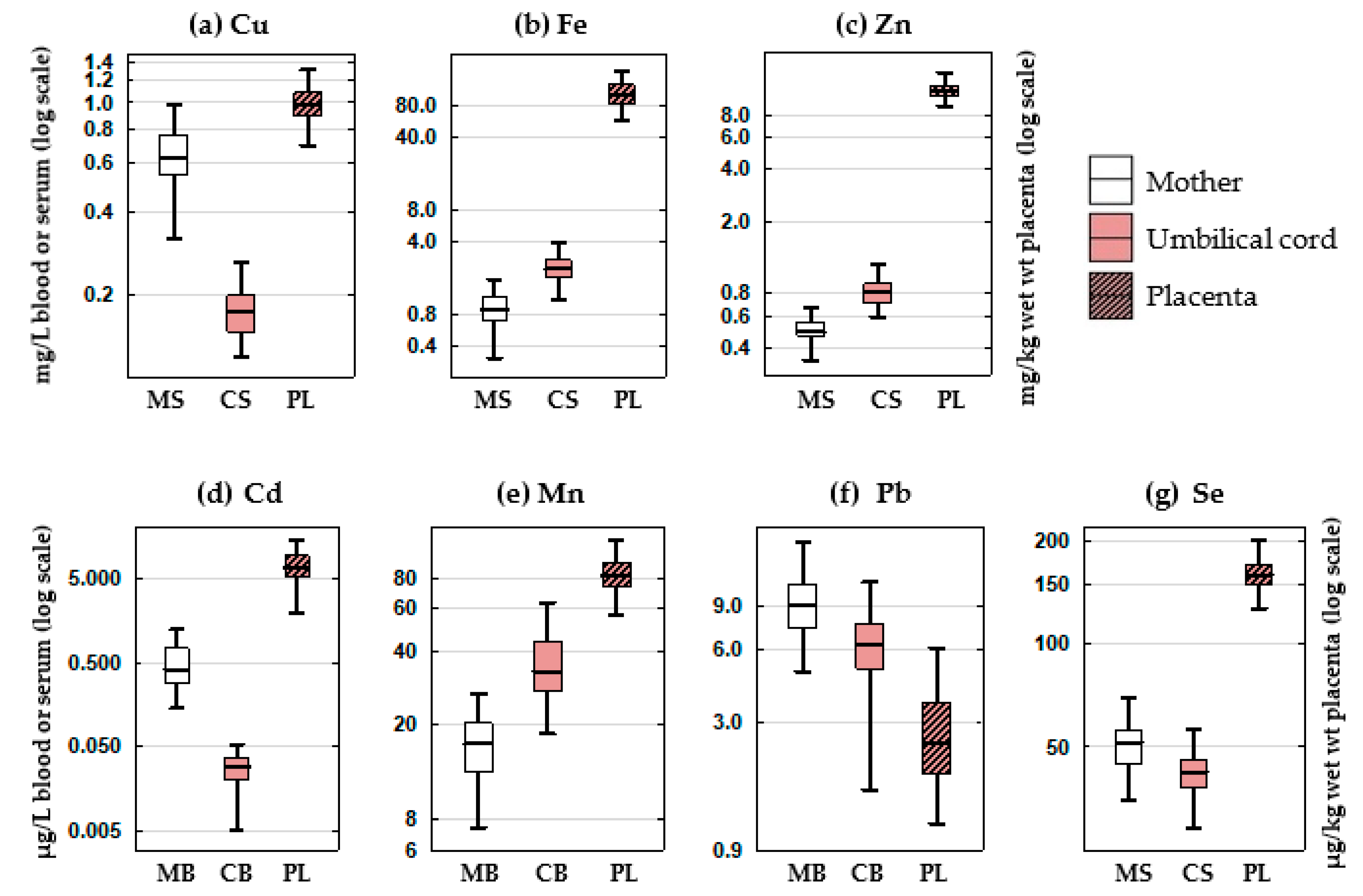
3.2.1. Element Concentrations
3.2.2. SOD and GPx Activity and MT Concentration
3.3. Comparison of Measured Parameters between Smokers and Non-Smokers
3.3.1. Element Concentrations
3.3.2. SOD and GPx Activity and MT Concentration
3.4. Association between Antioxidant Enzymes, MT and Elements
3.4.1. Association of Cd and Pb with Other Elements
3.4.2. Associations between Antioxidant Enzymes and MT
3.4.3. Association of Cd and Pb with SOD, GPx and MT
3.4.4. Identification of the Elements with the Strongest Impact on SOD, GPx and MT
3.4.5. Multiple Regression Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; Carmen González, M.; Arribas, S.M. Implication of oxidative stress in fetal programming of cardiovascular disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Ávila, J.G.O.; Echeverri, I.; de Plata, C.A.; Castillo, A. Impact of oxidative stress during pregnancy on fetal epigenetic patterns and early origin of vascular diseases. Nutr. Rev. 2015, 73, 12–21. [Google Scholar] [CrossRef]
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e769–e776. [Google Scholar] [CrossRef]
- Pintican, D.; Poienar, A.A.; Strilciuc, S.; Mihu, D. Effects of maternal smoking on human placental vascularization: A systematic review. Taiwan J. Obstet. Gynecol. 2019, 58, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Piasek, M.; Michael, H.C.; Blanuša, M.; Kostial, K. Assessment of steroid disruption and metal concentrations in human placenta: Effects of cigarette smoking. In Handbook of Smoking and Health; Koskinen, C.J., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 325–365. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis, and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Kossenjans, W.; Sahay, R.; Eis, A.; Brockman, D. Oxidative stress causes vascular dysfunction in the placenta. J. Matern. Fetal. Med. 2000, 9, 79–82. [Google Scholar] [PubMed]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Bolisetty, S.; Naidoo, D.; Lui, K.; Koh, T.H.H.G.; Watson, D.; Montgomery, R.; Whitehall, J. Postnatal changes in maternal and neonatal plasma antioxidant vitamins and the influence of smoking. Arch. Dis. Child Fetal Neonatal Ed. 2002, 86, F36–F40. [Google Scholar] [CrossRef]
- Chelchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Laskowska-Klita, T.; Leibschang, J. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 132–136. [Google Scholar] [CrossRef]
- Chełchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Lewandowski, L.; Reśko-Zachara, M.; Maciejewski, T.M. Influence of active exposure to tobacco smoke on nitric oxide status of pregnant women. Int. J. Environ. Res. Public Health 2018, 15, 2719. [Google Scholar] [CrossRef]
- Aycicek, A.; Ipek, A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur. J. Pediatr. 2008, 167, 81–85. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Panel on contaminants in the food chain: Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551. [Google Scholar] [CrossRef]
- Knopik, V.S.; MaCcani, M.A.; Francazio, S.; McGeary, J.E. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev. Psychopathol. 2012, 24, 1377–1390. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Milnerowicz, H.; Ściskalska, M.; Dul, M. Pro-inflammatory effects of metals in persons and animals exposed to tobacco smoke. J. Trace Elem. Med. Biol. 2015, 29, 1–10. [Google Scholar] [CrossRef]
- Terrin, G.; Canani, R.B.; di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; de Curtis, M. Zinc in early life: A key element in the fetus and preterm neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef] [PubMed]
- Sabolić, I.; Breljak, D.; Škarica, M.; Herak-Kramberger, C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. BioMetals 2010, 23, 897–926. [Google Scholar] [CrossRef]
- Sekovanić, A.; Jurasović, J.; Piasek, M. Metallothionein 2A gene polymorphisms in relation to diseases and trace element levels in humans. Arh. Hig. Rada Toksikol. 2020, 71, 27–47. [Google Scholar]
- Sekovanić, A.; Jurasović, J.; Piasek, M.; Pašalić, D.; Orct, T.; Sulimanec Grgec, A.; Stasenko, S.; Branović Čakanić, K.; Jazbec, A. Metallothionein 2A gene polymorphism and trace elements in mother-newborn pairs in the Croatian population. J. Trace Elem. Med. Biol. 2018, 45, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bizoń, A.; Milnerowicz-Nabzdyk, E.; Zalewska, M.; Zimmer, M.; Milnerowicz, H. Changes in pro/antioxidant balance in smoking and non-smoking pregnant women with intrauterine growth restriction. Reprod. Toxicol. 2011, 32, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Piasek, M.; Blanuša, M.; Kostial, K.; Laskey, J.W. Placental cadmium and progesterone concentrations in cigarette smokers. Reprod. Toxicol. 2001, 15, 673–681. [Google Scholar] [CrossRef]
- Higgins, S.T.; Heil, S.H.; Badger, G.J.; Mongeon, J.A.; Solomon, L.J.; McHale, L.; Bernstein, I.M. Biochemical verification of smoking status in pregnant and recently postpartum women. Exp. Clin. Psychopharmacol. 2007, 15, 58–66. [Google Scholar] [CrossRef]
- Stragierowicz, J.; Mikołajewska, K.; Zawadzka-Stolarz, M.; Polańska, K.; Ligocka, D. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. BioMed Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Skroch, P.; Buchman, C.; Karin, M. Regulation of human and yeast metallothionein gene transcription by heavy metal ions. Prog. Clin. Biol. Res. 1993, 380, 113–128. [Google Scholar]
- Ronco, A.M.; Garrido, F.; Llanos, M.N. Smoking specifically induces metallothionein-2 isoform in human placenta at term. Toxicology 2006, 223, 46–53. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Živković, T.; Tariba, B.; Pizent, A. Multielement analysis of human seminal plasma by octopole reaction cell ICP-MS. J. Anal. Atom. Spectrom. 2014, 29, 2114–2126. [Google Scholar] [CrossRef]
- Brčić Karačonji, I.; Skender, L.; Karačić, V. Determination of nicotine and cotinine in urine by headspace solid phase microextraction gas chromatography with mass spectrometric detection. Acta Chim. Slov. 2007, 54, 74–78. [Google Scholar]
- Clemmensen, L.; Hastie, T.; Witten, D.; Ersboll, B. Sparse discriminant analysis. Technometrics 2011, 53, 406–413. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry ATSDR. Toxicological Profile for Lead; Department of Health and Human Services, Public Health Service, Center for Disease Control: Atlanta, GA, USA, 2019. Available online: https://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=93&tid=22 (accessed on 9 June 2020).
- Agency for Toxic Substances and Disease Registry ATSDR. Toxicological Profile for Cadmium; Department of Health and Human Services, Public Health Service, Center for Disease Control: Atlanta, GA, USA, 2012. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15 (accessed on 9 June 2020).
- Stasenko, S.; Bradford, E.M.; Piasek, M.; Henson, M.C.; Varnai, V.M.; Jurasović, J.; Kušec, V. Metals in human placenta: Focus on the effects of cadmium on steroid hormones and leptin. J. Appl. Toxicol. 2010, 30, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Piasek, M.; Jurasović, J.; Sekovanić, A.; Brajenović, N.; Brčić Karačonji, I.; Mikolić, A.; Sulimanec Grgec, A.; Stasenko, S. Placental cadmium as an additional non-invasive bioindicator of active maternal tobacco smoking. J. Toxicol. Environ. Health Part A 2016, 79, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Hengstschläger, M. The role of the placenta in fetal exposure to heavy metals. Wien Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Hoque, A.M.W.; Raqib, R.; Öhrvik, H.; Ekström, E.C.; Vahter, M. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol. Lett. 2010, 192, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Tekin, D.; Kayaaltı, Z.; Aliyev, V.; Söylemezoğlu, T. The effects of metallothionein 2A polymorphism on placental cadmium accumulation: Is metallothionein a modifying factor in transfer of micronutrients to the fetus? J. Appl. Toxicol. 2012, 32, 270–275. [Google Scholar] [CrossRef]
- Sakamoto, M.; Yasutake, A.; Domingo, J.L.; Chan, H.M.; Kubota, M.; Murata, K. Relationships between trace element concentrations in chorionic tissue of placenta and umbilical cord tissue: Potential use as indicators for prenatal exposure. Environ. Int. 2013, 60, 106–111. [Google Scholar] [CrossRef]
- Phuapittayalert, L.; Saenganantakarn, P.; Supanpaiboon, W.; Cheunchoojit, S.; Hipkaeo, W.; Sakulsak, N. Increasing CACNA1C expression in placenta containing high Cd level: An implication of Cd toxicity. Environ. Sci. Pollut. Res. Int. 2016, 23, 24592–24600. [Google Scholar] [CrossRef]
- Ronco, A.M.; Arguello, G.; Suazo, M.; Llanos, M.N. Increased levels of metallothionein in placenta of smokers. Toxicology 2005, 208, 133–139. [Google Scholar] [CrossRef]
- Sorkun, H.C.; Bir, F.; Akbulut, M.; Divrikli, U.; Erken, G.; Demirhan, H.; Duzcan, E.; Elci, L.; Celik, I.; Yozgatli, U. The effects of air pollution and smoking on placental cadmium, zinc concentration and metallothionein expression. Toxicology 2007, 238, 15–22. [Google Scholar] [CrossRef]
- Esteban-Vasallo, M.D.; Aragonés, N.; Pollan, M.; López-Abente, G.; Perez-Gomez, B. Mercury, cadmium, and lead levels in human placenta: A systematic review. Environ. Health Perspect. 2012, 120, 1369–1377. [Google Scholar] [CrossRef]
- Brako, E.E.; Wilson, A.K.; Jonah, M.M.; Blum, C.A.; Cerny, E.A.; Williams, K.L.; Bhattacharyya, M.H. Cadmium pathways during gestation and lactation in control versus metallothionein 1,2-knockout mice. Toxicol. Sci. 2003, 71, 154–163. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohba, K.; Ohta, H. Participation of metal transporters in cadmium transport from mother rat to fetus. J. Toxicol. Sci. 2012, 37, 1035–1044. [Google Scholar] [CrossRef]
- Jacobo-Estrada, T.; Santoyo-Sánchez, M.; Thévenod, F.; Barbier, O. Cadmium handling, toxicity and molecular targets involved during pregnancy: Lessons from experimental models. Int. J. Mol. Sci. 2017, 18, 1590. [Google Scholar] [CrossRef] [PubMed]
- Espart, A.; Artime, S.; Tort-Nasarre, G.; Yara-Varón, E. Cadmium exposure during pregnancy and lactation: Materno-fetal and newborn repercussions of Cd(ii), and Cd-metallothionein complexes. Metallomics 2018, 10, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Metallothioneins: Historical Development and Overview. In Metal Ions in Life Sciences; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Royal Society of Chemistry: London, UK, 2009; Volume 5, pp. 1–29. [Google Scholar]
- Goyer, R.A. Transplacental transport of lead. Environ. Health Perspect. 1990, 89, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Tekin, D.; Kayaaltı, Z.; Söylemezoğlu, T. The effects of metallothionein 2A polymorphism on lead metabolism: Are pregnant women with a heterozygote genotype for metallothionein 2A polymorphism and their new-borns at risk of having higher blood lead levels? Int. Arch. Occup. Environ. Health 2012, 85, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Skerfving, S.; Bergdahl, I. Lead. In Handbook on the Toxicology of Metals, Specific Metals; Nordberg, G.F., Fowler, B., Nordberg, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Vahter, M.; Berglund, M.; Akesson, A.; Lidén, C. Metals and women’s health. Environ. Res. 2002, 88, 145–155. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress Part I: Mechanisms involved in metal induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Carri, M.T.; Galiazzo, F.; Ciriolo, M.R.; Rotilio, G. Evidence for co-regulation of Cu, Zn superoxide dismutase and metallothionein gene expression in yeast through transcriptional control by copper via the ACE 1 factor. FEBS Lett. 1991, 278, 263–266. [Google Scholar] [CrossRef]
- Ikebuchi, H.; Teshima, R.; Suzuki, K.; Terao, T.; Yamane, Y. Simultaneous induction of Pb-metallothionein-like protein and Zn-thionein in the liver of rats given lead acetate. Biochem. J. 1986, 233, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Palacios, Ò.; Leiva-Presa, À.; Atrian, S.; Lobinski, R. A study of the Pb (II) binding to recombinant mouse Zn7-metallothionein 1 and its domains by ESI TOF MS. Talanta 2007, 72, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Sabolić, I.; Breljak, D.; Herak-Kramberger, C.M.; Ljubojević, M. Cadmium and metallothionein. In Encyclopedia of Metalloproteins; Springer: New York, NY, USA, 2013; pp. 342–352. [Google Scholar]
- Furukawa, S.; Usuda, K.; Abe, M.; Hayashi, S.; Ogawa, I. Histological expression of metallothionein in the developing rat placenta. J. Toxicol. Pathol. 2008, 21, 223–227. [Google Scholar] [CrossRef][Green Version]
- Kowalska, K.; Bizoń, A.; Zalewska, M.; Milnerowicz, H. The influence of biological and environmental factors on metallothionein concentration in the blood. J. Trace Elem. Med. Biol. 2015, 29, 99–103. [Google Scholar] [CrossRef]
- Kantola, M.; Purkunen, R.; Kröger, P.; Tooming, A.; Juravskaja, J.; Pasanen, M.; Saarikoski, S.; Vartiainen, T. Accumulation of cadmium, zinc, and copper in maternal blood and developmental placental tissue: Differences between Finland, Estonia, and St. Petersburg. Environ. Res. 2000, 83, 54–66. [Google Scholar] [CrossRef]
- Pateva, I.B.; Kerling, E.H.; Reddy, M.; Chen, D.; Carlson, S.E.; Tancabelic, J. Effect of maternal cigarette smoking on newborn iron stores. Clin. Res. Trials 2015, 1, 4–7. [Google Scholar] [CrossRef]
- Alberg, A.J. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology 2002, 180, 121–137. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A.; Thurnham, D.I. Monitoring micronutrients in cigarette smokers. Clin. Chim. Acta 2007, 377, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Bo, Q.L.; Ji, Y.L.; Liu, L.; Hu, Y.F.; Chen, Y.H.; Zhang, J.; Zhao, L.L.; Xu, D.X. Maternal cadmium exposure reduces placental zinc transport and induces fetal growth restriction in mice. Reprod. Toxicol. 2016, 63, 174–182. [Google Scholar] [CrossRef]
- Jansson, L.T.; Perkkiö, M.V.; Willis, W.T.; Refino, C.J.; Dallman, P.R. Red cell superoxide dismutase is increased in iron deficient anemia. Acta Haematol. 1985, 74, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Jamba, L.; Nehru, B.; Bansal, M.P. Redox modulation of selenium binding proteins by cadmium exposures in mice. Mol. Cell Biochem. 1997, 177, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.F.; Yan, X.F.; Guo, F.Z.; Sun, N.Y.; Qian, Z.J.; Ding, D.Y. Effects of cigarette smoking and smoking cessation on plasma constituents and enzyme activities related to oxidative stress. Biomed. Environ. Sci. 2000, 13, 44–55. [Google Scholar] [PubMed]
- Aydogan, U.; Durmaz, E.; Ercan, C.M.; Eken, A.; Ulutas, O.K.; Kavuk, S.; Gursel, O.; Alanbay, I.; Akay, C.; Kurekci, A.E.; et al. Effects of smoking during pregnancy on DNA damage and ROS level consequences in maternal and newborn’s blood. Arh. Hig. Rada Toksikol. 2013, 64, 35–46. [Google Scholar] [CrossRef][Green Version]
- Ermis, B.; Yildirim, A.; Örs, R.; Tastekin, A.; Ozkan, B.; Akcay, F. Influence of smoking on serum and milk malondialdehyde, superoxide dismutase, glutathione peroxidase, and antioxidant potential levels in mothers at the postpartum seventh day. Biol. Trace Elem. Res. 2005, 105, 27–36. [Google Scholar] [CrossRef]
- Ermis, B.; Ors, R.; Yildirim, A.; Tastekin, A.; Kardas, F.; Akcay, F. Influence of smoking on maternal and neonatal serum malondialdehyde, superoxide dismutase, and glutathione peroxidase levels. Ann. Clin. Lab. Sci. 2004, 34, 405–409. [Google Scholar]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Qanungo, S.; Mukherjea, M. Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol. Cell Biochem. 2000, 215, 11–19. [Google Scholar] [CrossRef]
- Cerutti, P.; Ghosh, R.; Oya, Y.; Amstad, P. The role of the cellular antioxidant defence in oxidant carcinogenesis. Environ. Health Perspect. 1994, 102, 123–129. [Google Scholar] [PubMed]
| All (n = 74) | Non-Smokers (n = 37) | Smokers (n = 37) | p2 | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age (years) | 31 ± 5 | 33 ± 5 | 30 ± 5 | 0.003 |
| Education 3 | <0.001 | |||
| Primary school | 4 (5.41%) | 1 (2.70%) | 3 (8.11%) | |
| Secondary school | 33 (44.59%) | 8 (21.62%) | 25 (67.57%) | |
| University degree | 35 (47.30%) | 28 (75.68%) | 7 (18.92%) | |
| BMI before pregnancy (kg/m2) | 23.15 (14.69–36.59) | 23.26 (18.37–36.33) | 23.05 (14.69–36.59) | n.s. |
| BMI before delivery (kg/m2) | 28.13 (19.83–41.52) | 27.89 (22.57–41.52) | 28.31 (19.83–38.64) | n.s. |
| Weight gain during pregnancy (kg) | 14.29 ± 4.63 | 13.88 ± 4.18 | 14.70 ± 5.06 | n.s. |
| Parity | 2 (1–6) | 2 (1–6) | 2 (1–6) | n.s. |
| Urinary cotinine (ng/mL) | 1730 (<LOQ–7723) | <LOQ | 955 (101–7723) | <0.001 |
| Newborn characteristics | ||||
| Male | 52 (70) | 26 (70) | 26 (70) | n.s. |
| Birth weight (g) | 3494 ± 496 | 3558 ± 486 | 3430 ± 503 | n.s. |
| Birth body length (cm) | 50.47 ± 2.02 | 50.59 ± 1.77 | 50.35 ± 2.25 | n.s. |
| Birth weight/placental weight | 8.74 (6.49–14.91) | 9.19 (6.49–14.91) | 8.23 (6.98–11.70) | 0.017 |
| Placental characteristic | ||||
| Trimmed weight (g) | 401 ± 85.1 | 394 ± 90.2 | 408 ± 80.4 | n.s. |
| All (n = 74) | Non-Smokers (n = 37) | Smokers (n = 37) | p2 | |
|---|---|---|---|---|
| SOD (U/mL) | ||||
| Maternal plasma | 2.26 (2.00–2.67) | 2.42 (2.18–2.66) | 2.08 (1.87–2.60) | 0.025 |
| Cord plasma | 3.74 (3.40–4.26) | 4.13 (3.59–4.48) | 3.64 (3.04–3.81) | 0.004 |
| Placenta | 21.00 (8.09–39.52) | 16.36 (6.14–32.65) | 31.27 (12.44–43.38) | 0.047 |
| GPx (nmol/min/mL) | ||||
| Maternal plasma | 69.76 (58.23–80.04) | 70.22 (62.92–77.55) | 63.60 (56.10–81.84) | n.s. |
| Cord plasma | 41.33 (35.01–46.10) | 41.50 (36.76–46.75) | 39.90 (34.19–46.03) | n.s. |
| Placenta | 20.83 (16.93–24.36) | 20.36 (17.62–23.84) | 21.00 (15.11–26.36) | n.s. |
| MT (ng/mL) | ||||
| Maternal serum | 3.13 (2.46–4.01) | 3.16 (2.43–3.55) | 3.09 (2.56–4.21) | n.s. |
| Cord serum | 35.14 (30.54–42.48) | 34.03 (30.63–41.28) | 36.63 (29.91–42.64) | n.s. |
| Placenta | 178.5 (148.2–442.0) | 174.6 (156.1–251.0) | 197.8 (143.8–271.6) | n.s. |
| Dependent Variable | β [95% Confidence Interval] | p | Adj. R 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Element 1 | Element 2 | Element 3 | Element 4 | Smoking | Age | Education | |||
| log(SOD_MP) | 0.680 [0.144, 1.216] p = 0.014 | Fe_MS 0.133 [−0.022, 0.288] p = 0.092 | Mn_MB −0.006 [−0.018, 0.006] p = 0.340 | Pb_PL −0.017 [−0.036, 0.003] p = 0.087 | Zn_MS 0.049 [−0.762, 0.861] p = 0.904 | −0.052 [−0.210, 0.105] p = 0.508 | 0.007 [−0.007, 0.021] p = 0.296 | −0.060 [−0.214, 0.095] p = 0.445 | 0.081 | 0.09 |
| log(SOD_CP) | 1.527 [0.865, 2.189] p < 0.001 | Cd_CB −2.742 [−7.237, 1.753] p = 0.227 | Mn_MB −0.010 [−0.021, 0.001] p = 0.085 | Mn_PL 0.002 [−0.002, 0.006] p = 0.433 | Zn_PL −0.010 [−0.066, 0.046] p = 0.716 | 0.001 [−0.010, 0.012] p = 0.851 | −0.015 [−0.132, 0.102] p = 0.799 | 0.624 | <0.01 | |
| log(SOD_PL + 10) | 5.175 [3.489, 6.862] p < 0.001 | Cu_CS −2.013 [−4.899, 0.874] p = 0.168 | Fe_PL −0.017 [−0.020, -0.013] p < 0.001 | Se_PL 0.007 [0.000, 0.013] p = 0.043 | Zn_PL −0.024 [−0.131, 0.082] p = 0.650 | 0.082 [−0.173, 0.338] p = 0.521 | −0.012 [−0.034, 0.009] p = 0.243 | 0.012 [−0.239, 0.263] p = 0.923 | <0.001 | 0.66 |
| log(GPx_MP) | 3.203 [2.500, 3.905] p < 0.001 | Fe_CS −0.001 [−0.036, 0.033] p = 0.944 | Se_MS 0.002 [−0.004, 0.008] p = 0.526 | Se_CS 0.004 [−0.007, 0.015] p = 0.479 | Se_PL 0.004 [0.000, 0.008] p = 0.057 | −0.030 [−0.188, 0.128] p = 0.705 | 0.005 [−0.008, 0.018] p = 0.473 | −0.041 [−0.194, 0.112] p = 0.596 | 0.099 | 0.08 |
| log(GPx_CP) | 2.353 [1.645, 3.060] p < 0.001 | Cd_PL 0.003 [−0.011, 0.018] p = 0.637 | Cu_CS 2.726 [1.361, 4.090] p < 0.001 | Se_PL 0.004 [0.001, 0.007] p = 0.014 | Zn_PL 0.029 [−0.019, 0.078] p = 0.233 | −0.002 [−0.012, 0.008] p = 0.708 | −0.028 [−0.137, 0.080] p = 0.603 | <0.001 | 0.24 | |
| log(GPx_PL) | 2.374 [1.468, 3.280] p < 0.001 | Cd_PL −0.006 [−0.028, 0.015] p = 0.550 | Fe_MS −0.016 [−0.194, 0.163] p = 0.863 | Mn_MB −0.006 [−0.021, 0.008] p = 0.371 | Zn_PL 0.082 [0.014, 0.151] p = 0.019 | −0.003 [−0.018, 0.012] p = 0.702 | 0.036 [−0.128, 0.199] p = 0.666 | 0.253 | 0.03 | |
| log(MT_MS+5) | 1.946 [1.610, 2.282] p < 0.001 | Cd_CB 0.625 [−2.811, 4.061] p = 0.718 | Fe_CS 0.002 [−0.028, 0.033] p = 0.871 | Mn_PL 0.003 [0.000, 0.005] p = 0.061 | Pb_MB −0.006 [−0.015, 0.003] p = 0.211 | −0.001 [−0.009, 0.007] p = 0.857 | −0.007 [−0.092, 0.077] p = 0.868 | 0.382 | <0.01 | |
| log(MT_CP) | 3.845 [3.213, 4.477] p < 0.001 | Cu_PL −0.403 [−0.767, −0.038] p = 0.031 | Fe_PL −0.001 [−0.003, 0.001] p = 0.164 | Se_MS 0.001 [−0.005, 0.007] p = 0.752 | Zn_MS 0.186 [−0.628, 1.000] p = 0.650 | 0.039 [−0.100, 0.179] p = 0.575 | 0.003 [−0.010, 0.015] p = 0.654 | 0.047 [−0.095, 0.189] p = 0.512 | 0.338 | 0.02 |
| log(MT_PL) | 4.895 [3.859, 5.931] p < 0.001 | Fe_MS 0.212 [0.003, 0.422] p = 0.047 | Fe_PL −0.004 [−0.007, −0.002] p = 0.002 | Mn_PL 0.004 [−0.001, 0.009] p = 0.149 | Se_PL 0.003 [−0.002, 0.008] p = 0.259 | 0.117 [−0.092, 0.326] p = 0.268 | -0.009 [−0.026, 0.009] p = 0.325 | 0.076 [−0.127, 0.280] p = 0.455 | <0.001 | 0.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizent, A.; Lazarus, M.; Kovačić, J.; Tariba Lovaković, B.; Brčić Karačonji, I.; Živković Semren, T.; Sekovanić, A.; Orct, T.; Branović-Čakanić, K.; Brajenović, N.; et al. Cigarette Smoking during Pregnancy: Effects on Antioxidant Enzymes, Metallothionein and Trace Elements in Mother-Newborn Pairs. Biomolecules 2020, 10, 892. https://doi.org/10.3390/biom10060892
Pizent A, Lazarus M, Kovačić J, Tariba Lovaković B, Brčić Karačonji I, Živković Semren T, Sekovanić A, Orct T, Branović-Čakanić K, Brajenović N, et al. Cigarette Smoking during Pregnancy: Effects on Antioxidant Enzymes, Metallothionein and Trace Elements in Mother-Newborn Pairs. Biomolecules. 2020; 10(6):892. https://doi.org/10.3390/biom10060892
Chicago/Turabian StylePizent, Alica, Maja Lazarus, Jelena Kovačić, Blanka Tariba Lovaković, Irena Brčić Karačonji, Tanja Živković Semren, Ankica Sekovanić, Tatjana Orct, Karmen Branović-Čakanić, Nataša Brajenović, and et al. 2020. "Cigarette Smoking during Pregnancy: Effects on Antioxidant Enzymes, Metallothionein and Trace Elements in Mother-Newborn Pairs" Biomolecules 10, no. 6: 892. https://doi.org/10.3390/biom10060892
APA StylePizent, A., Lazarus, M., Kovačić, J., Tariba Lovaković, B., Brčić Karačonji, I., Živković Semren, T., Sekovanić, A., Orct, T., Branović-Čakanić, K., Brajenović, N., Jurič, A., Miškulin, I., Škrgatić, L., Stasenko, S., Mioč, T., Jurasović, J., & Piasek, M. (2020). Cigarette Smoking during Pregnancy: Effects on Antioxidant Enzymes, Metallothionein and Trace Elements in Mother-Newborn Pairs. Biomolecules, 10(6), 892. https://doi.org/10.3390/biom10060892








