The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention
Abstract
1. Introduction
2. Euterpe oleracea Mart. Plant Phytochemical Composition
3. Biological EO Activities: Application in the Prevention and Treatment of Diseases
3.1. Pharmacological Applications of Euterpe oleracea Mart. Fruit
3.1.1. Pro-Apoptotic Effect
3.1.2. Anticancer Effect
3.1.3. Anticlastogenic Effect
3.1.4. Anticonvulsant Effect
3.1.5. Antidepressive and Anti-Aging Effects
3.1.6. Antidiabetic Effect
3.1.7. Antihypertensive Effect
3.1.8. Anti-Inflammatory Effect
3.1.9. Antilipemic Effect
3.1.10. Antimicrobial Effect
3.1.11. Antinociceptive Effect
3.1.12. Antioxidant Effect
3.1.13. Antiplasmodial Effect
3.1.14. Antiproliferative Effect
3.1.15. Antiprotozoal Effect
3.1.16. Cardioprotective Effect
3.1.17. Healing Effect
3.1.18. Cytotoxic effect
3.1.19. Hepatoprotective Effect
3.1.20. Immune System Inhibition
3.1.21. Neuroprotective Effect
3.1.22. Lung Protective Effect
3.1.23. Renoprotective Effect
3.1.24. Hematopoietic Effect
3.2. Pharmacological Applications of Euterpe oleracea Mart. Oil
3.2.1. Antineoplasic Effect
3.2.2. Anti-Inflammatory Effect
3.2.3. Antilipemic Effect
3.2.4. Antimicrobial Effect
3.2.5. Antinociceptive Effect
3.2.6. Cytotoxic Effect
3.2.7. Genotoxic Effect
4. Inventions Related to EO as an Alternative Medicine
5. Survey Methodology and Criteria
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Bruning, M.C.R.; Mosegui, G.B.G.; Vianna, C.M.D.M. A utilização da fitoterapia e de plantas medicinais em unidades básicas de saúde nos municípios de Cascavel e Foz do Iguaçu–Paraná: A visão dos profissionais de saúde. Ciênc. Saúde Coletiva 2011, 17, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Oteng Mintah, S.; Asafo-Agyei, T.; Archer, M.A.; Atta-Adjei Junior, P.; Boamah, D.; Kumadoh, D.; Appiah, A.; Ocloo, A.; Boakye, Y.U.; Agyare, C. Medicinal Plants for Treatment of Prevalent Diseases. In Pharmacognosy Medicinal Plants; Perveen, S., Al-Taweel, A., Eds.; Intech Open: Londres, Reino Unido, 2019. [Google Scholar] [CrossRef]
- Lima-Saraiva, S.R.G.; De César, H.C.; Saraiva, C.; Oliveira-Júnior, R.G.; De Silva, J.C.; Damasceno, D.C.M.; Almeida, J.R.G.S.; Amorim, E.L.C. A implantação do programa de plantas medicinais e fitoterápicos no sistema público de saúde no Brasil: Uma revisão de literatura. Rev. Interdiscip. Pesqui. Inov. 2015, 1, 1–11. Available online: https://seer.ufs.br/index.php/revipi/article/view/3095/3406 (accessed on 2 February 2020).
- Silva, N.C.S.; Vítor, A.M.; Bessa, H.H.D.S.; Barros, R.M.S. A utilização de plantas medicinais e fitoterápicos em prol da saúde. UNICA Cad. Acad. 2017, 3, 1–5. [Google Scholar]
- Alves, R.R.N.; Rosa, I.M.L. Biodiversity, traditional medicine and public health: Where do they meet? J. Ethnobiol. Ethnomed. 2007, 3, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Lai, Y.; Yang, Q.; Hu, H.; Wang, Y. Sustainable utilization of traditional Chinese medicine resources: Systematic evaluation on different production modes. Evidence-Based Complement. Altern. Med. 2015, 2015, e218901. [Google Scholar] [CrossRef]
- Rinaldi, A.; Shetty, P. Traditional Medicine for Modern Times: Facts and Figures. 2015. Available online: https://www.scidev.net/global/medicine/feature/traditional-medicine-modern-times-facts-figures.html (accessed on 12 February 2020).
- Chugh, N.A.; Bali, S.; Koul, A. Integration of botanicals in contemporary medicine: Road blocks, checkpoints and go-ahead signals. Integr. Med. Res. 2018, 7, 109–125. [Google Scholar] [CrossRef]
- Klein, T.; Longhini, R.; Bruschi, M.L.; Mello, J.C.P. Fitoterápicos: Um mercado promissor. Rev. Cienc. Farm. Basica Apl. 2009, 30, 241–248. [Google Scholar]
- Santamarina, A.B.; Jamar, G.; Mennitti, L.V.; de Rosso, V.V.; Cesar, H.C.; Oyama, L.M.; Pisani, L.P. The use of juçara (Euterpe edulis Mart.) supplementation for suppression of nf-κb pathway in the hypothalamus after high-fat diet in wistar rats. Molecules 2018, 21, e1814. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Jamar, G.; Mennitti, L.V.; Ribeiro, D.A.; Cardoso, C.M.; de Rosso, V.V.; Oyama, L.M.; Pisani, L.P. Polyphenols-rich fruit (Euterpe edulis Mart.) prevents peripheral inflammatory pathway activation by the short-term high-fat diet. Molecules 2019, 24, e1655. [Google Scholar] [CrossRef] [PubMed]
- Čižauskaitė, U.; Jakubaitytė, G.; Žitkevičius, V.; Kasparavičienė, G. Natural ingredients-based gummy bear composition designed according to texture analysis and sensory evaluation in vivo. Molecules 2019, 24, e1442. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, M.J.; Balick, M.J. Medicinal uses of South American palms. J. Ethnopharmacol. 1984, 10, 157–179. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Costa, M.C.D.; Rocha, S.M.B.D.M. Benefícios funcionais do açaí na prevenção de doenças cardiovasculares. J. Amazon Health Sci. 2015, 1, 1–10. Available online: https://periodicos.ufac.br/revista/index.php/ahs/article/view/39 (accessed on 22 January 2020).
- Yamaguchi, K.K.D.L.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; Da Veiga-Junior, V.F. Amazon açai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef]
- Favacho, H.A.S.; Oliveira, B.R.; Santos, K.C.; Medeiros, B.J.L.; Sousa, P.J.C.; Perazzo, F.F.; Carvalho, J.C.T. Anti-inflammatory and antinociceptive activities of Euterpe oleracea oil. Braz. J. Pharmacogn. 2011, 21, 105–114. [Google Scholar] [CrossRef]
- Souza-Monteiro, J.R.; Hamoy, M.; Santana-Coelho, D.; Arrifano, G.P.F.; Paraense, R.S.O.; Costa-Malaquias, A.; Mendonça, J.R.; da Silva, R.F.; Monteiro, W.S.; Rogez, H.; et al. Anticonvulsant properties of Euterpe oleracea in mice. Neurochem. Int. 2015, 90, 20–27. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Alessandra-Perini, J.; Perini, J.A.; Rodrigues-Baptista, K.C.; Moura, R.S.D.; Junior, A.P.; Santos, T.A.D.; Souza, P.J.C.; Nasciutti, L.E.; Machado, D.E. Euterpe oleracea extract inhibits tumorigenesis effect of the chemical carcinogen DMBA in breast experimental cancer. BMC Complement. Altern. Med. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Melhorança Filho, A.L.; Pereira, M.R.R. Atividade antimicrobiana de óleos extraídos de açaí e de pupunha sobre o desenvolvimento de Pseudomonas aeruginosa e Staphylococcus aureus. Biosci. J. 2012, 28, 598–603. [Google Scholar]
- Dias-Souza, M.V.; Santos, R.M.D.; Cerávolo, I.P.; Cosenza, G.; Ferreira Marçal, P.H.; Figueiredo, F.J.B. Euterpe oleracea pulp extract: Chemical analyses, antibiofilm activity against Staphylococcus aureus, cytotoxicity and interference on the activity of antimicrobial drugs. Microb. Pathog. 2018, 114, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, T.S.S.D.A.; Macedo, P.C.D.O.; Pacheco, S.Y.K.; Silva, S.S.; da Barbosa, E.G.; Pereira, R.R.; Costa, R.M.R.; Junior, J.O.C.S.; Ferreira, M.A.D.S.; Almeida, J.C.D.; et al. Development and evaluation of antimicrobial and modulatory activity of inclusion complex of Euterpe oleracea Mart oil and β-cyclodextrin or hp-β–cyclodextrin. Int. J. Mol. Sci. 2020, 21, e942. [Google Scholar] [CrossRef]
- Silva, B.J.M.D.; Souza-Monteiro, J.R.; Rogez, H.; Crespo-López, M.E.; Nascimento, J.L.M.D.; Silva, E.O. Selective effects of Euterpe oleracea (açai) on Leishmania (Leishmania) amazonensis and Leishmania infantum. Biomed. Pharm. 2018, 97, 1613–1621. [Google Scholar] [CrossRef]
- Petruk, G.; Illiano, A.; Del Giudice, R.; Raiola, A.; Amoresano, A.; Rigano, M.M.; Piccoli, R.; Monti, D.M. Malvidin and cyanidin derivatives from açai fruit (Euterpe oleracea Mart.) counteract UV-A-induced oxidative stress in immortalized fibroblasts. J. Photochem. Photobiol. B Biol. 2017, 172, 42–51. [Google Scholar] [CrossRef]
- Crespo-López, M.E.; Soares, E.S.; Macchi, B.M.; Santos-Sacramento, L.; Takeda, P.Y.; Lopes-Araújo, A.; Paraense, R.S.O.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Luz, D.A.; et al. Towards therapeutic alternatives for mercury neurotoxicity in the amazon: Unraveling the pre-clinical effects of the superfruit açaí (Euterpe oleracea, Mart.) as juice for human consumption. Nutrients 2019, 26, e2585. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Talcott, S.T.; Safe, S.; Mertens-Talcott, S. Absorption and biological activity of phytochemical-rich extracts from açai (Euterpe oleracea Mart.) pulp and oil in vitro. J. Agric. Food Chem. 2008, 56, 3593–3600. [Google Scholar] [CrossRef]
- Agawa, S.; Sakakibara, H.; Iwata, R.; Shimoi, K.; Hergesheimer, A.; Kumazawa, S. Anthocyanins in mesocarp/epicarp and endocarp of fresh açai (Euterpe oleracea Mart.) and their antioxidant activities and bioavailability. Food Sci. Technol. Res. 2011, 17, 327–334. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of açai (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Cedrim, P.C.A.S.; Barros, E.M.A.; Nascimento, T.G.D. Antioxidant properties of acai (Euterpe oleracea) in the metabolic syndrome. Braz. J. Food Technol. 2018, 21, e2017092. [Google Scholar] [CrossRef]
- Mathias, L.M.B.S.; Alegre, P.H.C.; Santos, I.O.F.D.; Bachiega, T.; Figueiredo, A.M.; Chiuso-Minicucci, F.; Fernandes, A.A.; Bazan, S.G.Z.; Minicucci, M.F.; Azevedo, P.S.; et al. Euterpe oleracea Mart. (açai) supplementation attenuates acute doxorubicin-induced cardiotoxicity in rats. Cell. Physiol. Biochem. 2019, 53, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.F.; Lichtenstein, M.P.; Souza-Monteiro, J.R.; Farina, M.; Rogez, H.; Carvalho, J.C.T.; Suñol, C.; Crespo-López, M.E. Clarified açai (Euterpe oleracea) juice as an anticonvulsant agent: In vitro mechanistic study of GABAergic targets. Oxidative Med. Cell. Longev. 2018, 2018, e2678089. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.U.; Talcott, S.T. In vitro absorption and antiproliferative activities of monomeric and polymeric anthocyanin fractions from açai fruit Euterpe oleracea Mart. Food Chem. 2010, 119, 1071–1078. [Google Scholar] [CrossRef]
- Rocha, A.P.M.; Carvalho, L.C.R.M.; Sousa, M.A.V.; Madeira, S.V.F.; Sousa, P.J.C.; Tano, T.; Moura, R.S.D. Endothelium-dependent vasodilator effect of Euterpe oleracea Mart. (açai) extracts in mesenteric vascular bed of the rat. Vasc. Pharmacol. 2007, 46, 97–104. [Google Scholar] [CrossRef] [PubMed]
- De Bem, G.F.; Costa, C.A.; Santos, I.B.; Cristino Cordeiro, V.D.S.; Marins de Carvalho, L.C.R.; Vieira de Souza, M.A.; de Andrade Soares, R.; da Cunha Sousa, P.J.; Ognibene, D.T.; Resende, A.C.; et al. Antidiabetic effect of Euterpe oleracea mart. (açai) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: A positive interaction. PLoS ONE 2018, 13, e0199207. [Google Scholar] [CrossRef] [PubMed]
- Martino, H.S.D.; Dias, M.M.D.S.; Noratto, G.; Talcott, S.; Mertens-Talcott, S.U. Anti-lipidaemic and anti-inflammatory effect of açai (Euterpe oleracea Martius) polyphenols on 3T3-L1 adipocytes. J. Funct. Foods 2016, 23, 432–443. [Google Scholar] [CrossRef]
- Souza, B.S.F.; Carvalho, H.O.; Ferreira, I.M.; da Cunha, E.L.; Barros, A.S.; Taglialegna, T.; Carvalho, J.C.T. Effect of the treatment with Euterpe oleracea Mart. oil in rats with Triton-induced dyslipidemia. Biomed. Pharm. 2017, 90, 542–547. [Google Scholar] [CrossRef]
- Brunschwig, C.; Leba, L.J.; Saout, M.; Martial, K.; Bereau, D.; Robinson, J.C. Chemical composition and antioxidant activity of Euterpe oleracea roots and leaflets. Int. J. Mol. Sci. 2016, 18, e61. [Google Scholar] [CrossRef]
- Marinho, B.G.; Herdy, S.A.; Sá, A.C.; Santos, G.B.; Matheus, M.E.; Menezes, F.S.; Fernandes, P.D. Atividade antinociceptiva de extratos de açaí (Euterpe oleracea Mart.). Rev. Bras. Farmacogn. 2002, 12, 52–53. [Google Scholar] [CrossRef][Green Version]
- Arruda, D.C.; Felippi, R.; Mantovani, I.S.B.; Santos, G.B.; Gabriel, F.T.; de Sa, A.C.; Fernandez, S.B.D.O.; Ribeiro-do-Valle, R.M.; Menezes, F.S.; Ckless, K. Antioxidant activity and levels of phenolics in Euterpe oleracea Mart extract (açai). Rev. Bras. Plantas Med. 2004, 6, 5–10. [Google Scholar]
- Lichtenthäler, R.; Rodrigues, R.B.; Maia, J.G.S.; Papagiannopoulos, M.; Fabricius, H.; Marx, F. Total oxidant scavenging capacities of Euterpe oleracea Mart. (açai) fruit. Int. J. Food Sci. Nutr. 2005, 56, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.M.D.S.; Noratto, G.; Martino, H.S.D.; Arbizu, S.; Peluzio, M.D.C.G.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S.U. Pro-apoptotic activities of polyphenolics from açai (Euterpe oleracea Martius) in human SW-480 colon cancer cells. Nutr. Cancer 2014, 66, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Choi, Y.J.; Kim, N.; Nam, R.H.; Lee, S.; Lee, H.S.; Lee, H.-N.; Surh, Y.-J.; Lee, D.H. Açai berries inhibit colon tumorigenesis in azoxymethane/dextran sulfate sodium-treated mice. Gut Liver 2017, 11, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.F.; Romualdo, G.R.; Vanderveer, L.A.; Franco-Barraza, J.; Cukierman, E.; Clapper, M.L.; Carvalho, R.F.; Barbisan, L.F. Lyophilized açaí pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis while its main anthocyanin has the potential to affect the motility of colon cancer cells. Food Chem. Toxicol. 2018, 80, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Romualdo, G.R.; Fragoso, M.F.; Borguini, R.G.; Santiago, M.C.P.A.; Fernandes, A.A.H.; Barbisan, L.F. Protective effects of spray-dried açaí (Euterpe oleracea Mart) fruit pulp against initiation step of colon carcinogenesis. Food Res. Int. 2015, 77, 432–440. [Google Scholar] [CrossRef]
- Martinez, R.; Guimarães, D.; Berniz, C.; Abreu, J.; Rocha, A.; Moura, R.; Resende, A.C.; Teodoro, A. Açai (Euterpe oleracea Mart.) seed extract induces cell cycle arrest and apoptosis in human lung carcinoma cells. Foods 2018, 7, e178. [Google Scholar] [CrossRef]
- Silva, D.F.; Vidal, F.C.B.; Santos, D.M.C.P.C.; Morgado-Díaz, J.A.; Nascimento, M.D.D.S.B.; Moura, R.S.D. Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Complement. Altern. Med. 2014, 14, e175. [Google Scholar] [CrossRef]
- Brito, C.; Stavroullakis, A.T.; Ferreira, A.C.; Li, K.; Oliveira, T.; Nogueira-Filho, G.; Prakki, A. Extract of acai-berry inhibits osteoclast differentiation and activity. Arch. Oral Biol. 2016, 68, 29–34. [Google Scholar] [CrossRef]
- Souza-Monteiro, J.R.; Arrifano, G.P.F.; Queiroz, A.I.D.G.; Mello, B.S.F.; Custódio, C.S.; Macêdo, D.S.; Hamoy, M.; Paraense, R.S.O.; Bittencourt, L.O.; Lima, R.R.; et al. Antidepressant and antiaging effects of açaí (Euterpe oleracea Mart.) in mice. Oxidative Med. Cell. Longev. 2019, 2019, e3614960. [Google Scholar] [CrossRef]
- Cordeiro, V.S.C.; Carvalho, L.C.R.M.; de Bem, G.F.; Costa, C.A.; Souza, M.A.V.; Sousa, P.J.C.; Rocha, V.N.; Carvalho, J.J.; Moura, R.S.; Resende, A.C. Euterpe oleracea Mart extract prevents vascular remodeling and endothelial dysfunction in spontaneously hypertensive rats. Int. J. Appl. Res. Nat. Prod. 2015, 8, 6–16. [Google Scholar]
- Zhou, J.; Zhang, J.; Wang, C.; Qu, S.; Zhu, Y.; Yang, Z.; Wang, L. Açaí (Euterpe oleracea Mart.) attenuates alcohol-induced liver injury in rats by alleviating oxidative stress and inflammatory response. Exp. Ther. Med. 2017, 15, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from açai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.P.; Nesi, R.T.; Resende, A.C.; Souza, P.J.C.; Da Silva, A.J.R.; Borges, R.M.; Porto, L.C.; et al. Effects of Euterpe oleracea Mart. (açaí) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine 2012, 19, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.K.; Cadoná, F.C.; Assmann, C.E.; Andreazza, A.C.; Duarte, M.M.M.F.; dos Santos Branco, C.; Zhou, X.; Souza, D.V.D.; Ribeiro, E.E.; da Cruz, I.B.M. Açaí (Euterpe oleracea Mart.) has anti-inflammatory potential through NLRP3-inflammasome modulation. J. Funct. Foods 2019, 56, 364–371. [Google Scholar] [CrossRef]
- Dias, M.M.D.S.; Martino, H.S.D.; Noratto, G.; Roque-Andrade, A.; Stringheta, P.C.; Talcott, S.; Ramos, A.M.; Mertens-Talcott, S.U. Anti-inflammatory activity of polyphenolics from açai (Euterpe oleracea Martius) in intestinal myofibroblasts CCD-18Co cells. Food Funct. 2015, 6, 3249–3256. [Google Scholar] [CrossRef]
- Machado, D.E.; Rodrigues-Baptista, K.C.; Alessandra-Perini, J.; Soares de Moura, R.; Santos, T.A.D.; Pereira, K.G.; da Silva, Y.M.; Souza, P.J.C.; Nasciutti, L.E.; Perini, J.A. Euterpe oleracea extract (açaí) is a promising novel pharmacological therapeutic treatment for experimental endometriosis. PLoS ONE 2016, 11, e0166059. [Google Scholar] [CrossRef]
- De Oliveira, P.R.B.; da Costa, C.A.; de Bem, G.F.; Cordeiro, V.S.C.; Santos, I.B.; de Carvalho, L.C.R.M.; Conceição, E.P.S.D.; Lisboa, P.C.; Ognibene, D.T.; Sousa, P.J.C.; et al. Euterpe oleracea Mart.-derived polyphenols protect mice from diet-induced obesity and fatty liver by regulating hepatic lipogenesis and cholesterol excretion. PLoS ONE 2015, 10, e0143721. [Google Scholar] [CrossRef]
- Trindade, P.; Soares, E.; Monteiro, E.; Moura-Nunes, N.; Costa, D.; Daleprane, J. Antiadipogenic effects of açai polyphenols on high fat diet-fed mice and 3T3-L1 adipocytes: A potential mechanism of action (OR34-04-19). Curr. Dev. Nutr. 2019, 3, 500–502. [Google Scholar] [CrossRef]
- Oliveira de Souza, M.; Silva, M.; Silva, M.E.; de Paula Oliveira, R.; Pedrosa, M.L. Diet supplementation with açai (Euterpe oleracea Mart.) pulp improves biomarkers of oxidative stress and the serum lipid profile in rats. Nutrition 2010, 26, 804–810. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Batista, A.; Costa, D.C.F.D.; Moura-Nunes, N.; Koury, J.C.; da Costa, C.A.; Resende, Â.C.; Daleprane, J.B. Açai (Euterpe oleracea Mart.) seed flour prevents obesity-induced hepatic steatosis regulating lipid metabolism by increasing cholesterol excretion in high-fat diet-fed mice. Food Res. Int. 2018, 111, 408–415. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.O.; Souza e Silva, L.; de Brito Magalhães, C.L.; De Figueiredo, B.B.; Costa, D.C.; Silva, M.E.; Pedrosa, M.L. The hypocholesterolemic activity of açaí (Euterpe oleracea Mart.) is mediated by the enhanced expression of the ATP-binding cassette, subfamily G transporters 5 and 8 and low-density lipoprotein receptor genes in the rat. Nutr. Res. 2012, 32, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Feio, C.A.; Izar, M.C.; Ihara, S.S.; Kasmas, S.H.; Martins, C.M.; Feio, M.N.; Maués, L.A.; Borges, N.C.; Moreno, R.A.; Póvoa, R.M.; et al. Euterpe oleracea (açai) modifies sterol metabolism and attenuates experimentally-induced atherosclerosis. J. Atheroscler. Thromb. 2012, 19, 237–245. [Google Scholar] [CrossRef][Green Version]
- Pala, D.; Barbosa, P.O.; Silva, C.T.; De Souza, M.O.; Freitas, F.R.; Volp, A.C.P.; Maranhão, R.C.; Freitas, R.N.D. Açai (Euterpe oleracea Mart.) dietary intake affects plasma lipids, apolipoproteins, cholesteryl ester transfer to high-density lipoprotein and redox metabolism: A prospective study in women. Clin. Nutr. 2018, 37, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, L.K.; Giese, E.G.; Dos Santos, J.N.; Molento, M.B. Efeito antibacteriano in vitro de Euterpe oleracea Mart. e extratos hidroalcoólicos de Theobroma grandiflorum. Arq. Ciênc. Vet. 2016, 21, 21–32. [Google Scholar] [CrossRef]
- Borges, K.R.; Rodrigues, I.V.; Pereira, L.A.; Silva, G.X.; Filho, W.E.; Silva, M.A.C.N.; Alves, R.N.S.; Bezerra, C.R.F.; Rosa, I.G.; Brito, L.M.O.; et al. Euterpe oleracea Mart. inibe os fatores de virulência de Aspergillus fumigatus. Microbiol. Futuro 2019, 14, 717–728. [Google Scholar] [CrossRef]
- Jensen, G.S.; Ager, D.M.; Redman, K.A.; Mitzner, M.A.; Benson, K.F.; Schauss, A.G. Pain reduction and improvement in range of motion after daily consumption of an açai (Euterpe oleracea Mart.) pulp-fortified polyphenolic-rich fruit and berry juice blend. J. Food Med. 2011, 14, 702–711. [Google Scholar] [CrossRef]
- Sudo, R.T.; Neto, M.L.; Monteiro, C.E.S.; Amaral, R.V.; Resende, Â.C.; Souza, P.J.C.; Zapata-Sudo, G.; Moura, R.S. Antinociceptive effects of hydroalcoholic extract from Euterpe oleracea Mart. (açaí) in a rodent model of acute and neuropathic pain. BMC Complement. Altern. Med. 2015, 15, e208. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Chai, H.-B.; Keller, W.J.; Kinghorn, A.D. Lignans and other constituents of the fruits of Euterpe oleracea (açai) with antioxidant and cytoprotective activities. J. Agric. Food Chem. 2008, 56, 7759–7764. [Google Scholar] [CrossRef]
- Soares, E.R.; Monteiro, E.B.; de Bem, G.F.; Inada, K.O.P.; Torres, A.G.; Perrone, D.; Soulage, C.O.; Monteiro, M.C.; Resende, A.C.; Moura-Nunes, N.; et al. Up-regulation of Nrf2-antioxidant signaling by açaí (Euterpe oleracea Mart.) extract prevents oxidative stress in human endothelial cells. J. Funct. Foods 2017, 37, 107–115. [Google Scholar] [CrossRef]
- Nascimento, V.H.N.; do Lima, C.D.S.; Paixão, J.T.C.; Freitas, J.J.D.S.; Kietzer, K.S. Antioxidant effects of açaí seed (Euterpe oleracea) in anorexia-cachexia syndrome induced by Walker-256 tumor. Acta Cir. Bras. 2016, 31, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.M.J.; Viana, D.S.; Leite, D.D.C.; Peixoto, J.C.; Moura, M.R. Total phenolics and antioxidant activity of a functional gel based on açaí (Euterpe oleracea Martius) pulp. J. Adv. Agric. 2015, 3, 252–259. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Shanbrom, E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleracea Mart. (Açai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.T.; Venancio, V.P.; Kawano, T.; Abrão, L.C.C.; Tavella, T.A.; Almeida, L.D.; Pires, G.S.; Bilsland, E.; Sunnerhagen, P.; Azevedo, L.; et al. Chemical genomic profiling unveils the in vitro and in vivo antiplasmodial mechanism of açaí (Euterpe oleracea Mart.) polyphenols. Am. Chem. Soc. Omega 2019, 4, 15628–15635. [Google Scholar] [CrossRef]
- Pozo-Insfran, D.D.; Percival, S.S.; Talcott, S.T. Açai (Euterpe oleracea Mart.) polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. J. Agric. Food Chem. 2006, 54, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Zapata-Sudo, G.; da Silva, J.S.; Pereira, S.L.; Souza, P.J.; de Moura, R.S.; Sudo, R.T. Oral treatment with Euterpe oleracea Mart. (açaí) extract improves cardiac dysfunction and exercise intolerance in rats subjected to myocardial infarction. BMC Complement. Altern. Med. 2014, 14, e227. [Google Scholar] [CrossRef]
- Gale, A.M.; Kaur, R.; Baker, W.L. Hemodynamic and electrocardiographic effects of açaí berry in healthy volunteers: A randomized controlled trial. Int. J. Cardiol. 2014, 174, 421–423. [Google Scholar] [CrossRef]
- Kang, M.H.; Choi, S.; Kim, B. Skin wound healing effects and action mechanism of acai berry water extracts. Toxicol. Res. 2017, 33, 149–156. [Google Scholar] [CrossRef]
- Kang, M.H.; Kim, B.-H. Oral wound healing effects of acai berry water extracts in rat oral mucosa. Toxicol. Res. 2018, 34, 97–102. [Google Scholar] [CrossRef]
- Freitas, D.D.S.; Morgado-Díaz, J.A.; Gehren, A.S.; Vidal, F.C.B.; Fernandes, R.M.T.; Romão, W.; Tose, L.V.; Frazão, F.N.S.; Costa, M.C.P.; Silva, D.F.; et al. Cytotoxic analysis and chemical characterization of fractions of the hydroalcoholic extract of the Euterpe oleracea Mart. seed in the MCF-7 cell line. J. Pharm. Pharmacol. 2017, 69, 714–721. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Carvalho, M.M.; Lage, N.N.; de Souza Paulino, A.H.; Pereira, R.R.; de Almeida, L.T.; da Silva, T.F.; Magalhães, C.L.B.; Lima, W.G.; de Silva, M.E.; Pedrosa, M.L.; et al. Effects of açai on oxidative stress, ER stress, and inflammation-related parameters in mice with high fat diet-fed induced NAFLD. Sci. Rep. 2019, 9, e8107. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, T.; Ishiguro, N.; Chihara, K.; Ogi, K.; Nakashima, K.; Sada, K.; Hori-Tamura, N. Inhibitory effect of açaí (Euterpe oleracea Mart.) pulp on IgE-mediated mast cell activation. J. Agric. Food Chem. 2011, 59, 5595–5601. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Y.S.; Musgrave, I.F.; Harvey, B.S.; Smid, S.D. Açaí (Euterpe oleracea Mart.) berry extract exerts neuroprotective effects against β-amyloid exposure in vitro. Neurosci. Lett. 2013, 556, 221–226. [Google Scholar] [CrossRef]
- Machado, A.K.; Andreazza, A.C.; da Silva, T.M.; Boligon, A.A.; do Nascimento, V.; Scola, G.; Duong, A.; Cadoná, F.C.; Ribeiro, E.E.; da Cruz, I.B.M. Neuroprotective effects of açaí (Euterpe oleracea Mart.) against rotenone in vitro exposure. Oxidative Med. Cell. Longev. 2016, 2016, e8940850. [Google Scholar] [CrossRef]
- Holderness, J.; Schepetkin, I.A.; Freedman, B.; Kirpotina, L.N.; Quinn, M.T.; Hedges, J.F.; Jutila, M.A. Polysaccharides isolated from açai fruit induce innate immune responses. PLoS ONE 2011, 6, e17301. [Google Scholar] [CrossRef]
- Skyberg, J.A.; Rollins, M.F.; Holderness, J.S.; Marlenee, N.L.; Schepetkin, I.A.; Goodyear, A.; Dow, S.W.; Jutila, M.A.; Pascual, D.W. Nasal acai polysaccharides potentiate innate immunity to protect against pulmonary Francisella tularensis and Burkholderia pseudomallei infections. PLoS Pathog. 2012, 8, e1002587. [Google Scholar] [CrossRef]
- El Morsy, E.M.; Ahmed, M.A.E.; Ahmed, A.A.E. Attenuation of renal ischemia/reperfusion injury by açaí extract preconditioning in a rat model. Life Sci. 2015, 123, 35–42. [Google Scholar] [CrossRef]
- Cordeiro, V.S.C.; Bem, G.F.; Costa, C.A.; Santos, I.B.; Carvalho, L.C.R.M.; Ognibene, D.T.; Rocha, A.M.; Carvalho, J.J.; Moura, R.S.; Resende, A.C. Euterpe oleracea Mart. seed extract protects against renal injury in diabetic and spontaneously hypertensive rats: Role of inflammation and oxidative stress. Eur. J. Nutr. 2016, 57, 817–832. [Google Scholar] [CrossRef]
- Da Costa, C.A.; Ognibene, D.T.; Cordeiro, V.S.C.; de Bem, G.F.; Santos, I.B.; Soares, R.A.; Cunha, L.L.M.; Carvalho, L.C.R.M.; Moura, R.S.; de Resende, A.C. Effect of Euterpe oleracea Mart. seeds extract on chronic ischemic renal injury in renovascular hypertensive rats. J. Med. Food 2017, 20, 1002–1010. [Google Scholar] [CrossRef]
- Shibuya, S.; Toda, T.; Ozawa, Y.; Yata, M.J.V.; Shimizu, T. Acai extract transiently upregulates erythropoietin by inducing a renal hypoxic condition in mice. Nutrients 2020, 12, e533. [Google Scholar] [CrossRef] [PubMed]
- Maia, T.N.; de Araujo, G.B.R.; Teixeira, J.A.C.; Alves Junior, E.d.D.; Dias, K.P. Cardiotoxicity of Doxorubicin Treatment and Physical Activity: A Systematic Review. Int. J. Cardiovasc. Sci. 2015, 30, 70–80. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.F.; Silva, J.R.; Fascineli, M.L.; de Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Carneiro, F.P.; et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. B Biol. 2017, 166, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.S.; Froder, J.G.; Oliveira, P.R.; de Perazzo, F.F.; Rosa, P.C.P.; Gaivão, I.O.M.; Mathias, M.I.C.; Maistro, E.L. Cytotoxic effects of Euterpe oleracea fruit oil (açaí) in rat liver and thyroid tissues. Rev. Bras. Farmacogn. 2018, 29, 54–61. [Google Scholar] [CrossRef]
- Marques, E.S.; Froder, J.G.; Carvalho, J.C.T.; Rosa, P.C.P.; Perazzo, F.F.; Maistro, E.L. Evaluation of the genotoxicity of Euterpe oleracea Mart. (Arecaceae) fruit oil (açaí), in mammalian cells in vivo. Food Chem. Toxicol. 2016, 93, 13–19. [Google Scholar] [CrossRef]
- Soares de Moura, R.; inventor; Federal University of Rio de Janeiro, assignee. Process for Obtaining Euterpe oleracea (açaí) Fruit and Stone Clumps; Process of Obtaining Hydroalcoholic Extracts from Decollets; Process of Obtaining Lyophilisates and/or Spray Dryer; Pharmaceutical Compositions Containing the Lyophilisates and/or Spray Dryers of Said Extracts and Therapeutic Use of the Compositions in the Treatment of Pain in General. Brazilian Patent Application No. PI 0418614-1 A2, 25 November 2008. [Google Scholar]
- Santos, V. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models manganese-induced oxidative stress in rat primary astrocyte cultures. J. Toxicol. Environ. Health Part A Curr. Issues 2014, 77, 390–404. [Google Scholar] [CrossRef]
- Martinez, R.M.; Zarpelon, A.C.; Domiciano, T.P.; Georgetti, S.R.; Baracat, M.M.; Moreira, I.C.; Andrei, C.C.; Andrei, C.C.; Verri, W.A., Jr.; Casagrande, R. Antinociceptive effect of Tephrosia sinapou extract in the acetic acid, phenyl-p-benzoquinone, formalin, and complete Freund’s adjuvant models of overt pain-like behavior in mice. Scientifica 2016, 2016, e8656397. [Google Scholar] [CrossRef][Green Version]
- Soares de Moura, R.S.; inventor; Federal University of Rio de Janeiro, assignee. Process for Obtaining Fruit and Seed Decollets of Euterpe oleracea (açaí). Process for Obtaining Hydroalcoholic Extracts from the Decollets; Process of Obtaining Lyophilisate and/or Spray Dryer of the Hydroalcoholic Extract; Pharmaceutical Compositions Containing the Lyophilisates and/or Spray Dryer of Said Extracts and Therapeutic Use of the Compositions as a Vasodilator in the Treatment of Ischemic, Vaso-Spastic and Arterial Hypertension Syndromes. Brazilian Patent Application No. PI 0604281-3 A2, 4 March 2008. [Google Scholar]
- Ford, T.J.; Rocchiccioli, P.; Good, R.; McEntegart, M.; Eteiba, H.; Watkins, S.; Shaukat, A.; Lindsay, M.; Robertson, K.; Hood, S.; et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur. Heart J. 2018, 39, 4086–4097. [Google Scholar] [CrossRef]
- Soares de Moura, R.; inventor; Federal University of Rio de Janeiro, assignee. Method for Preparing Ointments Containing Antioxidants from Polyphenol-Rich Plants for Use in the Treatment of Wounds of Various Origins Which Involve an Increase in Oxidation-Promoting Agents and/or Increased Formation of Oxygen-Derived Reactive Species and/or Reduce Formation of Nitric Oxide. International Patent Application No. WO 2011/1036448 A1, 1 September 2011. [Google Scholar]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, e71. [Google Scholar] [CrossRef]
- Rodrigues, R.B.; Lichtenthäler, R.; Zimmermann, B.F.; Papagiannopoulos, M.; Fabricius, H.; Marx, F.; Maia, J.G.; Almeida, O. Total oxidant scavenging capacity of Euterpe oleracea Mart. (açaí) seeds and identification of their polyphenolic compounds. J. Agric. Food Chem. 2006, 54, 4162–4167. [Google Scholar] [CrossRef]
- Dreher, F.; Neocutis, S.A. Composiciones Antioxidantes y sus Métodos de Uso, Composición Farmaceutica, KIT. U.S. Patent Application No. US 61/814791, 22 April 2013. [Google Scholar]
- Nascimento, M.D.S.B.; inventor; Federal University of Maranhão, assignee. Composition of Alcoholic Extracts Obtained from Seeds of Euterpe oleracea Mart. (acai) and Euterpe edulis Mart. (Juçara) and its Pharmaceutical Forms Containing the Lyophilisates of Said Extracts and Therapeutic Use of the Formulations as Cancer Treatment. Brazilian Patent Application No. BR 102015017543-4 A2, 6 February 2018. [Google Scholar]
- Da Silva, H.R.; inventor; Federal University of Amapá, assignee. Technological Process for Obtaining Tablets Containing Ethanolic Extract (Standardized) from the Fruits of Euterpe oleracea Mart. (Acai), Its Application as an Antioxidant. Brazilian Patent Application No. BR 102017007451-0 A2, 30 October 2018. [Google Scholar]
- OECD 2001. Guideline 423: Acute Oral Toxicity-Acute Toxic Class Method Paris: Head of Publications Service. Available online: http://www.oecd.org/publications (accessed on 18 December 2008).
- Moreira Castilho, A.; Pianowski, L.F.; Soares de Moura, R.; inventor; Power Seed Comércio e Representações LTDA.; assignee. Açai Seed Extract; Use of Acai Seed Extracts; Process of Obtaining Food for the Treatment of Diseases or Disorders. Brazilian Patent Application No. BR 102018005450, 30 October 2018. [Google Scholar]
- Almeida, T.S.S.; de Lima, A.A.N.; de Ostroski, E.A. Sistemas Binários do Óleo Essencial de Açaí e Cicloamiloses. Brazilian Patent Application No. BR 10201807679332018, 20 December 2018. [Google Scholar]
- Figueirêdo, P.M.S.; inventor; Federal University of Maranhão, assignee. Antimicrobial Herbal Medicine Obtained from the Extract of the Leaves of Euterpe oleracea (açaí). Brazilian Patent Application No. BR 102017013494-6 A2, 15 January 2019. [Google Scholar]
- Cavalcante, P. Frutas Comestíveis da Amazônia; CEJUP: Belém, Brazil, 1991; p. 271. [Google Scholar]
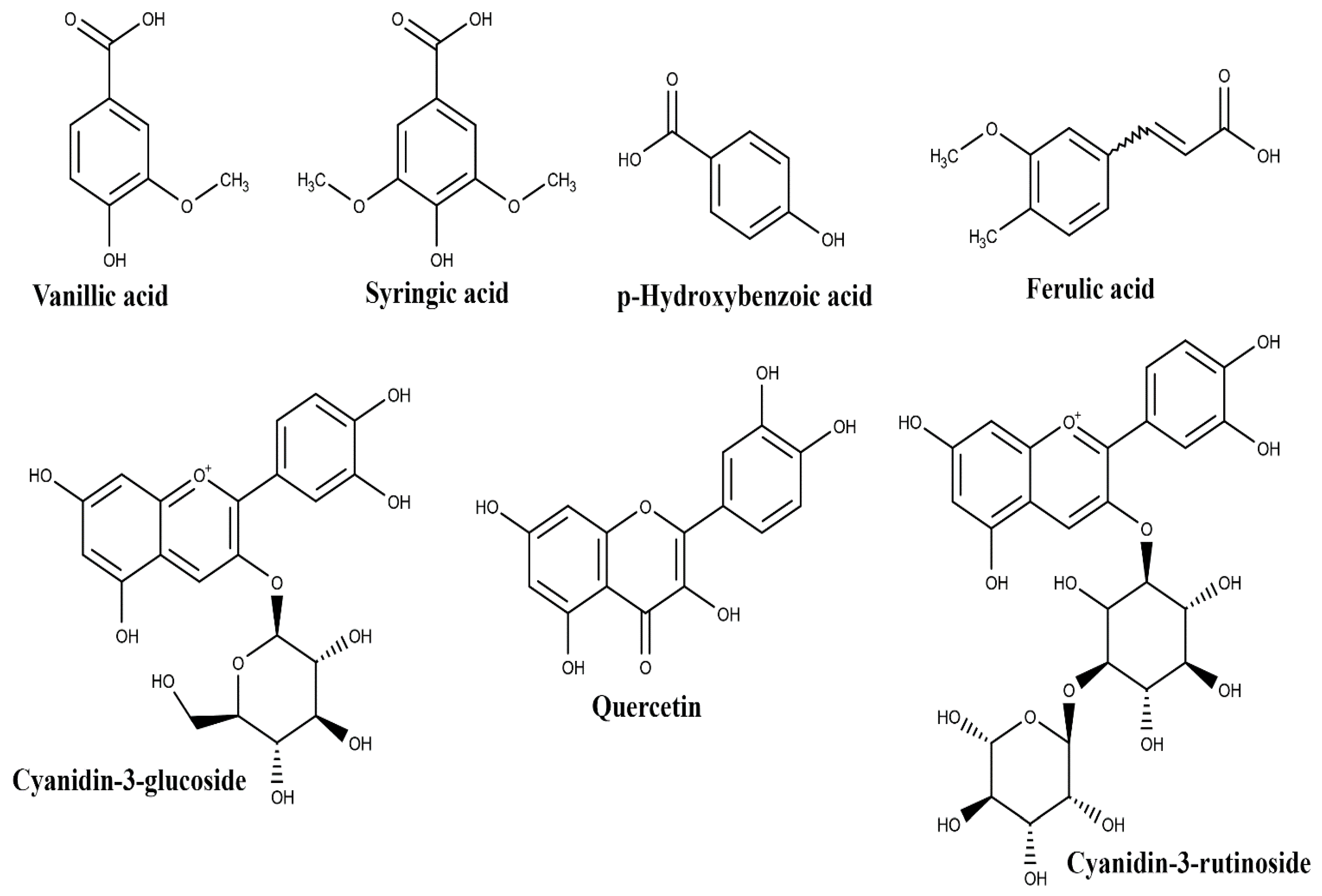
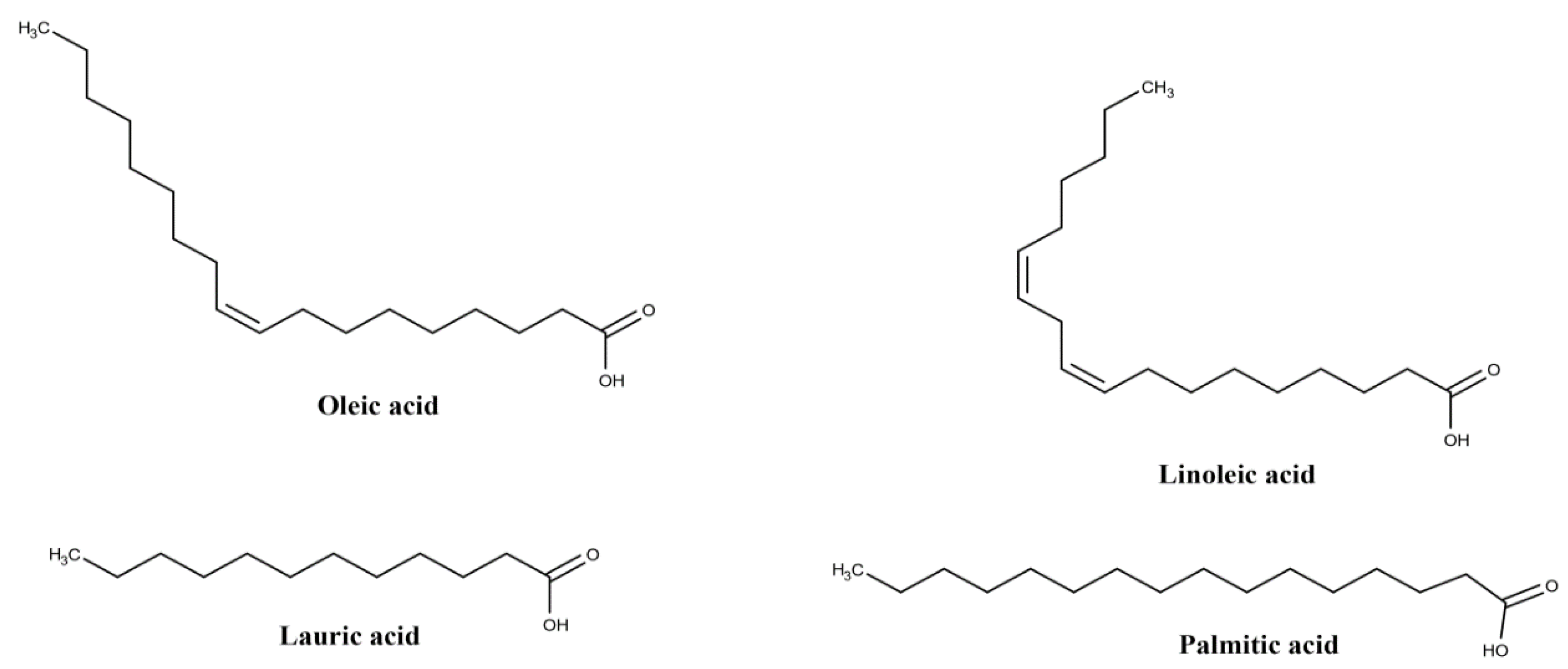
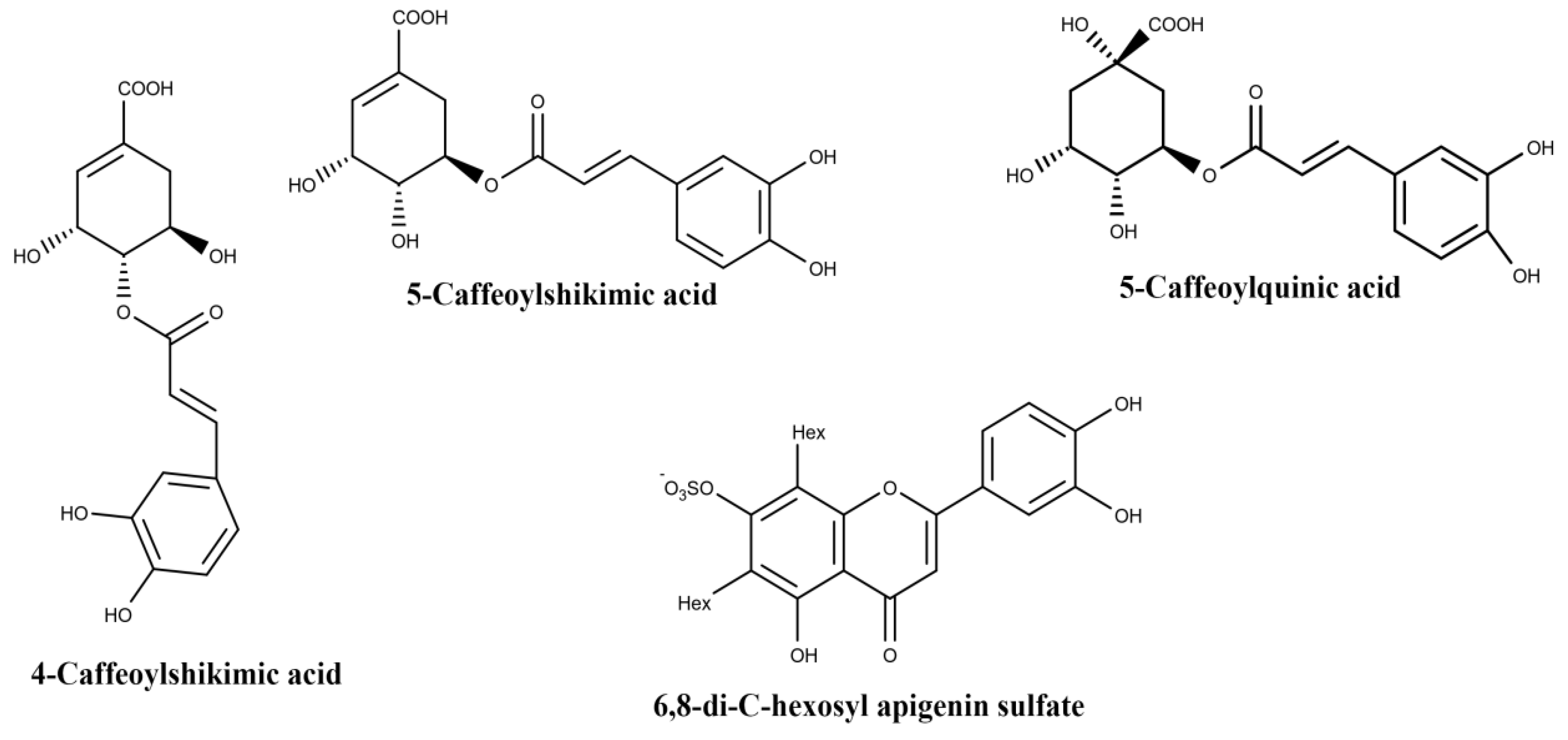
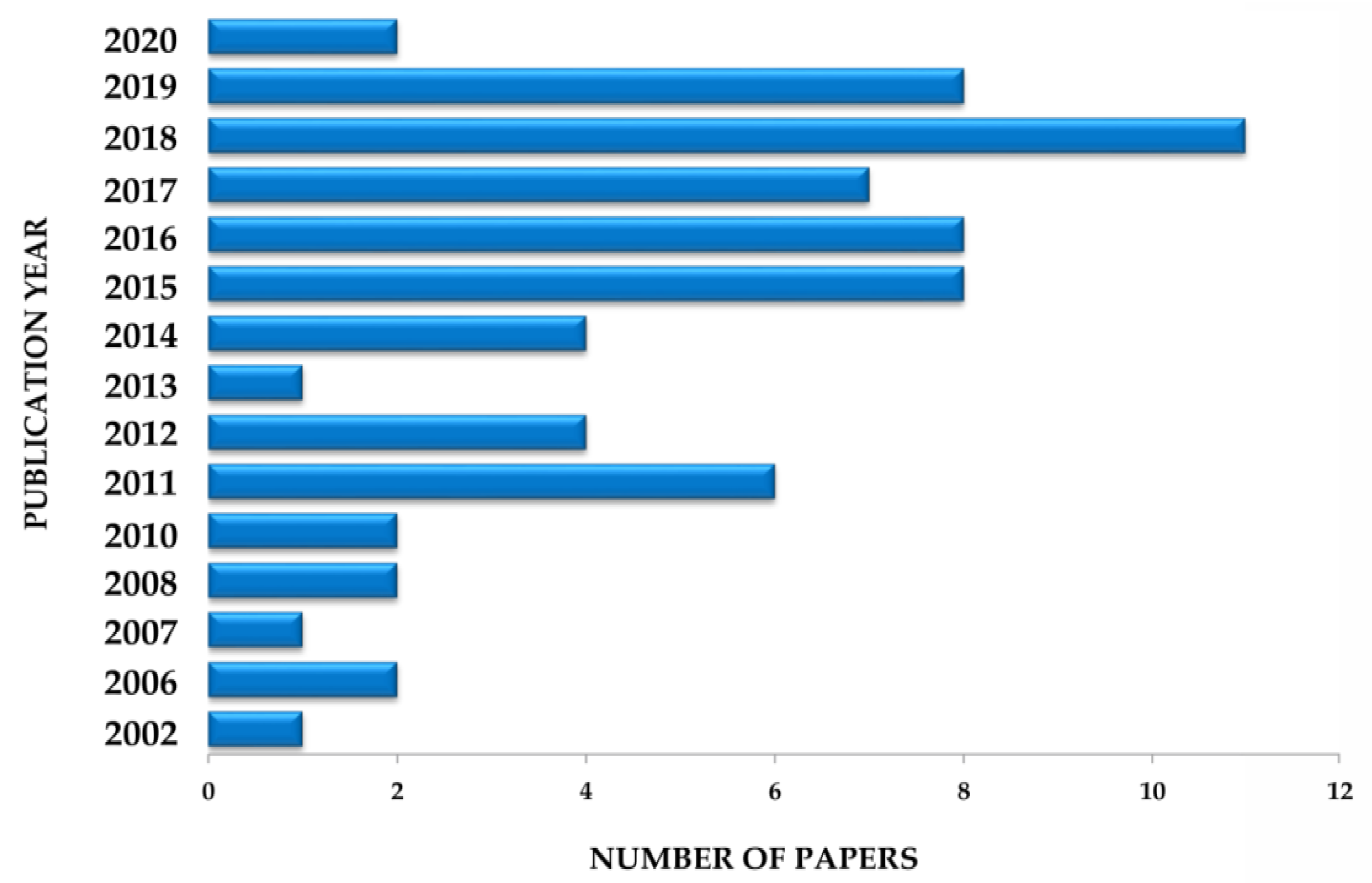
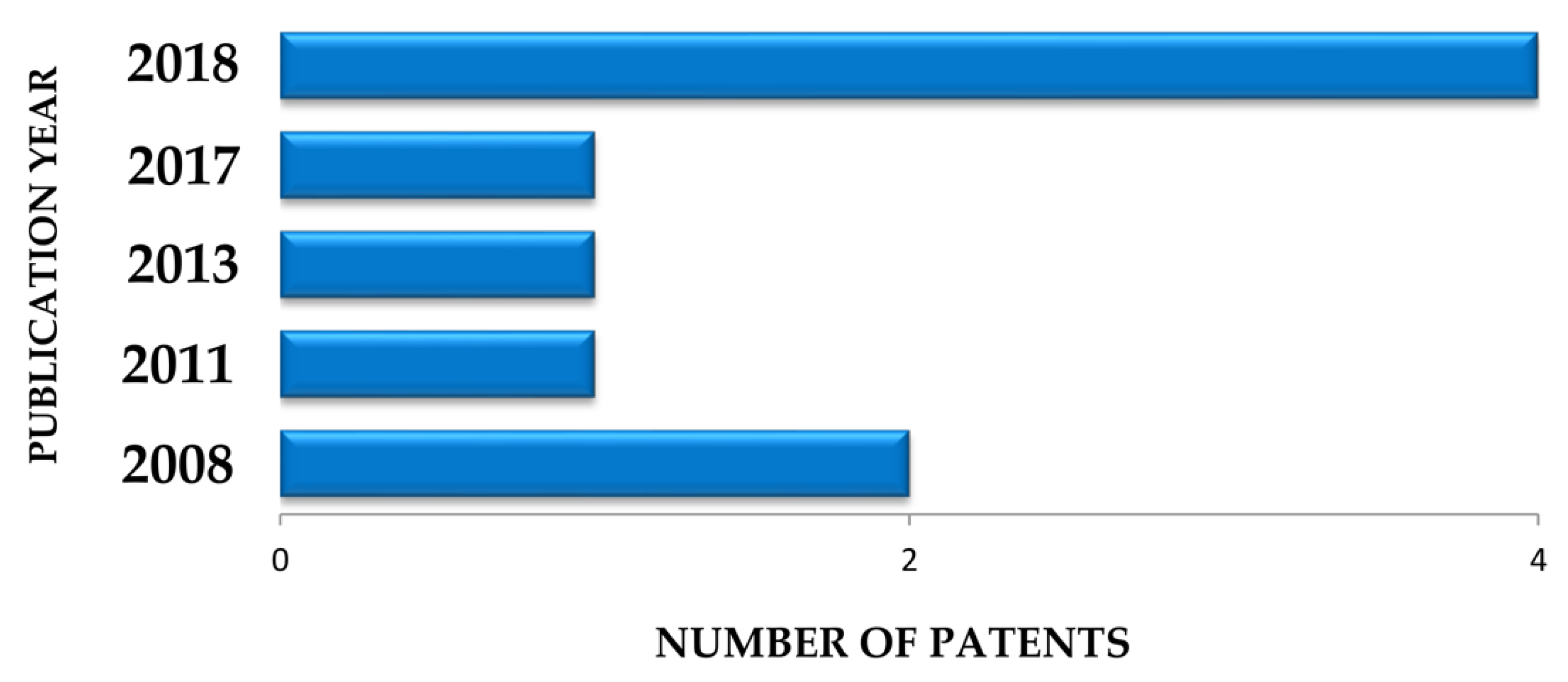
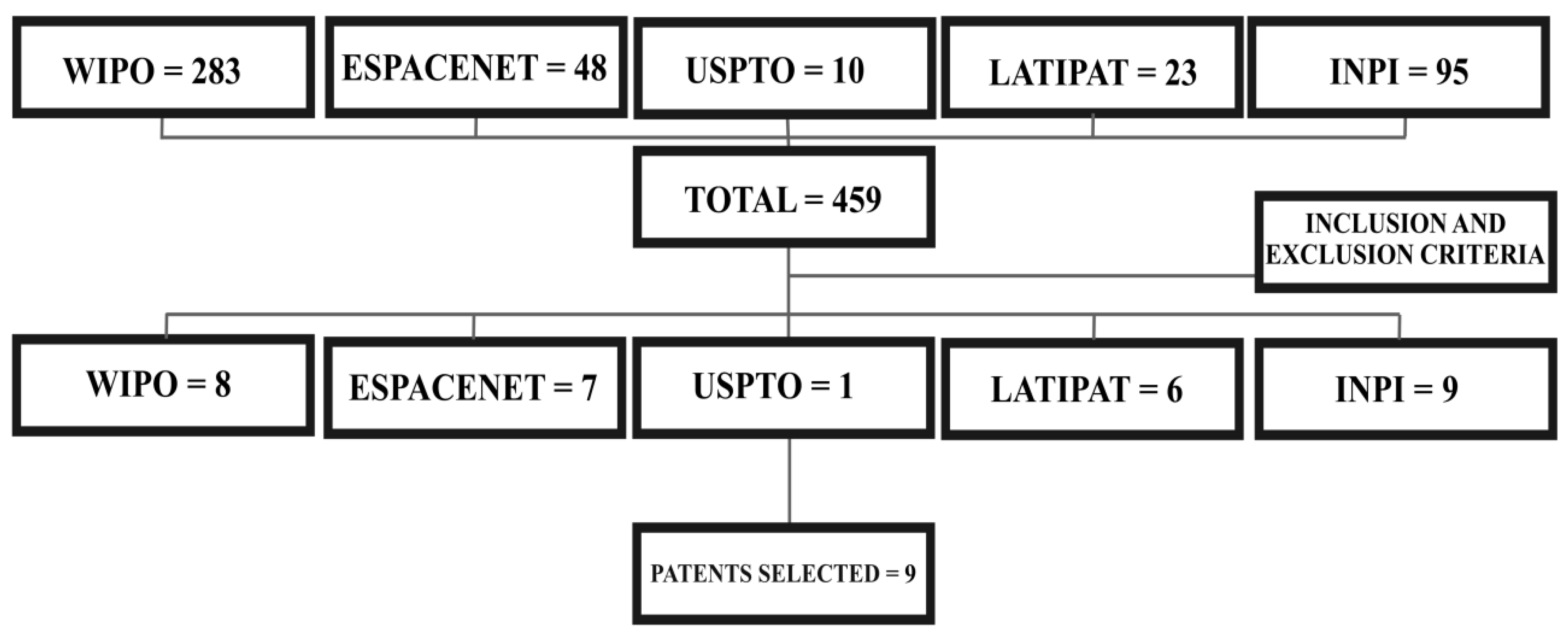
| Plant Part | Identified | References |
|---|---|---|
| vanillic acid | ||
| syringic acid | ||
| p-hydroxybenzoic acid | ||
| Pulp | protocatechuic acid | [29,30,31] |
| ferulic acid | ||
| quercetin | ||
| (+)-catechin | ||
| cyanidin-3-glucoside | ||
| cyanidin-3-rutinoside | ||
| oleic acid (18:1) | ||
| linoleic acid (18:2) | ||
| palmitic acid (16:0) | ||
| palmitoleic acid (16:1) | ||
| myristic acid (14:0) | ||
| lauric acid (12:0) | ||
| Oil | protocatechuic acid | [25,29] |
| p-hydroxybenzoic acid | ||
| catechin | ||
| vanillic acid | ||
| syringic acid | ||
| ferulic acid | ||
| procyanidin dimers | ||
| procyanidin trimers | ||
| 3-caffeoylquinic acid | ||
| 4-caffeoylquinic acid | ||
| 5-caffeoylquinic acid | ||
| 6,8-di-C-hexosyl apigenin | ||
| Leaf | 6,8-di-C-hexosyl apigenin sulfate | [41] |
| 6-C-hexosyl-8-C-pentosyl apigenin isomers | ||
| 6-C-pentosyl-8-C-hexosyl apigenin isomer | ||
| 8-C-glucosyl luteolin | ||
| 6-C-glucosyl luteolin | ||
| 6-C-glucosyl apigenin | ||
| 3-caffeoylquinic acid | ||
| 4-caffeoylquinic acid | ||
| Root | 5-caffeoylquinic acid | [41] |
| 4-caffeoylshikimic acid | ||
| 5-caffeoylshikimic acid |
| Model | Plant part | Assay/Dose | Results | Ref. |
|---|---|---|---|---|
| Pro-apoptotic activity | ||||
| In vitro method | Polyphenolic EO fraction | Analyses were conducted at concentrations of 5–20 mg/L. | Inhibition of HT-29 and SW-480 colon cancer cells. Absence of toxicity to CCD-18Co cells. Reduction of ROS induced by H2O2. | [45] |
| Antineoplasic activity | ||||
| In vivo method | Hydroalcoholic EO fruit extract | 8-Week-old female Winstar rats were treated with 200 mg/kg/day of lyophilized EO fruit extract for 16 weeks. | Decrease in the number of inflammatory cells and positive cells for macrophages in breast tumors. Reduction in immunostaining of VEGF, VEGFR-2 and COX-2. Lower concentrations of PGE2, VEGF and IL-10 in comparison with the control group. | [22] |
| In vivo method | Lyophilized EO berries | 4-Week-old male ICR mice received 2.5% and 50% açaí-containing diets for 14 weeks starting 1 week after colitis induction. | Reductions of the incidence of adenoma and cancer, MPO, TNF-α, IL-1β and IL-6 in colorectal cancer. Inhibition of PCNA and Bcl-2 expression and increased BAD and cleaved-caspase-3 expression. | [46] |
| In vivo method | Lyophilized EO pulp | Male Winstar rats received a basal diet supplemented with 5.0% and 7.5% of lyophilized açaí pulp for 22 weeks. | Reduction in the motility of colon carcinoma cells of the RKO line. Reduction in the total number of aberrant crypt foci, tumor cells, proliferation, and incidence of tumors with high-grade dysplasia. Increased gene expression of negative regulators of cell proliferation such as Dlc1 and Akt3. | [47] |
| In vivo method | Lyophilized EO pulp | 4-Week-old male Swiss albino mice received 2.5% and 5.0% of lyophilized açaí pulp as an additive to their low-fat diets for 4 weeks. | Reduction of damage to DNA from peripheral blood cells and reduction of multiplication of crypts and preneoplasic lesions of colon cancer. Total glutathione increase. | [48] |
| In vitro method | Hydroalcoholic EO seed extract | 1.25-200 µg/mL doses of the lyophilized extract were used for cell viability assay and 50 and 100 µg/mL for cell cycle performance and apoptosis. | High antioxidant activity by DPPH, ABTS, FRAP and ORAC. Decreased cell viability of lung cancer cell line (A549). Regulation of cell cycle, prevention of cell growth and increase in apoptotic cells. | [49] |
| In vitro method | Hydroalcoholic extract of EO seeds and peel | The extract was tested at concentrations of 10, 20 and 40 μg/mL in the MTT viability, Nuclear staining and Caspase-Glo® 3/7 luminescent assays. | The MCF-7 breast cancer line was the only one that responded to treatment with EO. Significant reduction in cell viability, and cell morphological characteristics altered by the appearance of autophagic vacuoles. | [50] |
| Anticlastogenic activity | ||||
| In vitro method | Dry EO pulp extract | The extract was tested at concentrations of 25, 50 or 100 μg/mL in the osteoclast differentiation, cell proliferation, hydroxyl apatite resorption and cytokine assays. | There was no evidence of toxicity to RAW 264.7 cells. Decreased secretion of IL-1α, IL-6 and TNF-α, but increased secretion of IL-3, IL-4, IL-13 and gamma interferon. | [51] |
| Anticonvulsant activity | ||||
| In vivo method | EO juice | Male Swiss rats were treated (10 μL/g body weight) with açaí juice by gavage for 4 days. | Delay of the first tonic–clonic seizure and decrease in its total duration. Prevention of total lipid peroxidation in the cerebral cortex. | [20] |
| In vitro method | Clarified EO juice | The EO juice was tested in neurons and astrocytes at concentrations of 0-50% to a final volume of 250 mL in HBSS | Increased binding to the agonist and decreased binding to the antagonist in cortical neurons. GABA uptake in the synaptic cleft points to an accumulation of endogenous GABA in the synaptic cleft. | [35] |
| Antidepressive activity | ||||
| In vitro and in vivo methods | Clarified EO juice | Male Swiss rats were treated (10 μL/g body weight) with açaí juice by gavage for 4 days. | Prevention of depressive behavior and changes in electromyography. Increased expression of TERT mRNA. Prevention of lipid peroxidation. Reduction of nitric oxide levels. | [52] |
| Antidiabetic activity | ||||
| In vivo method | Hydroalcoholic EO seed extract | The extract was administered by gavage (200/kg/day) to male Winstar rats for 4 weeks. Animals also exercised on a treadmill (30 min/day; 5 days/week). | Activation of the insulin signaling pathway in muscle and adipose tissue, increased levels of GLP-1 and anti-inflammatory action. Physical training enhanced the glucose-lowering effect, activating the phosphorylated adenosine monophosphate-activated protein kinase pathway and increasing the expression of insulin receptor. | [38] |
| Antihypertensive activity | ||||
| In vivo method | Aqueous commercialized extract;Hydroalcoholic EO peel and seed extracts | Concentrations in the range 0.3–100 μg/mL were tested in the mesenteric vascular bed of male Winstar rats. | Vasodilation by activation of the cyclic guanosine oxide-nitric-monophosphate pathway and possibly by the release of the endothelium-derived hyperpolarizing factor. | [37] |
| In vivo method | Hydroalcoholic EO seed extract | A dose of 200 mg/kg/day was tested in spontaneously hypertensive Winstar rats (50 days old) for 70 days. | Attenuation of protein carbonylation and nitrite levels. Up-regulation of eNOS and SOD1 expression. Increased activity of SOD. Prevention of the increase in media thickness and media:lumen ratio and decrease of elastic fibers. | [53] |
| Anti-inflammatory activity | ||||
| In vivo method | EO fresh pulp | Pure EO pulp (1 mL/100g) was administered to Winstar rats by gavage for 8 weeks. | EO intake alleviated chronic alcoholic liver injury in rats by attenuating oxidative stress and inflammatory response. | [54] |
| In vitro method | Extract from freeze-dried EO pulp | Isolated compounds were used in antioxidant and anti-inflammatory assays. | Five flavonoids were isolated and structurally identified. ORAC values varied significantly based on their chemical structures. Velutin strongly inhibited SEAP secretion in RAW-blue cells induced by LPS or ox-LDL, suggesting anti-inflammatory effects. | [55] |
| In vivo method | Hydroalcoholic EO seed extract | 8-Week-old male C57BL/6 mice were exposed to the smoke of 6 cigarettes for 5 days. Animals were treated with 300 mg/kg of the extract. | The group exposed to cigarette smoke exhibited lung morphology like that of the control group. The numbers of neutrophils and macrophages were lower than those not treated with EO. Reduction of pulmonary inflammation and oxidative stress markers. | [56] |
| In vitro method | Freeze-dried hydroalcoholic EO extract | Macrophages (RAW 264.7) were treated with different concentrations of the extract (0.001–1000 μg/mL) for 24 h at 37°C in a humid environment with 5% CO2. | The extract was able to reduce macrophage activation and proliferation through cell cycle arrest due to reduced activation of NLRP3 in response to the recovery of oxidative metabolism. | [57] |
| In vitro method | Polyphenolic compounds from EO juice | Polyphenolics were diluted to known concentration of total polyphenolics and normalized to contain a maximum concentration of 0.1% in DMSO (water: DMSO, 60:40). | The EO polyphenolic extract [1–5 mg gallic acid equivalent (GAE) L-1] had a protective effect against ROS production in human colon myofibroblastic CCD-18Co cells with and without LPS challenge. | [58] |
| In vitro method | EO fruit pulp fractions | The tested extract concentrations ranged from 50 to 1000 μg/mL for the methanol, ethyl acetate and acetone fractions, and from 10 to 250 μg/mL for the ethanol fraction. | LPS-mediated upregulation of p38-MAPK and NF-κB was attenuated by EO pulp fractions, which in turn down-regulated iNOS and COX-2 in BV-2 microglial cells. | [31] |
| In vivo method | Hydroalcoholic EO seed extract | Female Sprague-Dawley rats were treated with 200 mg/kg of extract dissolved in saline, by gastric tube for 30 days. | The EO extract effectively suppressed the establishment and growth of endometriotic lesions. | [59] |
| Antilipemic activity | ||||
| In vivo method | Hydroalcoholic EO seed extract | C57BL/6 male mice underwent a high fat (60%) diet. They also received 300 mg/kg/day of the extract by gavage for 12 weeks. | Mice fed with both high fat diet and EO seed extract showed a decreased food intake and body mass gain and had improved adiposity and hepatic steatosis. | [60] |
| In vitro method | Polyphenolic fraction from frozen pulp | Concentrations of EO polyphenols ranging from 2.5 to 10 μg GAE/mL were tested in mouse preadipocytes/fibroblast cells. | Reduced intracellular lipids accumulation during adipocyte differentiation in a dose-dependent manner and decrease in the expression of pro-inflammatory cytokines with and without TNFα challenge. | [39] |
| In vivo method | Ethanolic EO seed extract | N/D | Reduced proliferation of 3T3-L1 adipocytes. Inhibited proliferation of pre-adipocytes through transcription factors and adipogenic proteins such as PPARɣ, SREBP-1 and FAZ, suppressing lipid accumulation. | [61] |
| In vivo method | Pasteurized EO pulp | Two groups of female Fischer rats (standard diet + EO and hypercholesterolemic diet + EO) were supplemented with 2% of EO pulp for 6 weeks. | EO addition to diet had a hypocholesterolemic effect, reducing total and non-HDL cholesterol levels. | [62] |
| In vivo method | EO seed flour (ASF) | 3-Month-old male C57BL/6 mice received a high fat diet of 150 g/kg and 300 g/kg ASF daily for 12 weeks. | ASF treated groups showed lower triglyceride accumulation in hepatocytes compared to groups receiving just high fat diet. ASF consumption had a positive effect on liver steatosis. | [63] |
| In vivo method | Pasteurized EO pulp | Female Fischer rats were divided into groups with different diets, one of which receiving a standard AIN-93M diet and 2% EO and the other a hypercholesterolemic diet supplemented with 2% of EO daily for 6 weeks. | EO had a hypocholesterolemic effect on dietary-induced hypercholesterolemia through an increase in the expression of ABCGs and LDL-R genes. | [64] |
| In vivo method | EO pulp juice | Adult male New Zealand white rabbits were fed a regular diet plus 0.5% cholesterol for 12 weeks. | Consumption of EO extract markedly improved the lipid profile and attenuated atherosclerosis. These effects were related in part to a better balance in the synthesis and absorption of sterols. | [65] |
| Clinical study | EO pulp | EO pulp was provided in 200 g portions and consumed at leisure daily for 4 weeks. | EO consumption decreased ROS, ox-LDL and malondialdehyde while increasing antioxidative paraoxonase 1 activity. The increase in apolipoprotein A-I and cholesteryl ester transfer to HDL after the EO intake period suggested improved metabolism of this lipoprotein. EO also proved favorable for plasma HDL metabolism and antioxidant defense. | [66] |
| Antimicrobial activity | ||||
| In vitro method | Methanolic EO pulp extract | The extract was serially diluted to concentrations ranging from 1 mg/mL to 7.8 μg/ mL. | The methanolic extract was effective against cells and biofilms of S. aureus. Combinations of the methanolic extract and antimicrobial drugs resulted in statistically significant synergism. | [24] |
| In vitro method | Hydroalcoholic extract from EO leaves, fruits, and seeds | Using the serial dilution method, eight different concentrations (10–2.560 μg/mL) of plant extract were obtained and tested for antimicrobial activity. | The extracts showed antimicrobial activity against Clostridium perfringens, Streptococcus aureus and Pseudomonas aeruginosa, while none of them had any effect against Escherichia coli. | [67] |
| In vitro method | Hydroalcoholic EO pulp extract | Extracts with concentrations of 7.8, 15.6, 31.2, 62.5, 125, 250, 500, 1000 μg/mL were tested. | Two strains of Aspergillus fumigatus, AFAR and AF4091, showed poor adhesion. Extracts inhibited AFAR more than AF4091 strain growth. | [68] |
| Antinociceptive activity | ||||
| In vivo method | Ethanolic extract of EO flowers and stalks | Doses of 1, 10 and 30 mg/kg were administered. | Both extracts caused a 50% reduction in the total number of abdominal contortions. Increased rate of analgesia in the tail-removal model by stalks extract. Absence of hot plate analgesia for both extracts. | [42] |
| Clinical study | EO fruit and berry juice | Participants consumed 120 mL of EO fruit juice daily for 12 weeks. | Significant reduction of pain, better scores of amplitude and pain associated with activities of daily living in patients. | [69] |
| In vivo method | Hydroalcoholic EO seed extract (ASE) | 30, 100 or 30 mg/kg ASE samples were administered to male Swiss mice and male Winstar rats. | Reduction of nociception to acute/inflammatory pain, including thermal hyperalgesia, acetic acid-induced contortion and carrageenan-induced thermal hyperalgesia. Reduction of neurogenic and inflammatory phases after intraplantar injection of prevention of chronic pain in a rat spinal nerve attachment model. | [70] |
| Antioxidant activity | ||||
| In vitro method | Fractions from the mesocarp/epicarp and endocarp of EO fruit | Each sample extract was dissolved in 50% ethanol up to a concentration of 2 mg/mL. | In all antioxidant assays, including Hydrophilic (H-) and L-ORACFL assays, but except the ABTS radical quenching one, mesocarp/epicarp extracts showed stronger activity. | [30] |
| In vitro method | Compounds from powdered flakes of the fruit pulp and fractions | 10 µL of test samples in 25% DMSO solution were used. | Nine lignans and 2,6-dimethoxy-1,4-benzoquinone exhibited potent antioxidant activities by the Hydroxyl Radical Scavenging Activity assay, and 7 lignans by the DPPH assay. | [71] |
| In vitro method | Hydroalcoholic EO seed extract | Immortalized human umbilical vein endothelial cells (HUVEC) were treated with different concentrations of EO seed extract (0.1 – 100 μg/mL). | The EO seed extract was able to prevent the deleterious effects of H2O2 induced oxidative stress in HUVEC and positively modulated the antioxidant transcription factor (Nrf2) signaling pathway. | [72] |
| In vivo method | Hydroalcoholic EO seed extract. | Winstar rats were treated with 100 and 200 mg/mL extract by gavage for 14 days. | The EO seed extract showed no beneficial effect on the general framework of the cachectic syndrome in lab rats. | [73] |
| In vitro method | Lyophilized EO pulp | Gels were formulated with concentrations of 8, 12, 16 and 20% lyophilized EO pulp and sugars. | The gels with the highest concentration of freeze-dried EO pulp showed higher antioxidant activity. | [74] |
| In vitro method | Freeze-dried EO fruit pulp/skin powder. | For the neutrophils assay, EO powder was added to phosphate-buffered saline solution. For the COX assay, EO powder was extracted with 50% acetone and tested directly. | Freeze-dried EO showed a positive response as a COX-1 and COX-2 inhibitor. This study also proved that antioxidants in freeze-dried EO are able to enter human cells in a fully functional form in vitro. | [75] |
| Antiplasmodial activity | ||||
| In vitro and in vivo methods | Polyphenolic fractions from EO pulp | Fractions: Total phenolics (1), non-anthocyanin phenolics (2) and total anthocyanins (3). For the in vitro study 1.0 to 20.0 mg/L concentrations were used, while for the in vivo mice infected with two Plasmodium chabaudi strains were treated with fraction 1 at doses of 10, 15 and 20 mg/kg/day for 12 days. | None of the doses of 1 and 2 reduced the DNA content of either parasite strain tested. Fraction 2 showed moderate antiplasmodial activity in both strains, starting at 10.0 mg/L. During parasitemia peak in the in vivo assay, all concentrations of fraction 1 showed a decrease in parasite growth. | [76] |
| Antiproliferative activity | ||||
| In vitro method | Polyphenolic-enriched fractions from EO pulp and oil | Polyphenolic isolates were sterile filtered, normalized to a final concentration of 0.1% in DMSO and tested on HT-29 colon cancer cells. | Both polyphenolic extracts caused a significant decrease in total HT-29 cell number in a concentration-dependent manner. However, the oil polyphenols extract was more than twice as effective at all dilutions. | [29] |
| In vitro method | Monomeric and polymeric anthocyanin fractions from EO pulp | 50 and 500 µg of cyanidin-3-glucoside equivalent/mL of anthocyanin fractions were diluted in HBSS and tested on HT-29 colon cancer cells. | Both fractions and their mixtures decreased total cell numbers. The monomeric anthocyanin fractions (5-20 µg/mL) were more effective in reducing HT-29 cell proliferation. | [36] |
| In vitro method | EO pulp fractions | Nine fractions were obtained based on the solubility and affinity characteristics of the EO phytochemicals. | HL-60 leukemia cells showed a dose-dependent decrease in viability after 24 h-exposition to all EO fractions, with exception of the lipophilic and C18 non retained fractions (II and VI, respectively). Fractions I, III and V strongly suppressed HL-60 proliferation through apoptosis induction by caspase-3 activation. | [77] |
| In vitro method | Anthocyanin-rich EO pulp extract | The anthocyanin-rich extract was applied to cell cultures of C-6 rat brain microglia cells and MDA-468 breast cancer cells at concentrations of 50, 100 and 200 µg/mL. | The treatment suppressed proliferation of C-6 rat brain glioma cells significantly. However, the growth of MDA-468 breast cancer cells was not affected. | [78] |
| Antiprotozoal activity | ||||
| In vitro and in vivo method | Clarified EO juice | EO juice acquired from Amazon Dreams (Belém, PA, Brazil). Concentrations: 1: 12.5, 1:25 and 1:50 (v: v, EO and DMEM or RPMI medium culture). | Reduction in the number of Leishmania promastigotes and amastigotes. Reduction in cytokine levels of IL-17. Absence of cytotoxicity to macrophages infected by amastigotes | [26] |
| Cardioprotective activity | ||||
| In vivo method | Hydroalcoholic extract of EO seeds | Male Winstar rats were treated with 100 mg/kg/day of the extract for 4 weeks. | Positive modulation in systolic and diastolic blood pressure. Better distance covered when compared to the group with induced myocardial infarction. | [79] |
| Clinical study | EO fruit | Healthy individuals received gel capsule containing 500 mg of açaí provided by Nature’s Bounty Inc. (Bohemia, NY, USA). | Reduction of systolic blood pressure. Absence of variations in hemodynamic and electrocardiographic effects. | [80] |
| Healing | ||||
| In vitro and in vivo methods | EO aqueous extract | Concentrations of 0, 0.1, 0.3 and 1 mg/mL were tested in vitro, and a dose of 200 μL/wound/day for 18 days was tested in male Sprague-Dawley rats. | Increased expression of fibronectin mRNA. Decreased mRNA expression of MMP-1. Macroscopic and histopathological observations demonstrated healing. | [81] |
| In vivo method | EO aqueous extract | 6-Week-old male Sprague-Dawley rats had 50 μL of the extract applied to the wound area once a day for 6 days. | Macroscopic and histopathological observations demonstrated healing. Significantly high antioxidant effects in the Electron donating ability and ABTS assays, although they were slightly lower than in the control group. Low SOD value. | [82] |
| Cytotoxic activity | ||||
| In vivo method | Commercialized EO pulp | A mixture containing 5% EO was tested in cytotoxicity assays. | Improvement of fractional shortening of the left ventricle. Increase in β-hydroxyacyl-CoA dehydrogenase, phosphofructokinase, citrate synthase, complex enzyme II and ATP synthase activities. Decrease in myocardial lipid hydroperoxide and MMP-1 activities occurring in doxorubicin cardiotoxicity. | [34] |
| In vitro method | Fractions of hydroalcoholic EO seed extract after liquid-liquid partition | Chloroform (CF) and hexane (HF) fractions were diluted in DMSO, while the ethyl acetate fraction (EAF) was diluted in Milli-Q water. MCF-7 cells were treated with HF, CF and EAF at concentrations of 10, 20, 40 and 60 µg/mL. | HF, CF and EAF promoted cell viability reduction. However, EAF was significantly more cytotoxic when compared to HF and CF after 48 h, hence indicating antineoplastic potential. Cell death occurred by necrosis, not apoptosis | [83] |
| Hepatoprotective activity | ||||
| In vivo method | Hydroalcoholic EO seed extract | High-fat diet mice were treated with 300 mg/kg/day. | Reduction in body weight gain and food intake. Reduction of glycemic indexes, cholesterol and triglycerides in the liver. Reduction in the expression of SREBP-1c and HMG-CoA reductase. Increased expression of pAMPK, pACC, ACC, ABCG5 and ABCG8. | [60] |
| In vivo method | Marketed EO seed flour | Diet was prepared containing 15 or 30% of commercially available EO seed flour (Prag Soluções, Jaú, SP, Brazil). Diet was administered for 12 weeks to 3-months-old male C57BL/6 mice. | Reduction of lipid, glycemic indexes of insulin, leptin and lipogenesis. Reduced expression of SREBP-1c and HMG-CoA reductase. Increased expression of pAMPK, pACC, ACC, ABCG5/8. | [63] |
| In vitro and in vivo methods | Commercialized EO pulp | Doses tested in transit: 0, 12.5, 25, 50, 100, 200 and 400 mg/mL; Doses tested in steatotic mice: 3 g/kg/day for 6 weeks. | Inhibition of ROS and absence of cytotoxicity in liver carcinoma cells. Reduction of alanine aminotransferase, number of inflammatory cells, serum TNFα, lipid peroxidation and protein carbonylation. | [84] |
| Immune system inhibition | ||||
| In vitro method | EO pulp fruit | EO pulp was filtered and added to the cell culture medium at 1/30 or 1/60 dilution. | Reduction of antigen-induced degranulation of mouse primary cultured mast cells. Inhibition of FcεRI signaling pathways and suppression of FcεRI-mediated complementary signaling pathway. | [85] |
| Neuroprotective activity | ||||
| In vitro method | EO extract and isolated fractions | Concentrations in the range 0.5 - 50 μg/mL were tested. | Better viability of rat pheochromocytoma cells (PC12). Loss of ThT fluorescence. Disrupted human amyloid-protein (Aβ1–42) fibril and aggregate morphology. | [86] |
| In vitro method | Hydroalcoholic EO fruit extract | SH-SY5Y cells were exposed to 5 μg/mL of açaí extract. | Increased enzyme activity of the mitochondrial complex I, amount of proteins and overexpression of the mitochondrial complex I Q module subunits NDUFS7 and NDUFS8. Decreased levels of ROS in cells and lipid peroxidation. | [87] |
| Lung protective activity | ||||
| In vitro and in vivo methods | EO fruit pulp; polysaccharides isolated from EO fruit powder | TLR42/2 and TCRa2/2 mice (both on the C57BL/6 background) were treated with 5 μg to 500 μg of polysaccharides. Human monocyte-macrophage MonoMac-6 cells were also used for in vitro assays. | Polysaccharide fractions obtained from EO stimulated the activity of T γδ lymphocytes in cultures. Isolated fractions of polysaccharides with high molecular weight activated myeloid T cells and γδ in vitro and induced myeloid cell recruitment and IL-12 production in vivo. | [88] |
| In vitro and in vivo methods | Polysaccharides isolated from EO fruit powder | RAW264.7 cells were treated with varying doses of the isolated polysaccharides. The mice were treated with 1000 μg of polysaccharides before infection. | Isolated polysaccharide fraction reduced the replication of Francisella tularensis and Burkholderia pseudomallei in the lung of EO infected mice. Increased response of IFN-γ by NK in infections by F. tularensis. Increased IFN-γ response by NK and γδ T cells in infections by B. pseudomallei. | [89] |
| Renoprotective activity | ||||
| In vivo method | EO fruit extract | Doses of 500 and 1000 mg/kg/day were administered to male adult Wistar albino rats for 15 days. | Reduction of BUN, serum creatinine and renal tissue content of KIM-1. Reduction of levels of MDA, MPO, IFN-γ, caspase-3, collagen IV, endothelin-1 and IL-1. | [90] |
| In vivo method | Hydroalcoholic EO seed extract | A dose of 200 mg/kg/day was administered to male rats with streptozotocin (STZ)-induced diabetes for 45 days. | Decreased serum levels of urea, creatinine and albumin, renal fibrosis, TBARS, carbonyl and 8-isoprostane levels. No variation in the concentrations of IL-6, TNF-α, MCP-1 and caspase-3. Increase in the number of glomeruli and SOD, catalase and GPx. | [91] |
| In vivo method | Hydroalcoholic EO seed extract | Young male Winstar rats received 200 mg/kg of the extract for 40 days. | Prevention of the increase in systolic blood pressure, decrease in renal volume, glomeruli and collagen deposition. Decreased serum levels of urea, creatinine and urinary protein. Reduced MDA and carbonyl protein contents, increased nitrite, SOD, CAT and GPx contents. | [92] |
| Hematopoietic effect | ||||
| In vivo method | EO fruit pulp | C57BL/6NCrSlc mice were used for dosing and histopathology. A dose of 10 mL/kg/day was tested for 4 days | Increase in erythrocytes, hemoglobin and hematocrit. Increased erythropoietin hormone and transient gene expression hematological factor (EPO) and VEGFA. | [93] |
| Model | Plant Part | Assay/Dose | Results | Ref. |
|---|---|---|---|---|
| Anticancer | ||||
| In vitro and in vivo methods | EO oil-containing nanoemulsion (NanoA) | 14-Weeks-old C57BL/6 female mice were treated with 100 μL of NanoA directly injected into the tumor mass. The experiment was conducted for 15 days. | Application of photodynamic therapy showed death of B16F10 melanoma cells in vitro and of tumor-bearing C57BL/6 mice. | [95] |
| Anti-inflammatory activity | ||||
| In vivo method | EO oil from commercialized pulp | Doses of 500, 1000 and 1500 mg/kg of oil were administered to male Swiss rats for 6 days. | Inhibition in the formation of subcutaneous granulomatous tissue, reduction in ear edema, vascular permeability and migration of neutrophils in peritonitis. | [19] |
| Antilipemic activity | ||||
| In vivo method | EO oil obtained from commercialized pulp | Winstar rats were treated by gavage for 10 days at an effective dose of 1226 mg/kg. | The oil reduced levels of total cholesterol, triglycerides and direct low-density lipoprotein cholesterol (LDL-c) but increased that of HDL. | [40] |
| Antimicrobial activity | ||||
| In vitro method | EO oil from pulp and seeds | Discs impregnated with 10 μL of EO oil from the pulp and seeds were added to media containing Pseudomonas aeruginosa and Staphylococcus aureus. | Discs containing EO oil showed inhibition halo at first reading (after 24 h) on S. aureus. The oil showed no inhibitory effect on P. aeruginosa | [23] |
| In vitro method | EO oil and inclusion complexes with cyclodextrins. | The drug’s modulatory activity was tested at an initial concentration of 1024 μg/mL. | EO oil showed activity against Enterococcus faecalis, S. aureus, P. aeruginosa and Escherichia coli. Inclusion complexes with cyclodextrins showed a reduction in the Minimum Inhibitory Concentration of the oil, increasing its activity. | [25] |
| Antinociceptive activity | ||||
| In vivo method | EO oil from commercialized pulp. | Doses of 500, 1000 and 1500 mg/kg of oil were administered to male Swiss rats for 6 days. | Reduction of up to 55.58% in the total number of abdominal contortions. | [19] |
| Cytotoxic activity | ||||
| In vivo method | EO oil from pulp | EO oil was diluted in vehicle (1% Tween 80) and administered to male Winstar rats by gavage for 14 days at doses of 30, 100 and 300 mg/kg. | Changes in the thyroid gland directly related to the thyroid follicles. Hypertrophy associated with disorganization and alteration in the chemical composition of the colloid. Disorganization of hepatic tissue, alteration in the amount of lipids and vacuoles in the cytoplasm. The oil led to damage in cells and tissues of both organs. | [96] |
| Genotoxic activity | ||||
| In vivo method | EO oil from pulp | Oil was diluted in vehicle (1% Tween 80), and male Wistar rats were treated with EO by gavage at doses of 30, 100 and 300 mg/kg, for 14 days. | Peripheral blood leukocytes, liver, bone marrow and testicular cells indicated that the oil had no significant genotoxic effect. No chromosome breakage, aneugenicity, polychromatic erythrocytes were observed, which indicated no perturbation in hematopoiesis. | [97] |
| Reference | IPC | Applicant | Inventor /Year/ Country | Compound/Formulation | Indication/ Pharmacological Profile | Route of Administration/Dose | Assay |
|---|---|---|---|---|---|---|---|
| PI 0418614-1 A2 | A61K A61P | Federal University of Rio de Janeiro | Soares de Moura, 2008, Brazil. | Hydroalcoholic extracts of fruits and lumps (gelatin capsules, tablets) | Analgesic action for treatment and prevention of pain in humans and animals | Oral/10–1000 mg | Preclinical |
| PI 0604281-3 A2 | A61K A61P | Soares de Moura | Soares de Moura, 2008, Brazil. | Hydroalcoholic extracts of fruits and lumps (gelatin capsules, tablets) | Vasodilatory action in the treatment and prevention of vasospastic, ischemic diseases and hypertension. | Oral/10–1000 mg | Preclinical |
| WO 2011/1036448 A1 | A61K A61P | State University of Rio de Janeiro | Soares de Moura, 2011, Brazil. | Hydroalcoholic extracts of the fruits (hydrophobic and hydrophilic ointments) | Accelerated wound healing process. | Topical/0,001 - 100 mg/g. | N/D |
| US 61/814791 | A61K A61P | Neocutis S. A. | Dreher, 2013, USA. | Combined vitamin C, E and an antioxidant compounds, especially EO berries (hydrophobic and hydrophilic formulations for topical use) | Treatment and prevention of skin damage. | Topical and subcutaneous. | N/D |
| BR 102015017543-4 A2 | A61K A61P | Federal University of Maranhão | Nascimento, 2018, Brazil. | Hydroalcoholic extracts obtained from lumps (gelatin capsules, syrups, energy bars and flour) | Chemotherapeutic and chemopreventive activity. | Oral/10, 20 or 40 µg/ml. | Preclinical |
| BR 102017007451-0 A2 | A61K A61P | Federal University of Amapá | Ribeiro da Silva et al. 2018, Brazil. | Ethanolic extract of the fruits (tablet, capsule, granules, controlled release pharmaceutical form). | Antioxidant activity | Oral. | Preclinical |
| BR 102018005450 3 A2 | A61K A61P | Moreira Castilho et al. | Moreira Castilho et al., 2018, Brazil. | Seeds extract (solids, liquids, semisolids or pastes, tablets, hard or soft capsules, lozenges, powders, granules, suspensions, dispersions, emulsions, micro or nanoparticles, liposomes, micelles or vesicles). | Treatment of diseases and metabolic syndromes. | Oral, peroral, enteral, parenteral, topical, transdermal, inhaled, intrapulmonary, vaginal, rectal, intraocular and sublingual. | In vitro |
| BR 1020180767933 | A61K | Federal University of Rio Grande Grande do Norte | Almeida et al. 2018, Brazil. | Inclusion complexes of EO oil and cyclodextrins (tablet, capsule, powder, oral suspension, capsule, cream, ointment or gel). | Infectious processes/ antimicrobial antioxidant and anti-inflammatory activities. | Oral, intravenous, intramuscular, intraperitoneal, subcutaneous, topical. | N/D |
| BR 102017013494-6 A2 | A61K | Federal University of Maranhão | Figueirêdo 2019, Brazil. | Hydroalcoholic extract of the leaves (capsule, solution, syrup, tablets, gel, aerosols, mouthwash, cream, powder, paste, ointment, pellets, suppository and soap) | Infectious processes/ antimicrobial activity (against various bacteria and fungi of clinical interest). | Oral, topical, external and internal use/0,01 to 5g for 100g of product. | N/D |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, Á.A. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules 2020, 10, 813. https://doi.org/10.3390/biom10060813
de Almeida Magalhães TSS, de Oliveira Macedo PC, Converti A, Neves de Lima ÁA. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules. 2020; 10(6):813. https://doi.org/10.3390/biom10060813
Chicago/Turabian Stylede Almeida Magalhães, Thalita Sévia Soares, Pollyana Cristina de Oliveira Macedo, Attilio Converti, and Ádley Antonini Neves de Lima. 2020. "The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention" Biomolecules 10, no. 6: 813. https://doi.org/10.3390/biom10060813
APA Stylede Almeida Magalhães, T. S. S., de Oliveira Macedo, P. C., Converti, A., & Neves de Lima, Á. A. (2020). The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules, 10(6), 813. https://doi.org/10.3390/biom10060813