Cholinergic Stress Signals Accompany MicroRNA-Associated Stereotypic Behavior and Glutamatergic Neuromodulation in the Prefrontal Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Behavioural Tests
2.2. Histology and Immunolabeling
2.3. Measurements of Open Field Motor Activity
2.4. Telemetric Measurement of Motor Activity
2.5. Vestibular Test
2.6. Forced Swimming
2.7. Small RNA-Sequencing
2.8. Transcriptomic Analysis
2.9. Quantitative PCR (qPCR)
2.10. Statistical Analyses
3. Results
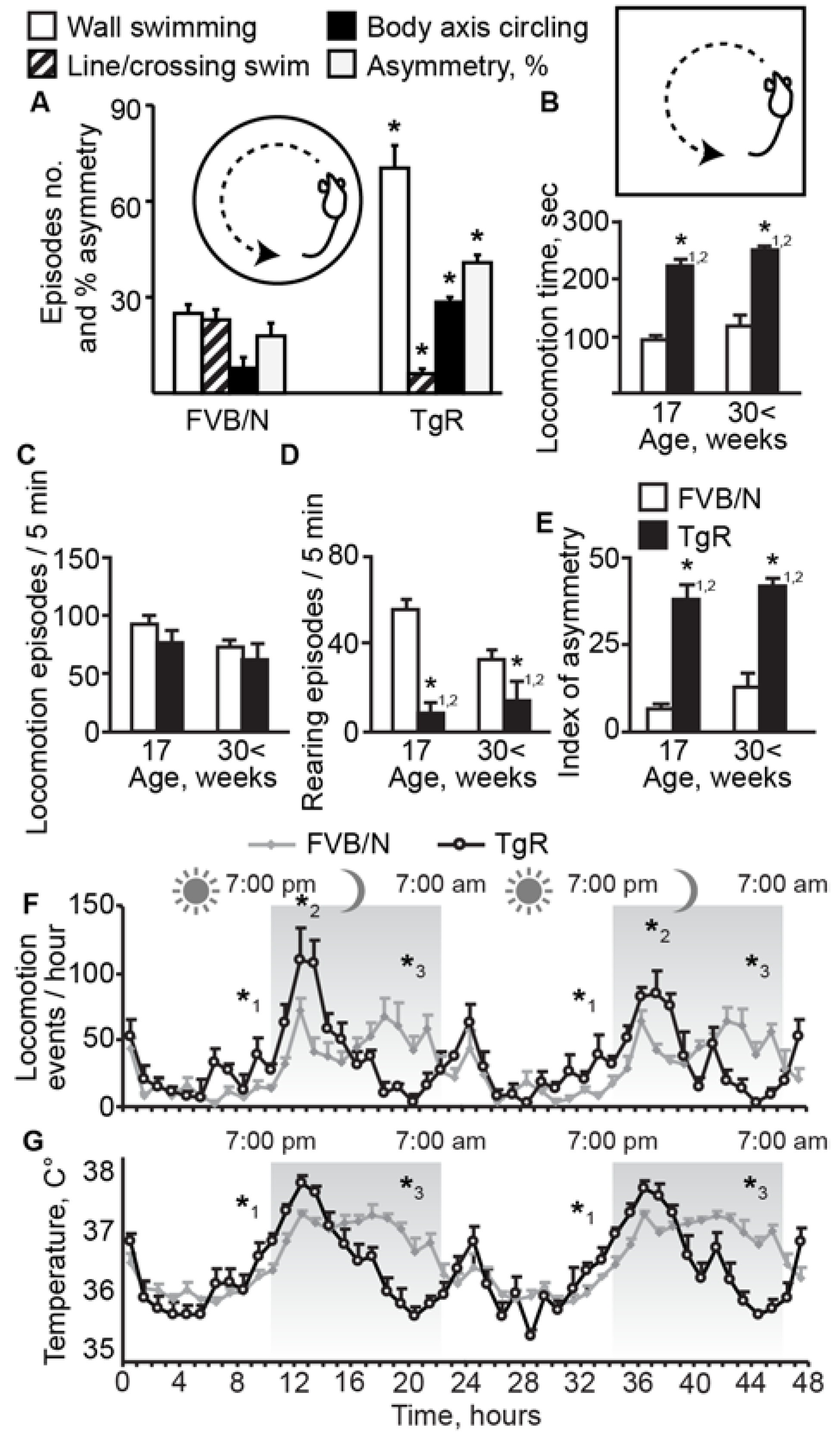
3.1. TgR Mice Show Clear SB Characteristics
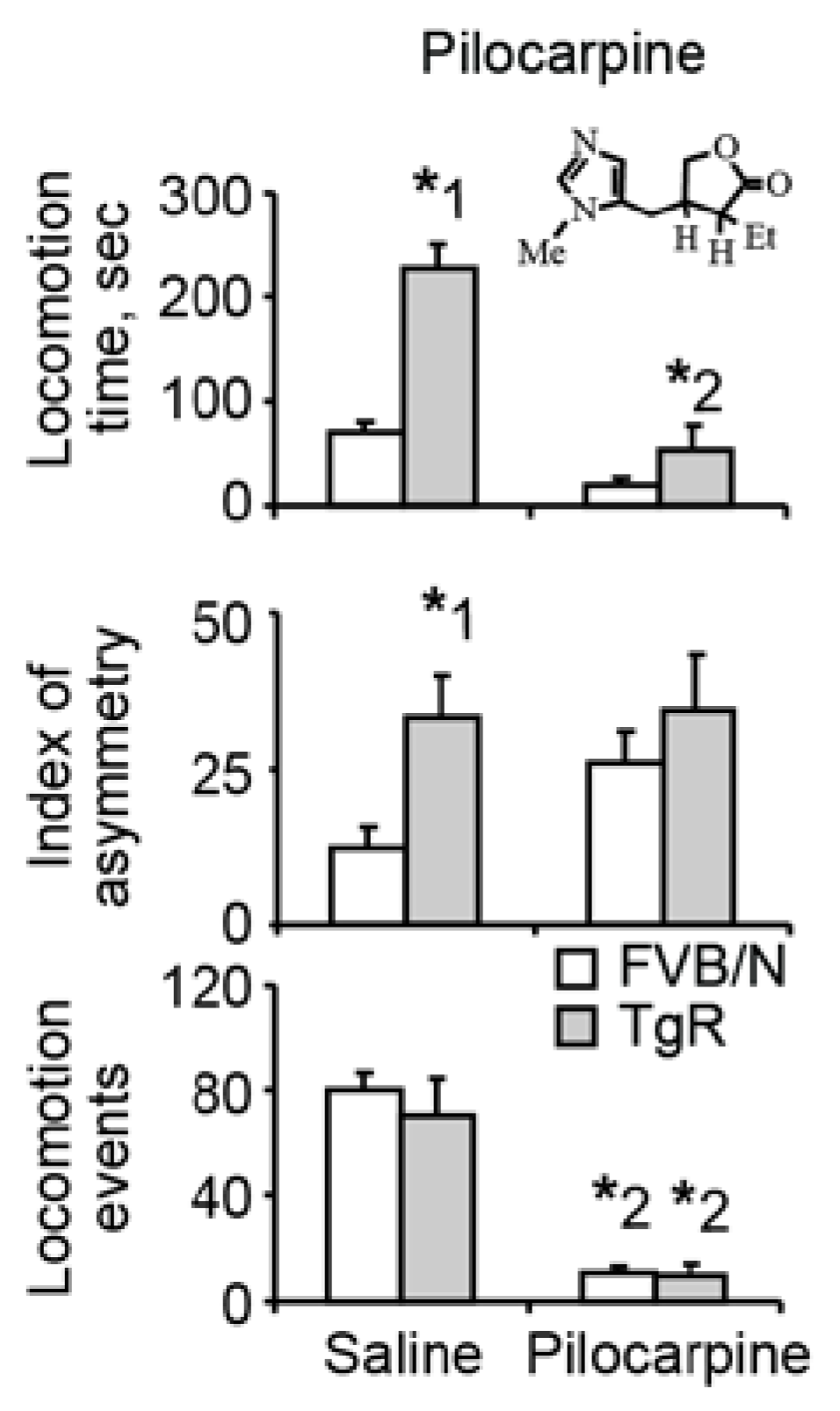
3.2. Muscarinic Stimulation Suppresses the Motor Hyperactivity but not the Locomotor Asymmetry Component of SB
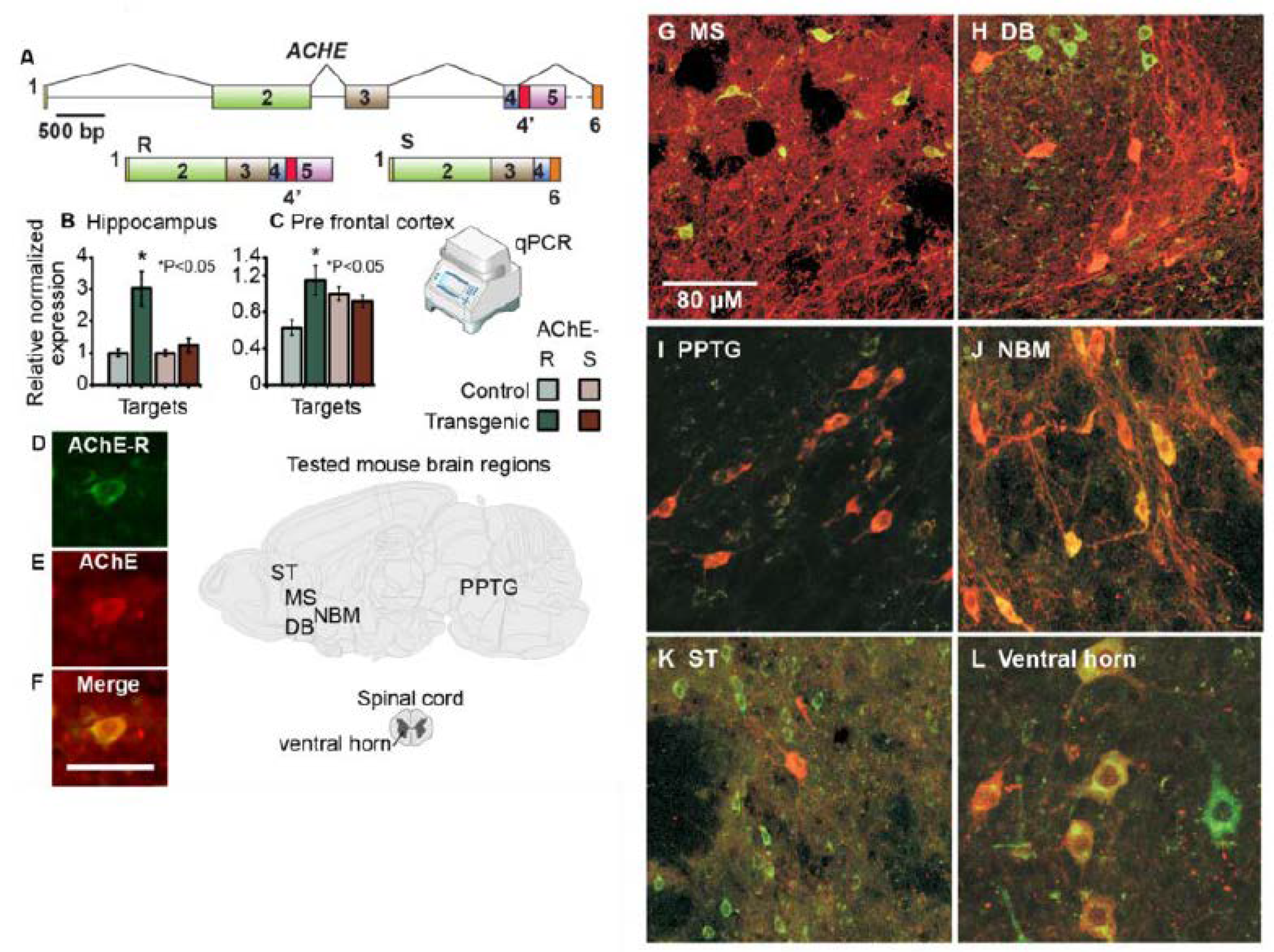
3.3. hAChE-R Excess Does Not Disrupt Normal Cholinergic and Glutamatergic Pathways and Is Inversely Correlated with ChAT Expression
3.4. AChE-R Distribution in Motor-Regulating Brain Regions
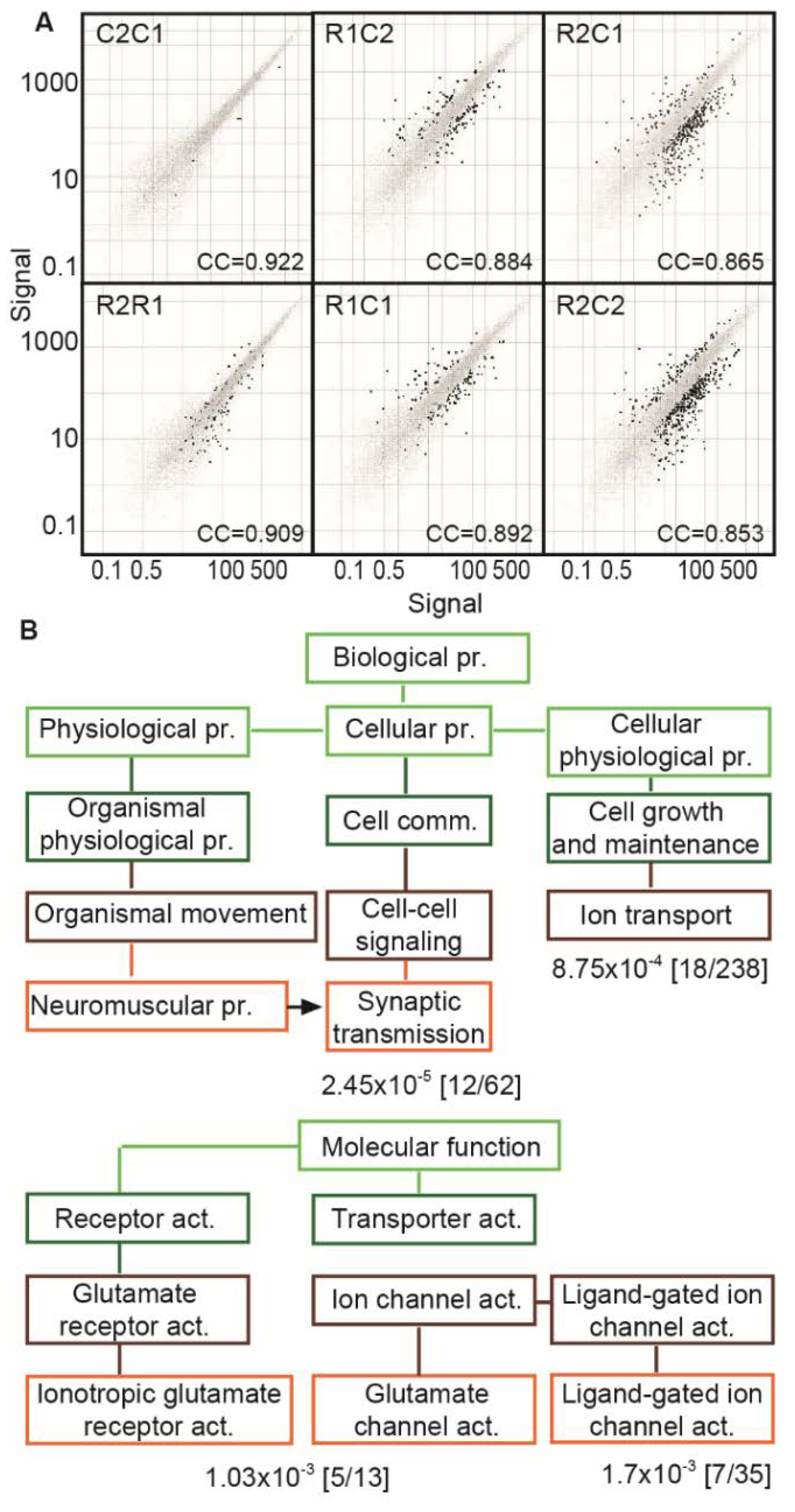
3.5. RNA-Seq Reveals Modified CholinomiRs in TgR Hippocampi
3.6. The TgR PFC Shows More Variable Cholinergic Transcripts than the Caudate Putamen
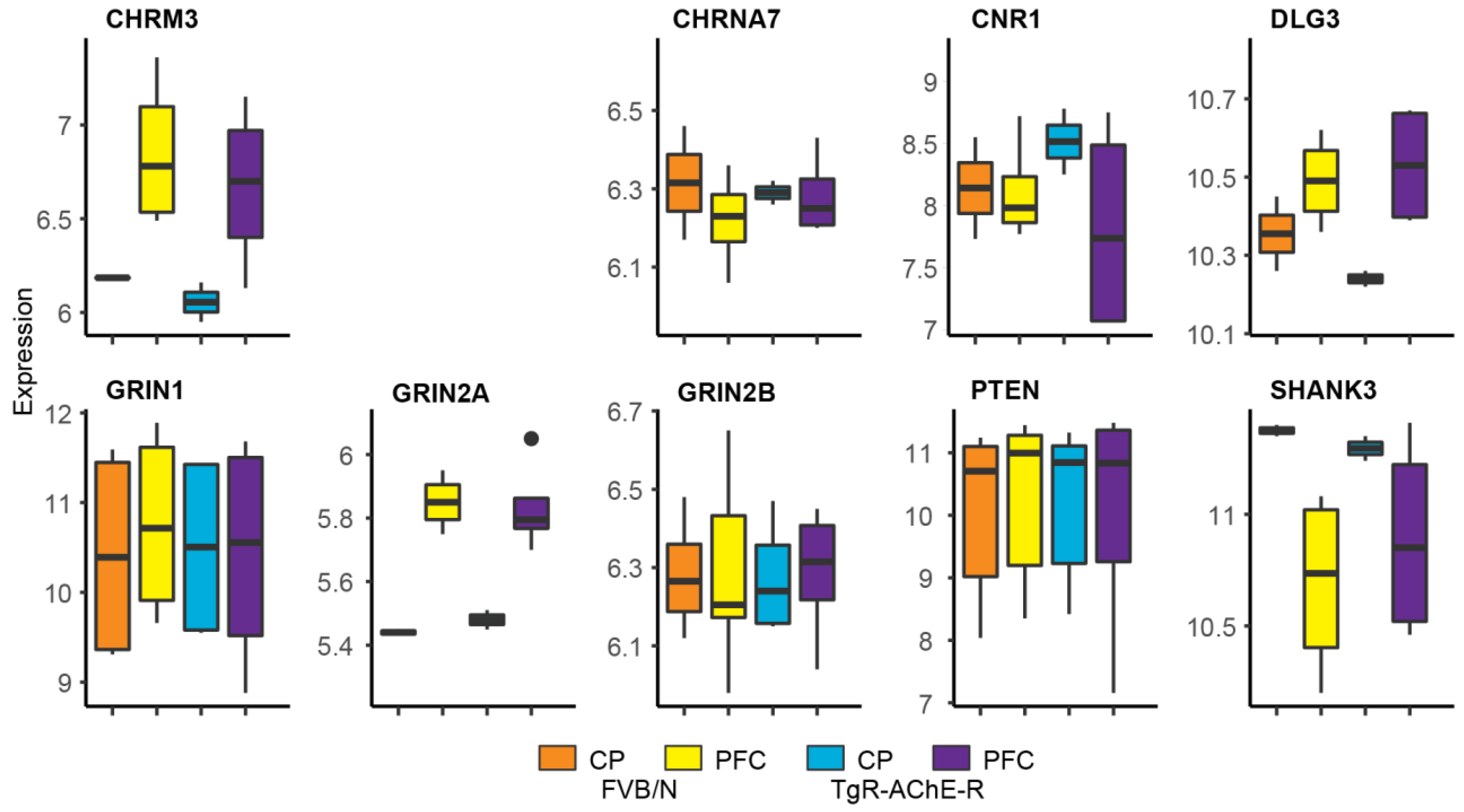
3.7. Suppressed Glutamatergic Neurotransmission-Related Genes in the TgR PFC
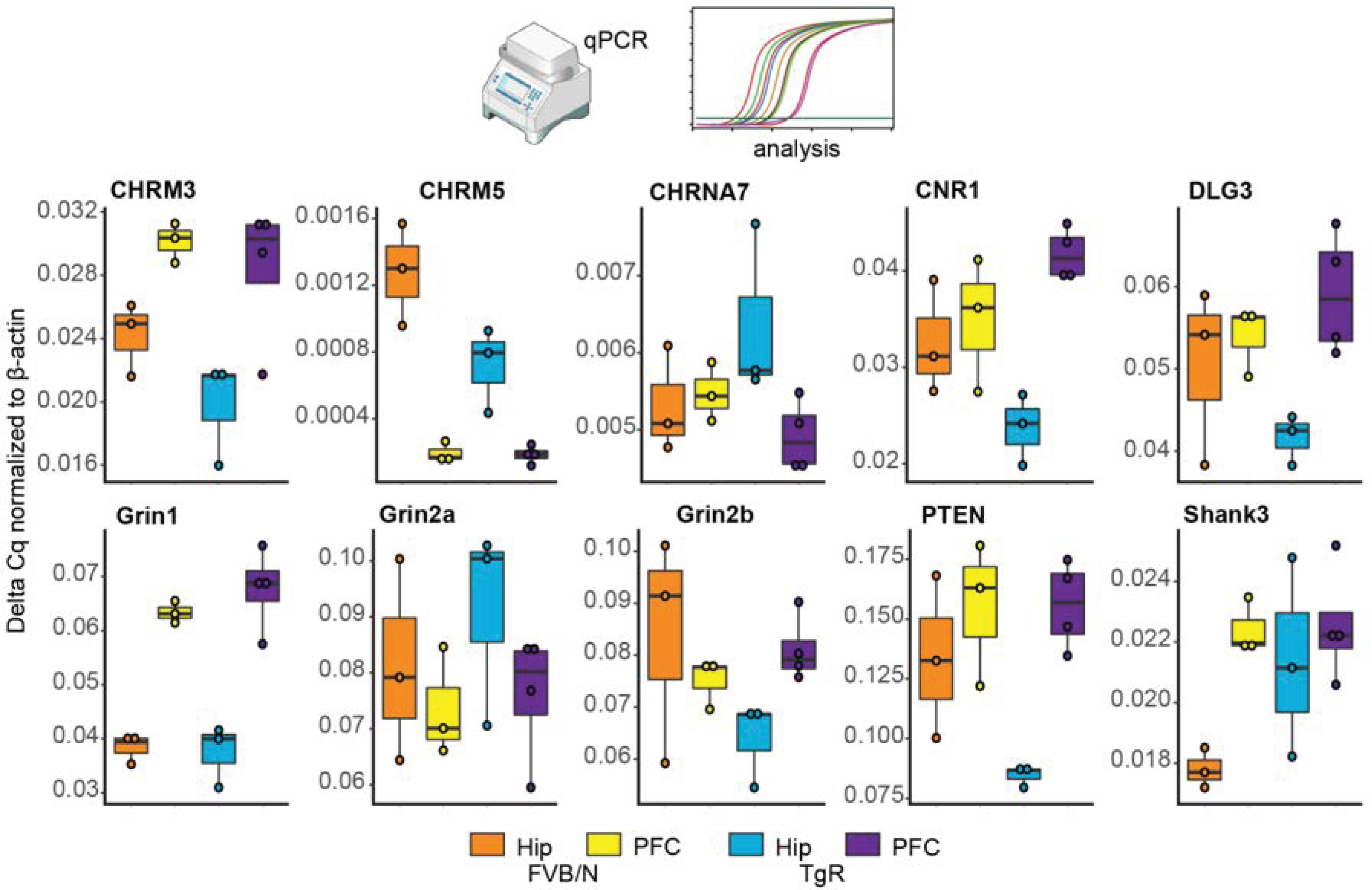
3.8. Validating Brain Region-Dependent Changes in Cholinergic and SB-Related Transcripts
4. Discussion
4.1. Modified Hippocampal miRNAs in TgR Mice with Altered Cholinergic Activities
4.2. Prefrontal Cortex and Striatal Transcriptomics of TgR vs FVB/N Mice
4.3. Implications for SB Studies
4.4. Clinical Relevance to Human SB
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, A.; Salgado, M.; Fahn, S. The characterization and outcome of stereotypical movements in nonautistic children. Mov. Disord. 1997, 12, 47–52. [Google Scholar] [CrossRef]
- Stern, E.; Silbersweig, D.A.; Chee, K.Y.; Holmes, A.; Robertson, M.M.; Trimble, M.; Frith, C.D.; Frackowiak, R.S.; Dolan, R.J. A functional neuroanatomy of tics in Tourette syndrome. Arch. Gen. Psychiatry 2000, 57, 741–748. [Google Scholar] [CrossRef]
- Peralta, V.; Cuesta, M.J. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophr. Res. 2001, 47, 107–116. [Google Scholar] [CrossRef]
- Pierce, K.; Courchesne, E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry 2001, 49, 655–664. [Google Scholar] [CrossRef]
- Turner, M. Annotation: Repetitive behaviour in autism: A review of psychological research. J. Child Psychol. Psychiatry 1999, 40, 839–849. [Google Scholar] [CrossRef]
- Kenworthy, L.; Charnas, L. Evidence for a discrete behavioral phenotype in the oculocerebrorenal syndrome of Lowe. Am. J. Med. Genet. 1995, 59, 283–290. [Google Scholar] [CrossRef]
- Kaytser, V.; Berry-Kravis, E.; Ouyang, B.; Doll, E.; Hall, D.A. Motor stereotypies in fragile X syndrome. J. Pediatr. Neurol. 2014, 12, 29–34. [Google Scholar] [CrossRef]
- Neul, J.L.; Zoghbi, H.Y. Rett syndrome: A prototypical neurodevelopmental disorder. Neuroscientist 2004, 10, 118–128. [Google Scholar] [CrossRef]
- Sadock, B.J.; Kaplan, H.I.; Sadock, V.A. Kaplan—Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; p. 1460. [Google Scholar]
- McGrath, M.J.; Campbell, K.M.; Parks, C.R.; Burton, F.H. Glutamatergic drugs exacerbate symptomatic behavior in a transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder. Brain Res. 2000, 877, 23–30. [Google Scholar] [CrossRef]
- Saka, E.; Graybiel, A.M. Pathophysiology of Tourette’s syndrome: Striatal pathways revisited. Brain Dev. 2003, 25, S15–S19. [Google Scholar] [CrossRef]
- Hoekstra, P.J.; Steenhuis, M.P.; Kallenberg, C.G.; Minderaa, R.B. Association of small life events with self reports of tic severity in pediatric and adult tic disorder patients: A prospective longitudinal study. J. Clin. Psychiatry 2004, 65, 426–431. [Google Scholar] [CrossRef]
- Wood, B.L.; Klebba, K.; Gbadebo, O.; Lichter, D.; Kurlan, R.; Miller, B. Pilot study of effect of emotional stimuli on tic severity in children with Tourette’s syndrome. Mov. Disord. 2003, 18, 1392–1395. [Google Scholar] [CrossRef]
- McKeown, K.A.; Moreno, R.; Hall, V.L.; Ribera, A.B.; Downes, G.B. Disruption of Eaat2b, a glutamate transporter, results in abnormal motor behaviors in developing zebrafish. Dev. Biol 2012, 362, 162–171. [Google Scholar] [CrossRef]
- Kim, J.; Yang, J.H.; Ryu, I.S.; Sohn, S.; Kim, S.; Choe, E.S. Interactions of Glutamatergic Neurotransmission and Brain-Derived Neurotrophic Factor in the Regulation of Behaviors after Nicotine Administration. Int. J. Mol. Sci. 2019, 20, 2943. [Google Scholar] [CrossRef]
- Canales, J.J.; Graybiel, A.M. A measure of striatal function predicts motor stereotypy. Nat. Neurosci. 2000, 3, 377–383. [Google Scholar] [CrossRef]
- Garner, J.P.; Mason, G.J. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behav. Brain Res. 2002, 136, 83–92. [Google Scholar] [CrossRef]
- Mason, W.A. Effects of social interaction on well-being: Development aspects. Lab. Anim. Sci. 1991, 41, 323–328. [Google Scholar]
- Wei, Q.; Lu, X.Y.; Liu, L.; Schafer, G.; Shieh, K.R.; Burke, S.; Robinson, T.E.; Watson, S.J.; Seasholtz, A.F.; Akil, H. Glucocorticoid receptor overexpression in forebrain: A mouse model of increased emotional lability. Proc. Natl. Acad. Sci. USA 2004, 101, 11851–11856. [Google Scholar] [CrossRef]
- Wurbel, H.; Stauffacher, M. Prevention of stereotypy in laboratory mice: Effects on stress physiology and behaviour. Physiol. Behav. 1996, 59, 1163–1170. [Google Scholar] [CrossRef]
- Powell, S.B.; Bodfish, J.W.; Parker, D.E.; Crawford, T.W.; Golden, R.N.; Lewis, M.H. Growth differences associated with compulsive and stereotyped behavior disorders in adults with mental retardation. Anxiety 1996, 2, 90–94. [Google Scholar] [CrossRef]
- Verhoeven, W.M.; Tuinier, S.; van den Berg, Y.W.; Coppus, A.M.; Fekkes, D.; Pepplinkhuizen, L.; Thijssen, J.H. Stress and self-injurious behavior; hormonal and serotonergic parameters in mentally retarded subjects. Pharmacopsychiatry 1999, 32, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef]
- Bloomfield, M.A.; McCutcheon, R.A.; Kempton, M.; Freeman, T.P.; Howes, O. The effects of psychosocial stress on dopaminergic function and the acute stress response. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.L.; Metherate, R.; Ashe, J.H. Modulation of cellular excitability in neocortex: Muscarinic receptor and second messenger-mediated actions of acetylcholine. Synapse 1994, 16, 123–136. [Google Scholar] [CrossRef]
- Day, L.B.; Schallert, T. Anticholinergic effects on acquisition of place learning in the Morris water task: Spatial mapping deficit or inability to inhibit nonplace strategies? Behav. Neurosci. 1996, 110, 998–1005. [Google Scholar] [CrossRef]
- Kaneko, S.; Hikida, T.; Watanabe, D.; Ichinose, H.; Nagatsu, T.; Kreitman, R.J.; Pastan, I.; Nakanishi, S. Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science 2000, 289, 633–637. [Google Scholar] [CrossRef]
- Kaufer, D.; Friedman, A.; Seidman, S.; Soreq, H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 1998, 393, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Meshorer, E.; Erb, C.; Gazit, R.; Pavlovsky, L.; Kaufer, D.; Friedman, A.; Glick, D.; Ben-Arie, N.; Soreq, H. Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science 2002, 295, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Meshorer, E.; Soreq, H. Virtues and woes of AChE alternative splicing in stress-related neuropathologies. Trends Neurosci. 2006, 29, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Picconi, B.; Passino, E.; Sgobio, C.; Bonsi, P.; Barone, I.; Ghiglieri, V.; Pisani, A.; Bernardi, G.; Ammassari-Teule, M.; Calabresi, P. Plastic and behavioral abnormalities in experimental Huntington’s disease: A crucial role for cholinergic interneurons. Neurobiol. Dis. 2006, 22, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sklan, E.H.; Berson, A.; Birikh, K.R.; Gutnick, A.; Shahar, O.; Shoham, S.; Soreq, H. Acetylcholinesterase Modulates Stress-Induced Motor Responses Through Catalytic and Noncatalytic Properties. Biol. Psychiatry 2006. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 2015, 38, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Tang, C.Z.; Zhang, D.F.; Yang, J.T.; Liu, Q.H.; Wang, Y.R.; Wang, W.S. Overexpression of microRNA-301b accelerates hippocampal microglia activation and cognitive impairment in mice with depressive-like behavior through the NF-kappaB signaling pathway. Cell Death Dis. 2019, 10, 316. [Google Scholar] [CrossRef]
- Tavakolizadeh, J.; Roshanaei, K.; Salmaninejad, A.; Yari, R.; Nahand, J.S.; Sarkarizi, H.K.; Mousavi, S.M.; Salarinia, R.; Rahmati, M.; Mousavi, S.F.; et al. MicroRNAs and exosomes in depression: Potential diagnostic biomarkers. J. Cell Biochem. 2018, 119, 3783–3797. [Google Scholar] [CrossRef]
- Ching, A.S.; Ahmad-Annuar, A. A Perspective on the Role of microRNA-128 Regulation in Mental and Behavioral Disorders. Front. Cell Neurosci. 2015, 9, 465. [Google Scholar] [CrossRef]
- Nadorp, B.; Soreq, H. Predicted overlapping microRNA regulators of acetylcholine packaging and degradation in neuroinflammation-related disorders. Front. Mol. Neurosci. 2014, 7, 9. [Google Scholar] [CrossRef]
- Allman, J.M.; Hakeem, A.; Erwin, J.M.; Nimchinsky, E.; Hof, P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann. N. Y. Acad. Sci. 2001, 935, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; McNaughton, N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System, 2nd ed.; Oxford University Press: New York, NY, USA, 2000; p. 424. [Google Scholar]
- Haber, S.N.; Fudge, J.L.; McFarland, N.R. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000, 20, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Emotion, memory and the brain. Sci. Am. 1994, 270, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Keay, K.A.; Bandler, R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci. Biobehav. Rev. 2001, 25, 669–678. [Google Scholar] [CrossRef]
- Kaczmarek, L.; Chaudhuri, A. Sensory regulation of immediate-early gene expression in mammalian visual cortex: Implications for functional mapping and neural plasticity. Brain Res. Brain Res. Rev. 1997, 23, 237–256. [Google Scholar] [CrossRef]
- Dori, A.; Cohen, J.; Silverman, W.F.; Pollack, Y.; Soreq, H. Functional manipulations of acetylcholinesterase splice variants highlight alternative splicing contributions to murine neocortical development. Cereb. Cortex 2005, 15, 419–430. [Google Scholar] [CrossRef]
- Cohen, O.; Erb, C.; Ginzberg, D.; Pollak, Y.; Seidman, S.; Shoham, S.; Yirmiya, R.; Soreq, H. Neuronal overexpression of ‘readthrough’ acetylcholinesterase is associated with antisense-suppressible behavioral impairments. Mol. Psychiatry 2002, 7, 874–885. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 12 January 2010).
- Wang, W.C.; Lin, F.M.; Chang, W.C.; Lin, K.Y.; Huang, H.D.; Lin, N.S. miRExpress: Analyzing high-throughput sequencing data for profiling microRNA expression. BMC Bioinform. 2009, 10, 328. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; Team R.C: Vienna, Austria, 2013. [Google Scholar]
- Beissbarth, T.; Speed, T.P. GOstat: Find statistically overrepresented Gene Ontologies within a group of genes. Bioinformatics 2004, 20, 1464–1465. [Google Scholar] [CrossRef]
- Chanales, A.J.H.; Oza, A.; Favila, S.E.; Kuhl, B.A. Overlap among Spatial Memories Triggers Repulsion of Hippocampal Representations. Curr. Biol. 2017, 27, 2307–2317. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Obayemi, A.; Wigestrand, M.B.; Fote, G.M.; Calarco, C.A.; Li, A.M.; Picciotto, M.R. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety-and depression-like behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 3573–3578. [Google Scholar] [CrossRef]
- Burke, R.E.; Karanas, A.L. Demonstration of a medial to lateral gradient in the density of cholinergic neuropil in the rat striatum. Neurosci. Lett. 1990, 108, 58–64. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Kreitzer, A.C. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology 2012, 27, 167–177. [Google Scholar] [CrossRef]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. The basal ganglia communicate with the cerebellum. Proc. Natl. Acad. Sci. USA 2010, 107, 8452–8456. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. NeuroPsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Cho, Y.T.; Ernst, M.; Fudge, J.L. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J. Neurosci. 2013, 33, 14017–14030. [Google Scholar] [CrossRef]
- Anderson, M.C.; Bunce, J.G.; Barbas, H. Prefrontal-hippocampal pathways underlying inhibitory control over memory. Neurobiol. Learn. Memory 2016, 134, 145–161. [Google Scholar] [CrossRef]
- Tewari, A.; Jog, R.; Jog, M.S. The Striatum and Subthalamic Nucleus as Independent and Collaborative Structures in Motor Control. Front. Syst. Neurosci. 2016, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Bullitt, E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J. Comp. Neurol. 1990, 296, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Van Elzakker, M.; Fevurly, R.D.; Breindel, T.; Spencer, R.L. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn. Memory 2008, 15, 899–908. [Google Scholar] [CrossRef]
- Montgomery, S.M.; Betancur, M.I.; Buzsaki, G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J. Neurosci. 2009, 29, 1381–1394. [Google Scholar] [CrossRef]
- Degroot, A.; Treit, D. Dorsal and ventral hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Brain Res. 2002, 949, 60–70. [Google Scholar] [CrossRef]
- Shaltiel, G.; Hanan, M.; Wolf, Y.; Barbash, S.; Kovalev, E.; Shoham, S.; Soreq, H. Hippocampal microRNA-132 mediates stress-inducible cognitive deficits through its acetylcholinesterase target. Brain Struct. Funct. 2013, 218, 59–72. [Google Scholar] [CrossRef]
- Hou, W.Z.; Chen, X.L.; Wu, W.; Hang, C.H. MicroRNA-370-3p inhibits human vascular smooth muscle cell proliferation via targeting KDR/AKT signaling pathway in cerebral aneurysm. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1080–1087. [Google Scholar]
- Gu, Y.; Becker, V.; Zhao, Y.; Menger, M.D.; Laschke, M.W. miR-370 inhibits the angiogenic activity of endothelial cells by targeting smoothened (SMO) and bone morphogenetic protein (BMP)-2. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 7213–7224. [Google Scholar] [CrossRef]
- Wa, Q.; Huang, S.; Pan, J.; Tang, Y.; He, S.; Fu, X.; Peng, X.; Chen, X.; Yang, C.; Ren, D.; et al. miR-204-5p Represses Bone Metastasis via Inactivating NF-kappaB Signaling in Prostate Cancer. Mol. Ther. Nucleic Acids 2019, 18, 567–579. [Google Scholar] [CrossRef]
- Hebert, S.S.; De Strooper, B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009, 32, 199–206. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Furey, M.L.; Pietrini, P.; Haxby, J.V. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science 2000, 290, 2315–2319. [Google Scholar] [CrossRef]
- Pittenger, C.; Kandel, E. A genetic switch for long-term memory. C. R. Acad. Sci. III 1998, 321, 91–96. [Google Scholar] [CrossRef]
- Farchi, N.; Shoham, S.; Hochner, B.; Soreq, H. Impaired hippocampal plasticity and errors in cognitive performance in mice with maladaptive neuronal splice site selection. Eur. J. Neurochem. 2007, 25, 87–98. [Google Scholar]
- Schoch, S.; Deak, F.; Konigstorfer, A.; Mozhayeva, M.; Sara, Y.; Sudhof, T.C.; Kavalali, E.T. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 2001, 294, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- De Ferrari, G.V.; Inestrosa, N.C. Wnt signaling function in Alzheimer’s disease. Brain Res. Brain Res. Rev. 2000, 33, 1–12. [Google Scholar] [CrossRef]
- Simantov, R. Multiple molecular and neuropharmacological effects of MDMA (Ecstasy). Life Sci. 2004, 74, 803–814. [Google Scholar] [CrossRef]
- Conti, F.; Minelli, A.; Melone, M. GABA transporters in the mammalian cerebral cortex: Localization, development and pathological implications. Brain Res. Brain Res. Rev. 2004, 45, 196–212. [Google Scholar] [CrossRef]
- Garty, H.; Lindzen, M.; Fuzesi, M.; Aizman, R.; Goldshleger, R.; Asher, C.; Karlish, S.J. A specific functional interaction between CHIF and Na,K-ATPase: Role of FXYD proteins in the cellular regulation of the pump. Ann. N. Y. Acad. Sci. 2003, 986, 395–400. [Google Scholar] [CrossRef]
- Pearl, P.L.; Gibson, K.M. Clinical aspects of the disorders of GABA metabolism in children. Curr. Opin. Neurol. 2004, 17, 107–113. [Google Scholar] [CrossRef]
- Contractor, A.; Heinemann, S.F. Glutamate receptor trafficking in synaptic plasticity. Sci. STKE 2002, 2002, RE14. [Google Scholar] [CrossRef]
- Dong, H.; Xiang, Y.Y.; Farchi, N.; Ju, W.; Wu, Y.; Chen, L.; Wang, Y.; Hochner, B.; Yang, B.; Soreq, H.; et al. Excessive expression of acetylcholinesterase impairs glutamatergic synaptogenesis in hippocampal neurons. J. Neurosci. 2004, 24, 8950–8960. [Google Scholar] [CrossRef]
- Farchi, N.; Ofek, K.; Podoly, E.; Dong, H.; Xiang, Y.-Y.; Diamant, S.; Livnah, O.; Hochner, B.; Lu, W.-Y.; Soreq, H. Peripheral site acetylcholinesterase blockade induces RACK-1 associated neuronal modeling. in press. Neurodegener. Dis. 2007, 4, 171–184. [Google Scholar] [CrossRef]
- Niwa, Y.; Kanda, G.N.; Yamada, R.G.; Shi, S.; Sunagawa, G.A.; Ukai-Tadenuma, M.; Fujishima, H.; Matsumoto, N.; Masumoto, K.H.; Nagano, M.; et al. Muscarinic Acetylcholine Receptors Chrm1 and Chrm3 Are Essential for REM Sleep. Cell Rep. 2018, 24, 2231–2247. [Google Scholar] [CrossRef]
- Sarter, M.; Hasselmo, M.E.; Bruno, J.P.; Givens, B. Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev. 2005, 48, 98–111. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.; Guo, H.; Ou, J.; Peng, Y.; Liu, Q.; Shen, Y.; Shi, L.; Liu, Y.; Xiong, Z.; et al. Association of genetic variants of GRIN2B with autism. Sci. Rep. 2015, 5, 8296. [Google Scholar] [CrossRef]
- Buxbaum, J.D.; Cai, G.; Chaste, P.; Nygren, G.; Goldsmith, J.; Reichert, J.; Anckarsater, H.; Rastam, M.; Smith, C.J.; Silverman, J.M.; et al. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am. J. Med. Genet. Part B NeuroPsychiatry Genet. Off. Publ. Int. Soc. Psychiatry Genet. 2007, 144B, 484–491. [Google Scholar] [CrossRef]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef]
- Amal, H.; Barak, B.; Bhat, V.; Gong, G.; Joughin, B.A.; Wang, X.; Wishnok, J.S.; Feng, G.; Tannenbaum, S.R. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol. Psychiatry 2018. [Google Scholar] [CrossRef]
- Mongeau, R.; Miller, G.A.; Chiang, E.; Anderson, D.J. Neural correlates of competing fear behaviors evoked by an innately aversive stimulus. J. Neurosci. 2003, 23, 3855–3868. [Google Scholar] [CrossRef]
- Neveu, P.J.; Moya, S. In the mouse, the corticoid stress response depends on lateralization. Brain Res. 1997, 749, 344–346. [Google Scholar] [CrossRef]
- Lobentanzer, S.; Hanin, G.; Klein, J.; Soreq, H. Integrative Transcriptomics Reveals Sexually Dimorphic Control of the Cholinergic/Neurokine Interface in Schizophrenia and Bipolar Disorder. Cell Rep. 2019, 29, 764–777. [Google Scholar] [CrossRef]
- Dudai, A.; Yayon, N.; Lerner, V.; Tasaka, G.; Deitcher, Y.; Niederhoffer, N.; Mizrahi, A.; Soreq, H.; London, M. Functional characterization of cortical ChAT/VIP interneurons and their effect on the circuit in vivo. PLoS Biol. 2020, in press. [Google Scholar]
- Birikh, K.R.; Sklan, E.H.; Shoham, S.; Soreq, H. Interaction of “readthrough” acetylcholinesterase with RACK1 and PKCbeta II correlates with intensified fear-induced conflict behavior. Proc. Natl. Acad. Sci. USA 2003, 100, 283–288. [Google Scholar] [CrossRef]
- Nijholt, I.; Farchi, N.; Kye, M.; Sklan, E.H.; Shoham, S.; Verbeure, B.; Owen, D.; Hochner, B.; Spiess, J.; Soreq, H.; et al. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol. Psychiatry 2004, 9, 174–183. [Google Scholar] [CrossRef]
- Graybiel, A.M.; Canales, J.J.; Capper-Loup, C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: A new hypothesis. Trends Neurosci. 2000, 23, S71–S77. [Google Scholar] [CrossRef]
- Jeffries, K.J.; Schooler, C.; Schoenbach, C.; Herscovitch, P.; Chase, T.N.; Braun, A.R. The functional neuroanatomy of Tourette’s syndrome: An FDG PET study III: Functional coupling of regional cerebral metabolic rates. Neuropsychopharmacology 2002, 27, 92–104. [Google Scholar] [CrossRef]
- Chappell, P.; Leckman, J.; Goodman, W.; Bissette, G.; Pauls, D.; Anderson, G.; Riddle, M.; Scahill, L.; McDougle, C.; Cohen, D. Elevated cerebrospinal fluid corticotropin-releasing factor in Tourette’s syndrome: Comparison to obsessive compulsive disorder and normal controls. Biol. Psychiatry 1996, 39, 776–783. [Google Scholar] [CrossRef]
- Moriarty, J.; Costa, D.C.; Schmitz, B.; Trimble, M.R.; Ell, P.J.; Robertson, M.M. Brain perfusion abnormalities in Gilles de la Tourette’s syndrome. Br. J. Psychiatry 1995, 167, 249–254. [Google Scholar] [CrossRef]
- Peterson, B.S.; Skudlarski, P.; Anderson, A.W.; Zhang, H.; Gatenby, J.C.; Lacadie, C.M.; Leckman, J.F.; Gore, J.C. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch. Gen. Psychiatry 1998, 55, 326–333. [Google Scholar] [CrossRef]
- Cabib, S.; Giardino, L.; Calza, L.; Zanni, M.; Mele, A.; Puglisi-Allegra, S. Stress promotes major changes in dopamine receptor densities within the mesoaccumbens and nigrostriatal systems. Neuroscience 1998, 84, 193–200. [Google Scholar] [CrossRef]
- Bracha, H.S. Asymmetric rotational (circling) behavior, a dopamine-related asymmetry: Preliminary findings in unmedicated and never-medicated schizophrenic patients. Biol. Psychiatry 1987, 22, 995–1003. [Google Scholar] [CrossRef]
- Sklan, E.H.; Lowenthal, A.; Korner, M.; Ritov, Y.; Landers, D.M.; Rankinen, T.; Bouchard, C.; Leon, A.S.; Rice, T.; Rao, D.C.; et al. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in Health, Risk Factors, Exercise Training, and Genetics study. Proc. Natl. Acad. Sci. USA 2004, 101, 5512–5517. [Google Scholar] [CrossRef]


| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| Shank3 | CCGGACCTGCAACAAACGA | GCGCGTCTTGAAGGCTATGAT |
| Pten | TGGATTCGACTTAGACTTGACCT | GCGGTGTCATAATGTCTCTCAG |
| CHRM3 | CCTCGCCTTTGTTTCCCAAC | TTGAGGAGAAATTCCCAGAGGT |
| CHRM5 | CCTCTACACGACCTACATCCTC | GTATGTCAGTGGTCTTGTGATGG |
| CHRNA7 | CACATTCCACACCAACGTCTT | AAAAGGGAACCAGCGTACATC |
| CNR1 | GTGTTCCACCGCAAAGATAGT | GCCTGTGAATGGATATGTACCTG |
| Grin2b | CAGCAAAGCTCGTTCCCAAAA | GTCAGTCTCGTTCATGGCTAC |
| Grin2a | ACGTGACAGAACGCGAACTT | TCAGTGCGGTTCATCAATAACG |
| Grin1 | AGAGCCCGACCCTAAAAAGAA | CCCTCCTCCCTCTCAATAGC |
| DLG3 | AACAGATCGGTGTGATCCCTA | CTGTCCTGGCATGGAACTTCA |
| AChE-S | CTGAACCTGAAGCCCTTAGAG | CCGCCTCGTCCAGAGTAT |
| AChE-R | CTGAACCTGAAGCCCTTAGAG | GGGGAGGTGGAGAAGAGAG |
| Beta-actin | CCACACCCGCCACCAGTT | TACAGCCCGGGGAGCAT |
| RPL-19 | GATTGACCGCCATATGTATCAC | GTCAGCCAGGAGCTTCTTG |
| Test Paradigm | Variable | F Value | Degrees of Freedom | p Value | Significant Post Hoc N.K. Tests, p < 0.05 |
|---|---|---|---|---|---|
| 1. Open field | Age 17 weeks | ||||
| Locomotion time | 16.5 | (2.16) | 0.0001 | TgR-H > TgR-L, FVB/N | |
| Locomotion episodes | N.S. | ||||
| Locomotor asymmetry | 31.7 | (2.16) | 0.0001 | TgR-H > TgR-L, FVB/N | |
| Rearing | 29.6 | (2.16) | 0.005 | TgR-H < TgR-L, FVB/N | |
| Age 30 weeks | |||||
| Locomotion time | 19.9 | (2.32) | 0.0001 | TgR-H > TgR-L, FVB/N | |
| Locomotion episodes | N.S. | ||||
| Locomotor asymmetry | 20.7 | (2.32) | 0.0001 | TgR-H > TgR-L, FVB/N | |
| Rearing | 7.8 | (2.32) | 0.005 | TgR-H < TgR-L, FVB/N | |
| 2. Forced swim | Swims along wall | 18.6 | (2.29) | 0.0001 | TgR-H > TgR-L, FVB/N |
| Swims across pool | 10.5 | (2.29) | 0.0005 | TgR-H < TgR-L, FVB/N | |
| Body-wise circling | 9.2 | (2.29) | 0.0005 | TgR-H > TgR-L, FVB/N | |
| % Asymmetry | 21.1 | (2.29) | 0.0001 | TgR-H > TgR-L, FVB/N | |
| 3. Serial choice maze | Right/left choice errors | 19.1 | (2.47) | 0.0001 | TgR-H > TgR-L>FVB/N |
| Retrace errors | 14.0 | (2.47) | 0.001 | TgR-H > FVB/N | |
| Errorless runs | 11.0 | (2.47) | 0.0001 | TgR-H < FVB/N |
| Biological Activity | Circadian Time Interval | F Value | Degrees of Freedom | p Value | Significant Post Hoc N.K. Tests, p < 0.05 |
|---|---|---|---|---|---|
| 1. Locomotion | Light phase, 1st half | N.S. | |||
| Light phase, 2nd half | 11.2 | (2.21) | 0.0005 | TgR-H > TgR-L, FVB/N | |
| Dark phase, 1st half | 11.8 | (2.21) | 0.0005 | TgR-H > TgR-L, FVB/N | |
| Dark phase, 2nd half | 42 | (2.21) | 0.0001 | FVB/N > TgR-H, TgR-L | |
| 2. Temperature | Light phase, 1st half | 4.3 | (2.21) | 0.03 | FVB/N > TgR-H, TgR-L |
| Light phase, 2nd half | 7.5 | (2.21) | 0.0001 | FVB/N < TgR-H, TgR-L | |
| Dark phase, 1st half | N.S. | ||||
| Dark phase, 2nd half | 38 | (2.21) | 0.0005 | FVB/N > TgR-H, TgR-L | |
| 3. Circadian correlation: locomotion vs. temperature | Over the entire 24 h cycle | N.S. | |||
| Light phase, 1st half | N.S. | ||||
| Light phase, 2nd half | N.S. | ||||
| Dark phase, 1st half | 9.8 | (2.21) | 0.001 | FVB/N < TgR-H, TgR-L | |
| Dark phase, 2nd half | N.S. |
| Brain Region | F Value | p Value | Significant Post Hoc N.K. Tests, p < 0.05 |
|---|---|---|---|
| Dorsolateral striatum | 3.81 | 0.036 | TgRL > TgRH, FVB/N |
| Medial striatum | 4.47 | 0.0223 | TgRH < TgRL, FVB/N |
| Anterior cingulate cortex | 3.19 | 0.059 | TgRH < FVB/N |
| Lateral septum | 3.0 | 0.068 | TgRH < FVB/N |
| Hippocampus, CA1 | 2.8 | N.S. | |
| Hippocampus, CA3 | 2.23 | N.S. | |
| Hippocampus, dentate gyrus | 0.04 | N.S. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshitzky, G.; Shoham, S.; Madrer, N.; Husain, A.M.; Greenberg, D.S.; Yirmiya, R.; Ben-Shaul, Y.; Soreq, H. Cholinergic Stress Signals Accompany MicroRNA-Associated Stereotypic Behavior and Glutamatergic Neuromodulation in the Prefrontal Cortex. Biomolecules 2020, 10, 848. https://doi.org/10.3390/biom10060848
Moshitzky G, Shoham S, Madrer N, Husain AM, Greenberg DS, Yirmiya R, Ben-Shaul Y, Soreq H. Cholinergic Stress Signals Accompany MicroRNA-Associated Stereotypic Behavior and Glutamatergic Neuromodulation in the Prefrontal Cortex. Biomolecules. 2020; 10(6):848. https://doi.org/10.3390/biom10060848
Chicago/Turabian StyleMoshitzky, Gilli, Shai Shoham, Nimrod Madrer, Amir Mouhammed Husain, David S. Greenberg, Raz Yirmiya, Yoram Ben-Shaul, and Hermona Soreq. 2020. "Cholinergic Stress Signals Accompany MicroRNA-Associated Stereotypic Behavior and Glutamatergic Neuromodulation in the Prefrontal Cortex" Biomolecules 10, no. 6: 848. https://doi.org/10.3390/biom10060848
APA StyleMoshitzky, G., Shoham, S., Madrer, N., Husain, A. M., Greenberg, D. S., Yirmiya, R., Ben-Shaul, Y., & Soreq, H. (2020). Cholinergic Stress Signals Accompany MicroRNA-Associated Stereotypic Behavior and Glutamatergic Neuromodulation in the Prefrontal Cortex. Biomolecules, 10(6), 848. https://doi.org/10.3390/biom10060848





