The S100B Protein and Partners in Adipocyte Response to Cold Stress and Adaptive Thermogenesis: Facts, Hypotheses, and Perspectives
Abstract
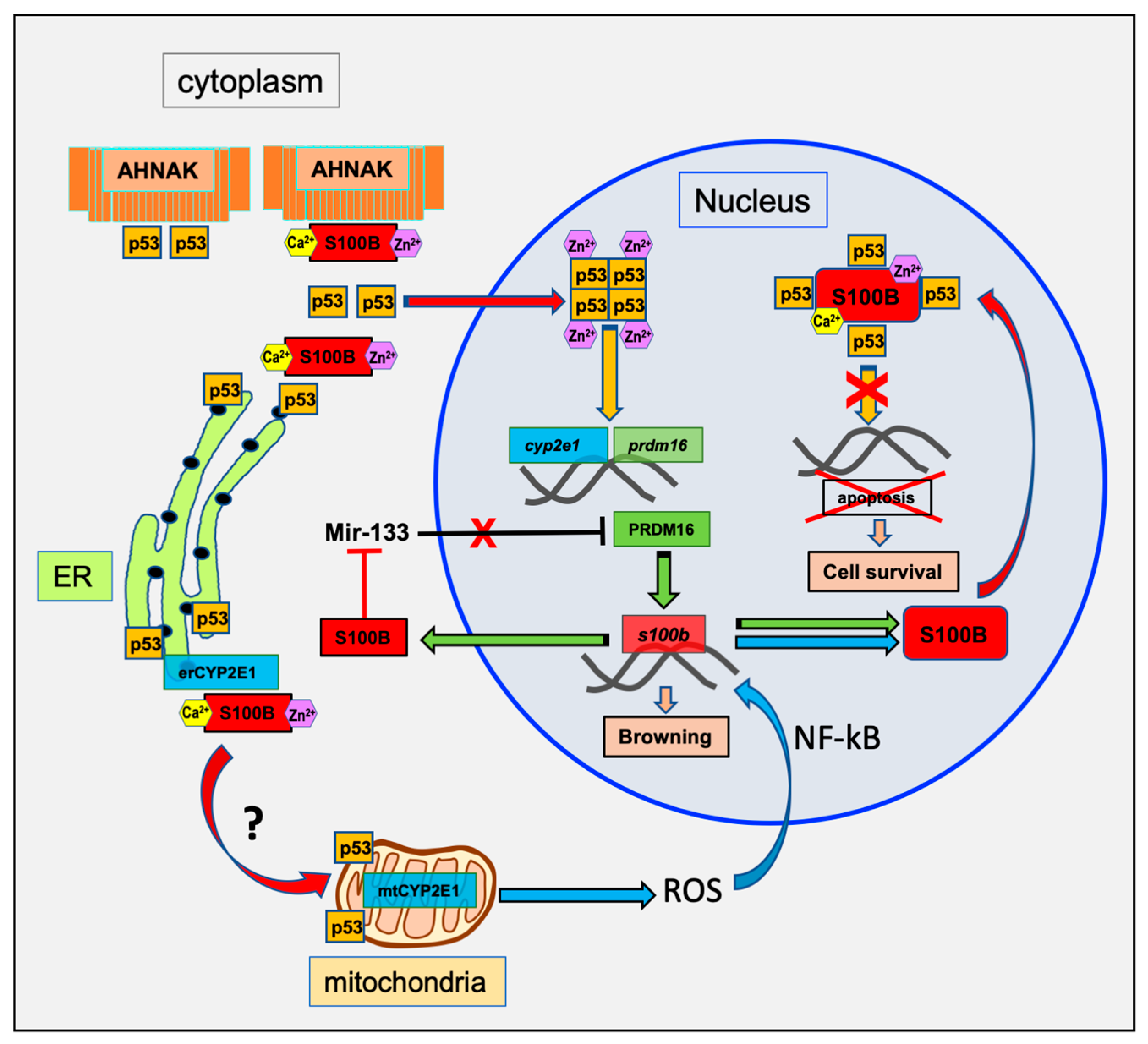
1. Introduction
2. Transcriptional Regulation of S100B in BAT Differentiation
3. The Intracellular S100B Targets in BAT Differentiation
3.1. P53
3.2. ATAD3A
3.3. CYP2E1
4. S100B secretion by Adipocyte
4.1. Non-Classical Interstitial S100B Secretion by Adipocytes, a Role for AHNAK
4.2. Alternative Mechanism for S100B Release from Circulating Extracellular Vesicles
5. The S100B Interaction with Extracellular Targets
6. Conclusions
Acknowledgments
Funding
Conflicts of Interest
References
- Fujita, J. Cold shock response in mammalian cells. J. Mol. Microbiol. Biotechnol. 1999, 1, 243–255. [Google Scholar] [PubMed]
- Rzechorzek, N.; Connick, P.; Patani, R.; Selvaraj, B.T.; Chandran, S. Hypothermic Preconditioning of Human Cortical Neurons Requires Proteostatic Priming. EBioMedicine 2015, 2, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Mollereau, B. Cooling-Induced ER Stress is Good for Your Brain. EBioMedicine 2015, 2, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Peretti, D.; Bastide, A.; Radford, H.; Verity, N.; Molloy, C.; Martin, M.G.; Moreno, J.A.; Steinert, J.; Smith, T.; Dinsdale, D.; et al. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature 2015, 518, 236–239. [Google Scholar] [CrossRef]
- Berry, R.; López-Martínez, G. A dose of experimental hormesis: When mild stress protects and improves animal performance. Comp. Biochem. Physiol. Part. A: Mol. Integr. Physiol. 2020, 242, 1–11. [Google Scholar] [CrossRef]
- Wek, R.C.; Anthony, T.G. Obesity: Stressing about unfolded proteins. Nat. Med. 2010, 16, 374–376. [Google Scholar] [CrossRef]
- Matai, L.; Sarkar, G.C.; Chamoli, M.; Malik, Y.; Kumar, S.S.; Rautela, U.; Jana, N.R.; Chakraborty, K.; Mukhopadhyay, A. Dietary restriction improves proteostasis and increases life span through endoplasmic reticulum hormesis. Proc. Natl. Acad. Sci. USA 2019, 116, 17383–17392. [Google Scholar] [CrossRef]
- Luna–López, A.; González-Puertos, V.Y.; López-Diazguerrero, N.E.; Königsberg, M. New considerations on hormetic response against oxidative stress. J. Cell Commun. Signal. 2014, 8, 323–331. [Google Scholar] [CrossRef]
- Roobol, A.; Carden, M.; Newsam, R.J.; Smales, C.M. Biochemical insights into the mechanisms central to the response of mammalian cells to cold stress and subsequent rewarming. FEBS J. 2008, 276, 286–302. [Google Scholar] [CrossRef]
- Bartelt, A.; Widenmaier, S.; Schlein, C.; Johann, K.; Goncalves, R.L.S.; Eguchi, K.; Fischer, A.W.; Parlakgul, G.; Snyder, N.A.; Nguyen, T.B.; et al. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 2018, 24, 292–303. [Google Scholar] [CrossRef]
- Bartness, T.J.; Ryu, V. Neural control of white, beige and brown adipocytes. Int. J. Obes. Suppl. 2015, 5, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ding, X.; Cao, Y.; Wang, H.; Zeng, W. Dense Intra-adipose Sympathetic Arborizations Are Essential for Cold-Induced Beiging of Mouse White Adipose Tissue. Cell Metab. 2017, 26, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Bachman, E.S.; Dhillon, H.; Zhang, C.-Y.; Cinti, S.; Bianco, A.; Kobilka, B.K.; Lowell, B.B. beta AR Signaling Required for Diet-Induced Thermogenesis and Obesity Resistance. Science 2002, 297, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes[S]. J. Lipid Res. 2012, 53, 619–629. [Google Scholar] [CrossRef]
- Zeng, X.; Ye, M.; Resch, J.; Jedrychowski, M.P.; Hu, B.; Lowell, B.B.; Ginty, D.D.; Spiegelman, B.M. Innervation of thermogenic adipose tissue via a calsyntenin 3β–S100b axis. Nature 2019, 569, 229–235. [Google Scholar] [CrossRef]
- Zimmer, D.B.; Eubanks, J.O.; Ramakrishnan, D.; Criscitiello, M.F. Evolution of the S100 family of calcium sensor proteins. Cell Calcium 2013, 53, 170–179. [Google Scholar] [CrossRef]
- Michetti, F.; Dell’Anna, E.; Tiberio, G.; Cocchia, D. Immunochemical and immunocytochemical study of S-100 protein in rat adipocytes. Brain Res. 1983, 262, 352–356. [Google Scholar] [CrossRef]
- Baudier, J.; Glasser, N.; Gerard, D. Ions binding to S100 proteins. I. Calcium- and zinc-binding properties of bovine brain S100 alpha alpha, S100a (alpha beta), and S100b (beta beta) protein: Zn2+ regulates Ca2+ binding on S100b protein. J. Boil. Chem. 1986, 261, 8192–8203. [Google Scholar]
- Ostendorp, T.; Diez, J.; Heizmann, C.W.; Fritz, G.; Fritz, G. The crystal structures of human S100B in the zinc- and calcium-loaded state at three pH values reveal zinc ligand swapping. BBA Bioenerg. 2011, 1813, 1083–1091. [Google Scholar] [CrossRef]
- Baudier, J.; Deloulme, J.C.; Shaw, G.S. The Zn 2+ and Ca 2+ -binding S100B and S100A1 proteins: Beyond the myths. Boil. Rev. 2020, 95, 738–758. [Google Scholar] [CrossRef]
- Ostendorp, T.; Leclerc, E.; Galichet, A.; Koch, M.; Demling, N.; Weigle, B.; Heizmann, C.W.; Kroneck, P.M.H.; Fritz, G. Structural and functional insights into RAGE activation by multimeric S100B. EMBO J. 2007, 26, 3868–3878. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.G.; Vitolo, M.I.; Pierce, A.D.; Fox, J.M.; Shapiro, P.; Martin, S.S.; Wilder, P.T.; Weber, D.J. Complex Formation between S100B Protein and the p90 Ribosomal S6 Kinase (RSK) in Malignant Melanoma Is Calcium-dependent and Inhibits Extracellular Signal-regulated Kinase (ERK)-mediated Phosphorylation of RSK*. J. Boil. Chem. 2014, 289, 12886–12895. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Blake, M.; Tang, C.; Zimmer, D.; Rustandi, R.R.; Weber, D.J.; Carrier, F. Inhibition of p53 Transcriptional Activity by the S100B Calcium-binding Protein. J. Boil. Chem. 2001, 276, 35037–35041. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-H.; Duda, T.; Pertzev, A.; Venkataraman, V.; Makino, C.L.; Sharma, R.K. S100B serves as a Ca(2+) sensor for ROS-GC1 guanylate cyclase in cones but not in rods of the murine retina. Cell. Physiol. Biochem. 2012, 29, 417–430. [Google Scholar] [CrossRef]
- Baudier, J.; Cole, R.D. Interactions between the microtubule-associated tau proteins and S100b regulate tau phosphorylation by the Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 1988, 263, 5876–5883. [Google Scholar]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef]
- Barbatelli, G.; Morroni, M.; Vinesi, P.; Cinti, S.; Michetti, F. S-100 Protein in Rat Brown Adipose Tissue under Different Functional Conditions: A Morphological, Immunocytochemical, and Immunochemical Study. Exp. Cell Res. 1993, 208, 226–231. [Google Scholar] [CrossRef]
- Morozzi, G.; Beccafico, S.; Bianchi, R.; Riuzzi, F.; Bellezza, I.; Giambanco, I.; Arcuri, C.; Minelli, A.; Donato, R. Oxidative stress-induced S100B accumulation converts myoblasts into brown adipocytes via an NF-κB/YY1/miR-133 axis and NF-κB/YY1/BMP-7 axis. Cell Death Differ. 2017, 24, 2077–2088. [Google Scholar] [CrossRef]
- Suzuki, F.; Kato, K.; Nakajima, T. Enhancement of Adipose S-100 Protein Release by Catecholamines. J. Biochem. 1983, 94, 1707–1710. [Google Scholar] [CrossRef]
- Suzuki, F.; Kato, K. Inhibition of adipose S-100 protein release by insulin. BBA Bioenerg. 1985, 845, 311–316. [Google Scholar] [CrossRef]
- Fujiya, A.; Nagasaki, H.; Seino, Y.; Okawa, T.; Kato, J.; Fukami, A.; Himeno, T.; Uenishi, E.; Tsunekawa, S.; Kamiya, H.; et al. The role of S100B in the interaction between adipocytes and macrophages. Obesity 2013, 22, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Buckman, L.B.; Anderson-Baucum, E.K.; Hasty, A.H.; Ellacott, K.L.J. Regulation of S100B in white adipose tissue by obesity in mice. Adipocyte 2014, 3, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Deloulme, J.-C.; Raponi, E.; Gentil, B.; Bertacchi, N.; Marks, A.; Labourdette, G.; Baudier, J. Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol. Cell. Neurosci. 2004, 27, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Donno, C.; Giannetti, S.; Perić, M.; Andjus, P.; D’Ambrosi, N.; Michetti, F. The Astrocytic S100B Protein with Its Receptor RAGE Is Aberrantly Expressed in SOD1G93A Models, and Its Inhibition Decreases the Expression of Proinflammatory Genes. Mediat. Inflamm. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, S.; Seto-Ohshima, A.; Shinohara, Y.; Yamamoto, Y.; Yamamoto, H.; Itohara, S.; Hirase, H. Neural-Activity-Dependent Release of S100B from Astrocytes Enhances Kainate-Induced Gamma Oscillations In Vivo. J. Neurosci. 2008, 28, 10928–10936. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Riuzzi, F.; Arcuri, C.; Tubaro, C.; Bianchi, R.; Giambanco, I.; Donato, R. S100B protein in tissue development, repair and regeneration. World, J. Boil. Chem. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B story: From biomarker to active factor in neural injury. J. Neurochem. 2018, 148, 168–187. [Google Scholar] [CrossRef]
- Scotto, C.; Deloulme, J.-C.; Rousseau, D.; Chambaz, E.; Baudier, J. Calcium and S100B Regulation of p53-Dependent Cell Growth Arrest and Apoptosis. Mol. Cell. Boil. 1998, 18, 4272–4281. [Google Scholar] [CrossRef][Green Version]
- Gu, J.; Mao, W.; Ren, W.; Xu, F.; Zhu, Q.; Lu, C.; Lin, Z.; Zhang, Z.; Chu, Y.; Liu, R.; et al. Ubiquitin-protein ligase E3C maintains non-small-cell lung cancer stemness by targeting AHNAK-p53 complex. Cancer Lett. 2019, 443, 125–134. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Q.; Wilder, P.T.; Carrier, F.; Weber, D.J. The Calcium-binding Protein S100B Down-regulates p53 and Apoptosis in Malignant Melanoma. J. Boil. Chem. 2010, 285, 27487–27498. [Google Scholar] [CrossRef]
- Haimoto, H.; Kato, K.; Suzuki, F.; Nagura, H. The ultrastructural changes of S-100 protein localization during lipolysis in adipocytes. An immunoelectron-microscopic study. Am. J. Pathol. 1985, 121, 185–191. [Google Scholar] [PubMed]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Boil. 2015, 16, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.D.; Galbraith, M.D.; Andrysik, Z.; Espinosa, J.M. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2017, 25, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Laptenko, O.; Prives, C. Transcriptional regulation by p53: One protein, many possibilities. Cell Death Differ. 2006, 13, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, P.; Gorina, S.; Pavletich, N. Crystal structure of the tetramerization domain of the p53 tumor suppressor at 1.7 angstroms. Science 1995, 267, 1498–1502. [Google Scholar] [CrossRef]
- Park, J.-H.; Zhuang, J.; Li, J.; Hwang, P.M. p53 as guardian of the mitochondrial genome. FEBS Lett. 2016, 590, 924–934. [Google Scholar] [CrossRef]
- Giorgi, C.; Bonora, M.; Sorrentino, G.; Missiroli, S.; Poletti, F.; Suski, J.M.; Ramirez, F.G.; Rizzuto, R.; Di Virgilio, F.; Zito, E.; et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA 2015, 112, 1779–1784. [Google Scholar] [CrossRef]
- Krstić, J.; Reinisch, I.; Schupp, M.; Schulz, T.; Prokesch, A. p53 Functions in Adipose Tissue Metabolism and Homeostasis. Int. J. Mol. Sci. 2018, 19, 2622. [Google Scholar] [CrossRef]
- Boregowda, S.V.; Krishnappa, V.; Strivelli, J.; Haga, C.L.; Booker, C.N.; Phinney, D. Basal p53 expression is indispensable for mesenchymal stem cell integrity. Cell Death Differ. 2018, 25, 679–692. [Google Scholar] [CrossRef]
- Molchadsky, A.; Ezra, O.; Amendola, P.G.; Krantz, D.; Kogan-Sakin, I.; Buganim, Y.; Rivlin, N.; Goldfinger, N.; Folgiero, V.; Falcioni, R.; et al. p53 is required for brown adipogenic differentiation and has a protective role against diet-induced obesity. Cell Death Differ. 2013, 20, 774–783. [Google Scholar] [CrossRef]
- Gentil, B.; Delphin, C.; Mbele, G.O.; Deloulme, J.-C.; Ferro, M.; Garin, J.; Baudier, J. The Giant Protein AHNAK Is a Specific Target for the Calcium- and Zinc-binding S100B Protein. J. Boil. Chem. 2001, 276, 23253–23261. [Google Scholar] [CrossRef] [PubMed]
- Scotto, C.; Delphin, C.; Deloulme, J.-C.; Baudier, J. Concerted Regulation of Wild-Type p53 Nuclear Accumulation and Activation by S100B and Calcium-Dependent Protein Kinase, C. Mol. Cell. Boil. 1999, 19, 7168–7180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, J.H.; Lee, S.H.; Na Kim, Y.; Kim, I.Y.; Kim, Y.J.; Kyeong, D.S.; Lim, H.J.; Cho, S.Y.; Choi, J.; Wi, Y.J.; et al. AHNAK deficiency promotes browning and lipolysis in mice via increased responsiveness to β-adrenergic signalling. Sci. Rep. 2016, 6, 23426–23436. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.; Rajendran, R.; Singh, S.; Garva, R.; Demonacos, C.; Demonacos, C. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast Cancer Res. 2013, 15, R107. [Google Scholar] [CrossRef]
- Ohsie, S.J.; Sarantopoulos, G.P.; Cochran, A.J.; Binder, S.W. Immunohistochemical characteristics of melanoma. J. Cutan. Pathol. 2008, 35, 433–444. [Google Scholar] [CrossRef]
- Robidoux, J.; Martin, T.L.; Collins, S. β-ADRENERGIC RECEPTORS AND REGULATION OF ENERGY EXPENDITURE: A Family Affair. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 297–323. [Google Scholar] [CrossRef]
- Baudier, J. ATAD3 proteins: Brokers of a mitochondria-endoplasmic reticulum connection in mammalian cells. Boil. Rev. 2017, 93, 827–844. [Google Scholar] [CrossRef]
- Peralta, S.; Goffart, S.; Williams, S.L.; Díaz, F.; Garcia, S.; Nissanka, N.; Area-Gomez, E.; Pohjoismäki, J.; Moraes, C.T. ATAD3 controls mitochondrial cristae structure in mouse muscle, influencing mtDNA replication and cholesterol levels. J. Cell Sci. 2018, 131, jcs217075. [Google Scholar] [CrossRef]
- Yang, C.; Suda, T. Hyperactivated mitophagy in hematopoietic stem cells. Nat. Immunol. 2017, 19, 2–3. [Google Scholar] [CrossRef]
- Jin, G.; Xu, C.; Zhang, X.; Long, J.; Rezaeian, A.H.; Liu, C.; Furth, M.E.; Kridel, S.; Pasche, B.; Bian, X.-W.; et al. Atad3a suppresses Pink1-dependent mitophagy to maintain homeostasis of hematopoietic progenitor cells. Nat. Immunol. 2017, 19, 29–40. [Google Scholar] [CrossRef]
- Gilquin, B.; Taillebourg, E.; Cherradi, N.; Hubstenberger, A.; Gay, O.; Merle, N.; Assard, N.; Fauvarque, M.-O.; Tomohiro, S.; Kuge, O.; et al. The AAA+ ATPase ATAD3A Controls Mitochondrial Dynamics at the Interface of the Inner and Outer Membranes. Mol. Cell. Boil. 2010, 30, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Gilquin, B.; Cannon, B.R.; Hubstenberger, A.; Moulouel, B.; Falk, E.; Merle, N.; Assard, N.; Kieffer, S.; Rousseau, D.; Wilder, P.T.; et al. The Calcium-Dependent Interaction between S100B and the Mitochondrial AAA ATPase ATAD3A and the Role of This Complex in the Cytoplasmic Processing of ATAD3A. Mol. Cell. Boil. 2010, 30, 2724–2736. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fujioka, H.; Joshi, D.; Li, Q.; Sangwung, P.; Hsieh, P.; Zhu, J.; Torio, J.; Sweet, D.; Wang, L.; et al. Mitophagy is required for brown adipose tissue mitochondrial homeostasis during cold challenge. Sci. Rep. 2018, 8, 8251–8264. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T. Regulation of cytochrome p450 by inflammatory mediators: Why and how? Drug Metab. Dispos. 2001, 29, 207–212. [Google Scholar]
- Knockaert, L.; Fromenty, B.; Robin, M.-A. Mechanisms of mitochondrial targeting of cytochrome P450 2E1: Physiopathological role in liver injury and obesity. FEBS J. 2011, 278, 4252–4260. [Google Scholar] [CrossRef]
- Yoshinari, K.; Sato, T.; Okino, N.; Sugatani, J.; Miwa, M.; Zhang, X.; Cutler, T.L.; Caggana, M.; Ding, X. Expression and Induction of Cytochromes P450 in Rat White Adipose Tissue. J. Pharmacol. Exp. Ther. 2004, 311, 147–154. [Google Scholar] [CrossRef]
- Robin, M.-A.; Anandatheerthavarada, H.K.; Biswas, G.; Sepuri, N.B.V.; Gordon, D.M.; Pain, D.; Avadhani, N.G. Bimodal Targeting of Microsomal CYP2E1 to Mitochondria through Activation of an N-terminal Chimeric Signal by cAMP-mediated Phosphorylation. J. Boil. Chem. 2002, 277, 40583–40593. [Google Scholar] [CrossRef]
- Davey, G.E.; Murmann, P.; Heizmann, C.W. Intracellular Ca2+and Zn2+Levels Regulate the Alternative Cell Density-dependent Secretion of S100B in Human Glioblastoma Cells. J. Boil. Chem. 2001, 276, 30819–30826. [Google Scholar] [CrossRef]
- Rabouille, C. Pathways of Unconventional Protein Secretion. Trends Cell Boil. 2017, 27, 230–240. [Google Scholar] [CrossRef]
- Maia, J.; Caja, S.; Moraes, M.C.S.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Boil. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, K.M.; Bister, N.; Koistinaho, J.; Malm, T. Exosomes as new diagnostic tools in CNS diseases. Biochim. Biophys. Acta-Mol. Basis Dis. 2016, 1862, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Haase, H. Ahnak, a new player in ?-adrenergic regulation of the cardiac L-type Ca2+ channel. Cardiovasc. Res. 2007, 73, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Bhatti, D.L.; Lee, K.W.; Medrihan, L.; Cheng, J.; Wei, J.; Zhong, P.; Yan, Z.; Kooiker, C.; Song, C.; et al. Ahnak scaffolds p11/Anxa2 complex and L-type voltage-gated calcium channel and modulates depressive behavior. Mol. Psychiatry 2019, 25, 1–15. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, I.Y.; Na Kim, Y.; Shin, S.M.; Roh, K.J.; Lee, S.H.; Sohn, M.; Cho, S.Y.; Lee, S.H.; Ko, C.-Y.; et al. Obesity Resistance and Enhanced Insulin Sensitivity in Ahnak -/- Mice Fed a High Fat Diet Are Related to Impaired Adipogenesis and Increased Energy Expenditure. PLoS ONE 2015, 10, e0139720. [Google Scholar] [CrossRef]
- Ramdas, M.; Harel, C.; Armoni, M.; Karnieli, E. AHNAK KO Mice are Protected from Diet-Induced Obesity but are Glucose Intolerant. Horm. Metab. Res. 2014, 47, 265–272. [Google Scholar] [CrossRef]
- Woo, J.K.; Shin, J.H.; Lee, S.H.; Park, H.-M.; Cho, S.Y.; Sung, Y.M.; Kim, I.Y.; Seong, J.K. Essential role of Ahnak in adipocyte differentiation leading to the transcriptional regulation of Bmpr1α expression. Cell Death Dis. 2018, 9, 864–877. [Google Scholar] [CrossRef]
- Davis, T.; Loos, B.; Engelbrecht, A.-M. AHNAK: The giant jack of all trades. Cell. Signal. 2014, 26, 2683–2693. [Google Scholar] [CrossRef]
- Benaud, C.; Gentil, B.; Assard, N.; Court, M.; Garin, J.; Delphin, C.; Baudier, J. AHNAK interaction with the annexin 2/S100A10 complex regulates cell membrane cytoarchitecture. J. Cell Boil. 2003, 164, 133–144. [Google Scholar] [CrossRef]
- De Seranno, S.; Benaud, C.; Assard, N.; Khediri, S.; Gerke, V.; Baudier, J.; Delphin, C. Identification of an AHNAK Binding Motif Specific for the Annexin2/S100A10 Tetramer. J. Boil. Chem. 2006, 281, 35030–35038. [Google Scholar] [CrossRef]
- Dempsey, B.R.; Rezvanpour, A.; Lee, T.-W.; Barber, K.R.; Junop, M.; Shaw, G.S. Structure of an Asymmetric Ternary Protein Complex Provides Insight for Membrane Interaction. Structrue 2012, 20, 1737–1745. [Google Scholar] [CrossRef]
- Gabel, M.; Delavoie, F.; Demais, V.; Royer, C.; Bailly, Y.; Vitale, N.; Bader, M.-F.; Chasserot-Golaz, S. Annexin A2–dependent actin bundling promotes secretory granule docking to the plasma membrane and exocytosis. J. Cell Boil. 2015, 210, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; You, J.O.; Ha, K.S.; Bae, D.S.; Suh, P.-G.; Rhee, S.G.; Bae, Y.S. AHNAK-mediated Activation of Phospholipase C-γ1 through Protein Kinase, C. J. Boil. Chem. 2004, 279, 26645–26653. [Google Scholar] [CrossRef] [PubMed]
- Lang, T. SNARE proteins and ‘membrane rafts’. J. Physiol. 2007, 585, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Chasserot-Golaz, S.; Coorssen, J.R.; Meunier, F.A.; Vitale, N. Lipid Dynamics in Exocytosis. Cell. Mol. Neurobiol. 2010, 30, 1335–1342. [Google Scholar] [CrossRef]
- Borgonovo, B.; Cocucci, E.; Racchetti, G.; Podini, P.; Bachi, A.; Meldolesi, J. Regulated exocytosis: A novel, widely expressed system. Nature 2002, 4, 955–963. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Rupnik, M.S.; Meldolesi, J. The regulated exocytosis of enlargeosomes is mediated by a SNARE machinery that includes VAMP4. J. Cell Sci. 2008, 121, 2983–2991. [Google Scholar] [CrossRef]
- Lorusso, A.; Covino, C.; Priori, G.; Bachi, A.; Meldolesi, J.; Chieregatti, E. Annexin2 coating the surface of enlargeosomes is needed for their regulated exocytosis. EMBO J. 2006, 25, 5443–5456. [Google Scholar] [CrossRef]
- Lee, J.-E.; Moon, P.-G.; Lee, I.-K.; Baek, M.-C. Proteomic Analysis of Extracellular Vesicles Released by Adipocytes of Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Protein J. 2015, 34, 220–235. [Google Scholar] [CrossRef]
- Kirov, A.; Kacer, R.; Conley, B.A.; Vary, C.P.; Prudovsky, I. AHNAK2 Participates in the Stress-Induced Nonclassical FGF1 Secretion Pathway. J. Cell. Biochem. 2015, 116, 1522–1531. [Google Scholar] [CrossRef]
- Gentil, B.; Delphin, C.; Benaud, C.; Baudier, J. Expression of the giant protein AHNAK (desmoyokin) in muscle and lining epithelial cells. J. Histochem. Cytochem. 2003, 51, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; De Morrée, A.; Van Remoortere, A.; Bushby, K.; Frants, R.R.; Dunnen, J.T.; Van Der Maarel, S.M. Calpain 3 is a modulator of the dysferlin protein complex in skeletal muscle. Hum. Mol. Genet. 2008, 17, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Fukuda, T.; Nagayasu, S.; Nakanishi, J.; Yoshida, K.; Hirata-Tsuchiya, S.; Nakao, Y.; Sano, T.; Yamashita, A.; Yamada, S.; et al. Dental pulp cell-derived powerful inducer of TNF-α comprises PKR containing stress granule rich microvesicles. Sci. Rep. 2019, 9, 3825–3842. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, U.; Purfürst, B.; Schoewel, V.; Morano, I.; Spuler, S.; Haase, H. Ahnak1 abnormally localizes in muscular dystrophies and contributes to muscle vesicle release. J. Muscle Res. Cell Motil. 2011, 32, 271–280. [Google Scholar] [CrossRef]
- Han, R.; Campbell, K.P. Dysferlin and muscle membrane repair. Curr. Opin. Cell Boil. 2007, 19, 409–416. [Google Scholar] [CrossRef]
- Komai, A.M.; Musovic, S.; Peris, E.; Alrifaiy, A.; El Hachmane, M.F.; Johansson, M.; Asterholm, I.W.; Olofsson, C. White Adipocyte Adiponectin Exocytosis Is Stimulated via β3-Adrenergic Signaling and Activation of Epac1: Catecholamine Resistance in Obesity and Type 2 Diabetes. Diabetes 2016, 65, 3301–3313. [Google Scholar] [CrossRef]
- Gonçalves, C.A.; Leite, M.C.; Guerra, M.C. Adipocytes as an Important Source of Serum S100B and Possible Roles of This Protein in Adipose Tissue. Cardiovasc. Psychiatry Neurol. 2010, 2010, 1–7. [Google Scholar] [CrossRef][Green Version]
- Kleindienst, A.; Meissner, S.; Eyupoglu, I.Y.; Parsch, H.; Schmidt, C.; Buchfelder, M. Dynamics of S100B Release into Serum and Cerebrospinal Fluid Following Acute Brain Injury. Pain 2009, 106, 247–250. [Google Scholar] [CrossRef]
- Rontogianni, S.; Synadaki, E.; Li, B.; Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Wu, W.; Altelaar, M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun. Boil. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Uozumi, N.; Gao, C.; Yoshioka, T.; Nakano, M.; Moriwaki, K.; Nakagawa, T.; Masuda, T.; Tanabe, M.; Miyoshi, E. Identification of a Novel Type of CA19-9 Carrier in Human Bile and Sera of Cancer Patients: An Implication of the Involvement in Nonsecretory Exocytosis. J. Proteome Res. 2010, 9, 6345–6353. [Google Scholar] [CrossRef]
- Silva, T.A.; Smuczek, B.; Valadão, I.C.; Dzik, L.M.; Iglesia, R.; Cruz, M.C.; Zelanis, A.; De Siqueira, A.S.; Serrano, S.M.T.; Goldberg, G.S.; et al. AHNAK enables mammary carcinoma cells to produce extracellular vesicles that increase neighboring fibroblast cell motility. Oncotarget 2016, 7, 49998–50016. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hagen, J.; Guntur, K.V.; Allette, K.; Schuyler, S.; Ranjan, J.; Petralia, F.; Gesta, S.; Sebra, R.; Mahajan, M.; et al. A next generation sequencing based approach to identify extracellular vesicle mediated mRNA transfers between cells. BMC Genom. 2017, 18, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Peluso, G.; Melone, M.A.B. RAGE recycles at the plasma membrane in S100B secretory vesicles and promotes Schwann cells morphological changes. J. Cell. Physiol. 2008, 217, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, C.H.; Ruiz, H.H.; Arivazhagan, L.; Aranda, J.F.; Shim, C.; Daya, P.; Derk, J.; MacLean, M.; He, M.; Frye, L.; et al. A Receptor of the Immunoglobulin Superfamily Regulates Adaptive Thermogenesis. Cell Rep. 2019, 28, 773–791. [Google Scholar] [CrossRef]
- Coles, C.H.; Jones, E.Y.; Aricescu, A.R. Extracellular regulation of type IIa receptor protein tyrosine phosphatases: Mechanistic insights from structural analyses. Semin. Cell Dev. Boil. 2014, 37, 98–107. [Google Scholar] [CrossRef]
- Wu, C.-L.; Hardy, S.; Aubry, I.; Landry, M.; Haggarty, A.; Saragovi, H.U.; Tremblay, M.L. Identification of function-regulating antibodies targeting the receptor protein tyrosine phosphatase sigma ectodomain. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Yi, J.-H.; Katagiri, Y.; Yu, P.; Lourie, J.; Bangayan, N.J.; Symes, A.J.; Geller, H.M. Receptor protein tyrosine phosphatase σ binds to neurons in the adult mouse brain. Exp. Neurol. 2014, 255, 12–18. [Google Scholar] [CrossRef]
- Chagnon, M.J.; Uetani, N.; Tremblay, M.L. Functional significance of the LAR receptor protein tyrosine phosphatase family in development and diseases. Biochem. Cell Boil. 2004, 82, 664–675. [Google Scholar] [CrossRef]
- Norris, K.; Norris, F.; Kono, D.H.; Vestergaard, H.; Pedersen, O.; Theofilopoulos, A.N.; Møller, N.P.H. Expression of protein-tyrosine phosphatases in the major insulin target tissues. FEBS Lett. 1997, 415, 243–248. [Google Scholar] [CrossRef]
- Leclerc, E.; Fritz, G.; Vetter, S.W.; Heizmann, C.W.; Fritz, G. Binding of S100 proteins to RAGE: An update. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 993–1007. [Google Scholar] [CrossRef]
- Medrikova, D.; Sijmonsma, T.P.; Sowodniok, K.; Richards, D.M.; Delacher, M.; Sticht, C.; Gretz, N.; Schafmeier, T.; Feuerer, M.; Herzig, S. Brown Adipose Tissue Harbors a Distinct Sub-Population of Regulatory T Cells. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Nguyen, K.; Odegaard, J.I.; Cui, X.; Tian, X.Y.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Roder, J.K.; Gerlai, R. Memory and the effect of cold shock in the water maze in S100 beta transgenic mice. Physiol. Behav. 1996, 60, 611–615. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudier, J.; Gentil, B.J. The S100B Protein and Partners in Adipocyte Response to Cold Stress and Adaptive Thermogenesis: Facts, Hypotheses, and Perspectives. Biomolecules 2020, 10, 843. https://doi.org/10.3390/biom10060843
Baudier J, Gentil BJ. The S100B Protein and Partners in Adipocyte Response to Cold Stress and Adaptive Thermogenesis: Facts, Hypotheses, and Perspectives. Biomolecules. 2020; 10(6):843. https://doi.org/10.3390/biom10060843
Chicago/Turabian StyleBaudier, Jacques, and Benoit J Gentil. 2020. "The S100B Protein and Partners in Adipocyte Response to Cold Stress and Adaptive Thermogenesis: Facts, Hypotheses, and Perspectives" Biomolecules 10, no. 6: 843. https://doi.org/10.3390/biom10060843
APA StyleBaudier, J., & Gentil, B. J. (2020). The S100B Protein and Partners in Adipocyte Response to Cold Stress and Adaptive Thermogenesis: Facts, Hypotheses, and Perspectives. Biomolecules, 10(6), 843. https://doi.org/10.3390/biom10060843





