Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins
Abstract
1. Introduction
Membrane Protein
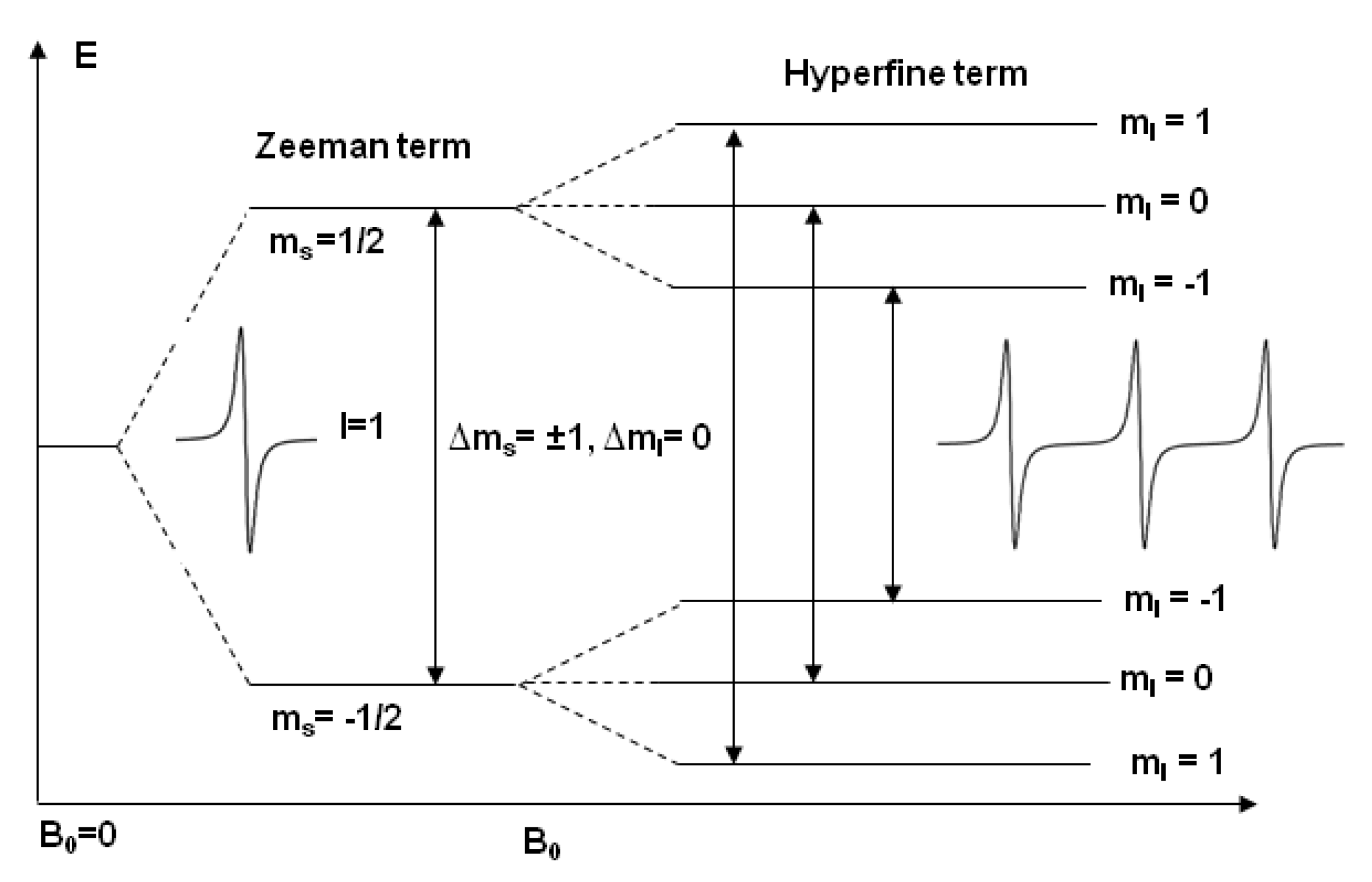
2. Electron Paramagnetic Resonance
2.1. Continuous Wave Electron Paramagnetic Resonance (CW-EPR) Spectroscopy
2.2. Pulse Electron Paramagnetic Resonance Spectroscopy
3. Biological EPR
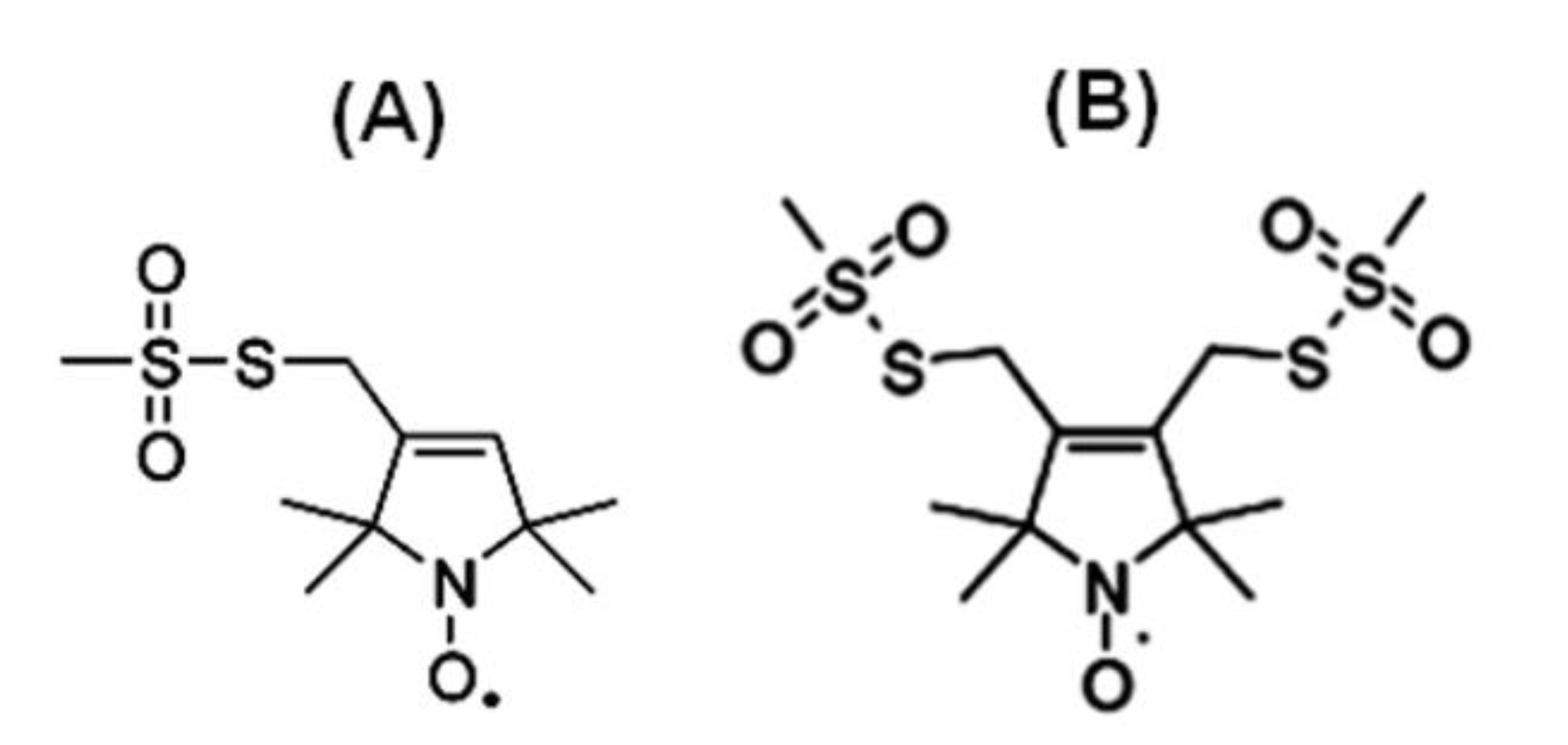
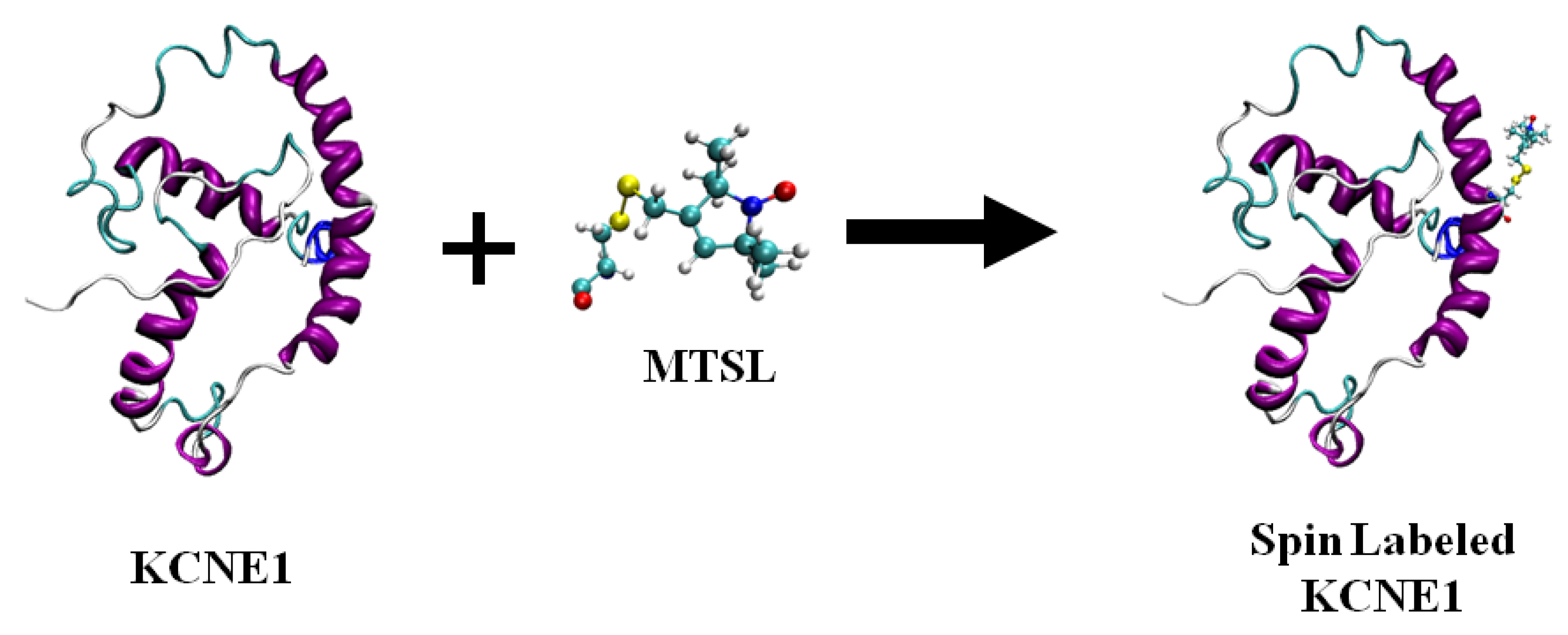
Nitroxide Based Spin Labeling EPR
4. Nitroxide Based Site-Directed Spin Labeling EPR for Studying Membrane Proteins
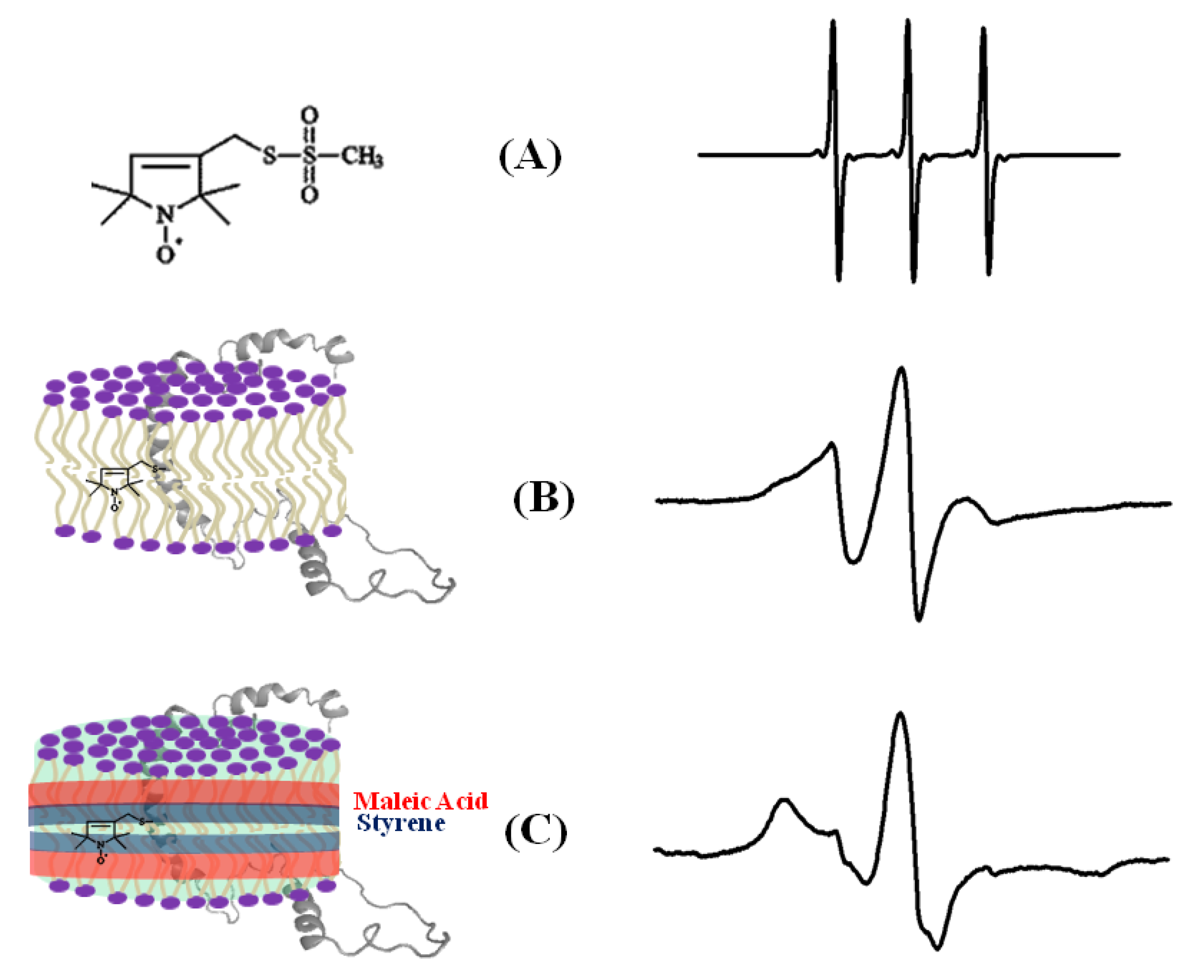
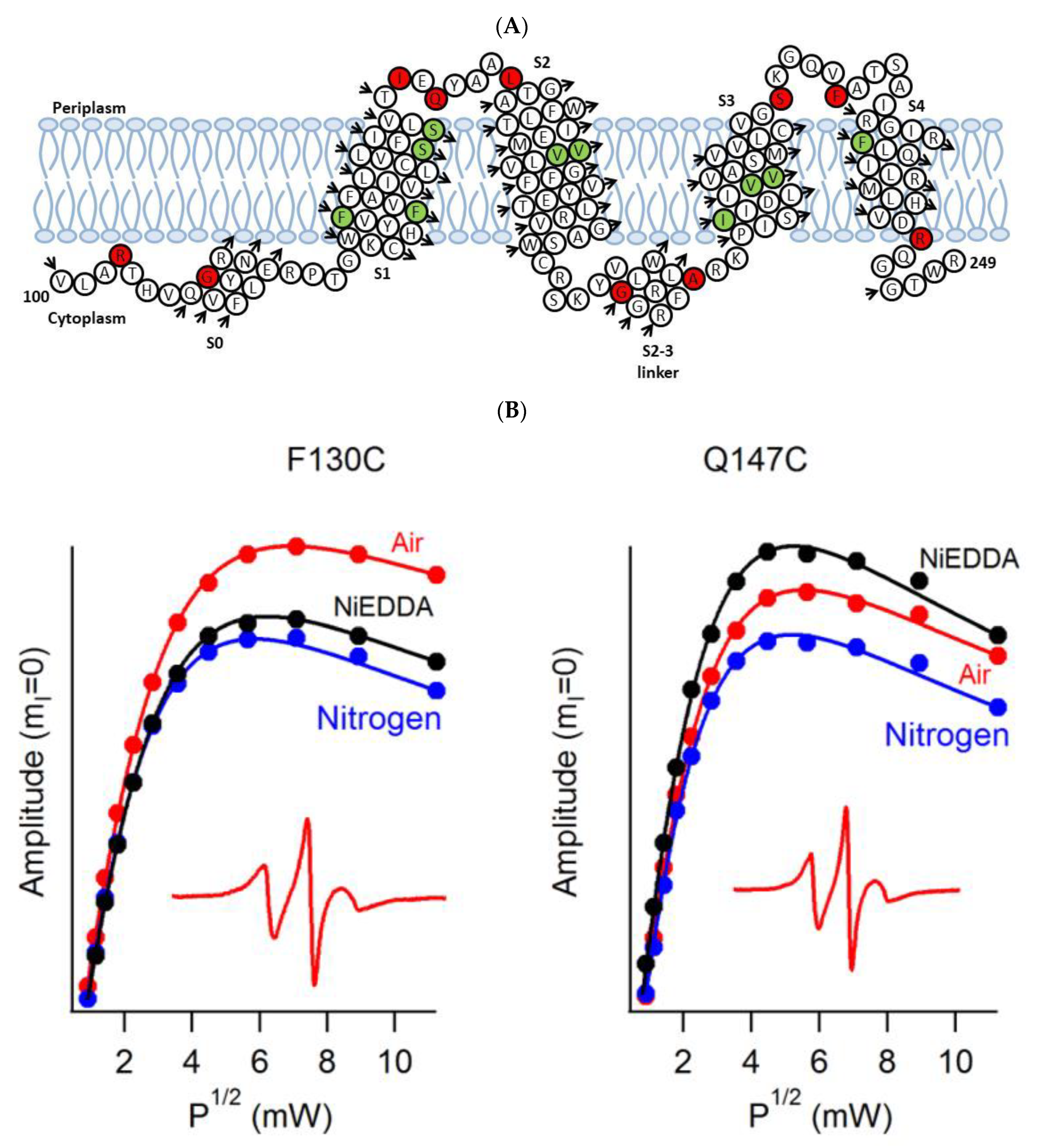
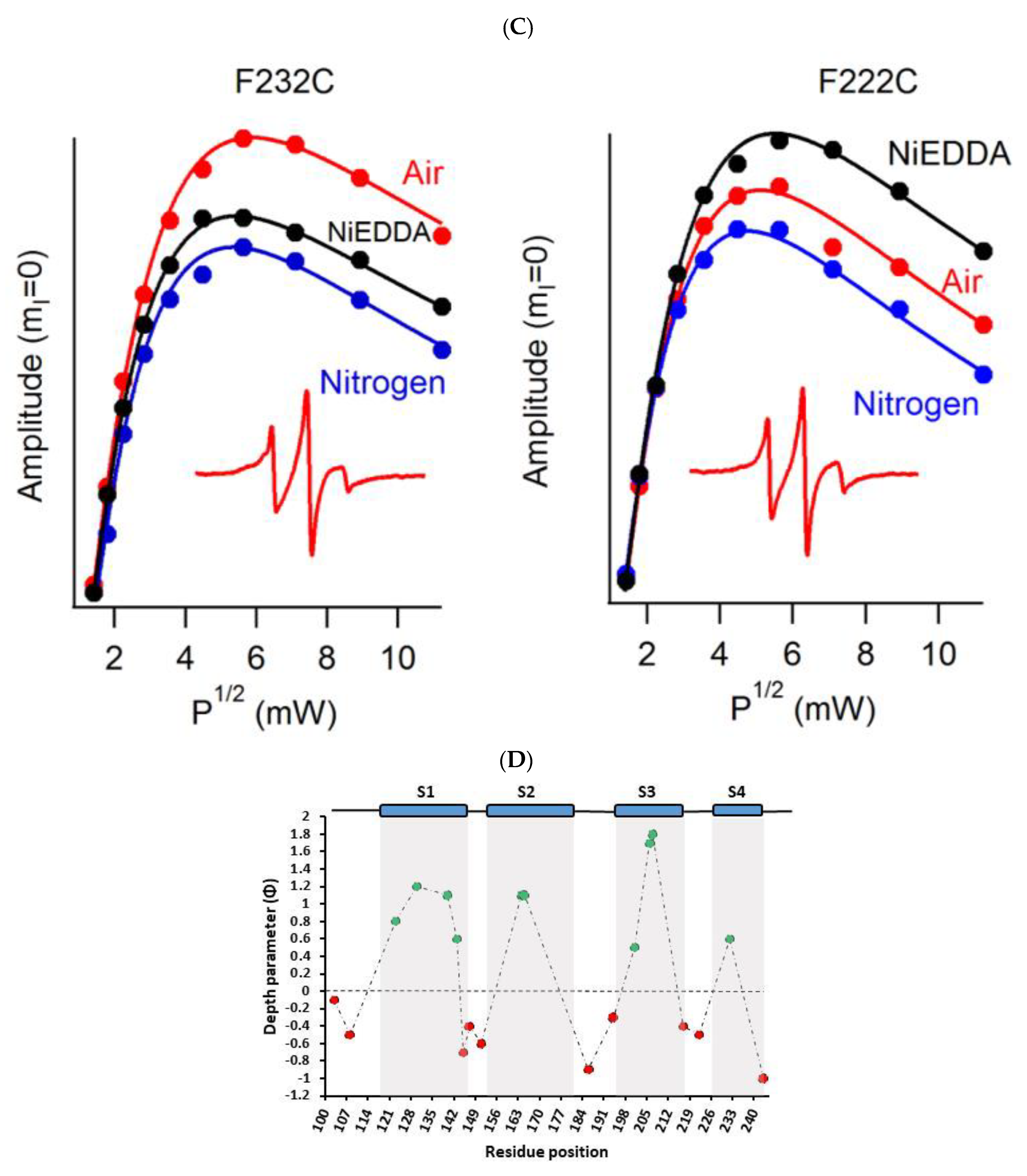
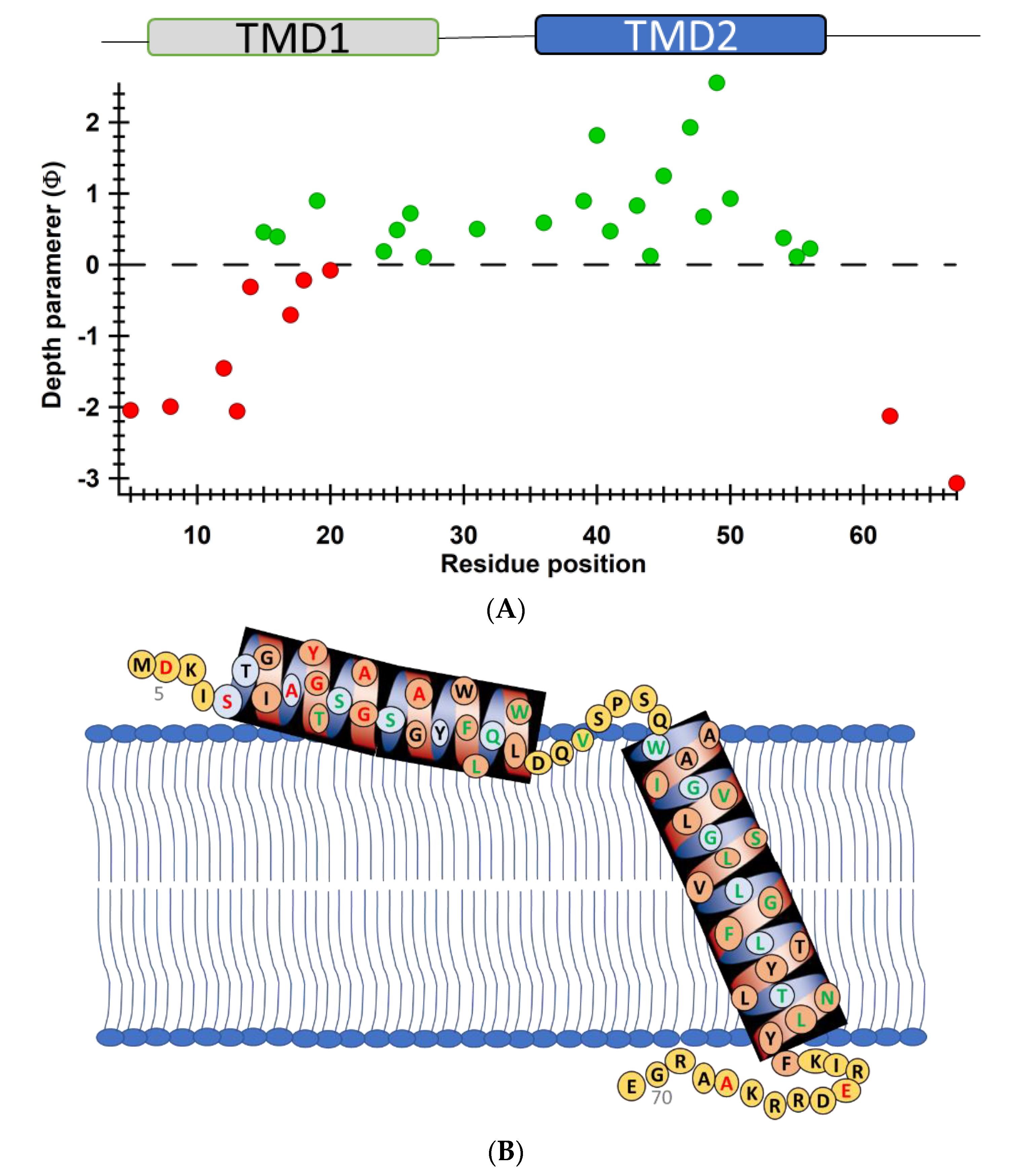
4.1. SDSL CW-EPR for Studying Structural Topology and Dynamic Properties of Membrane Proteins
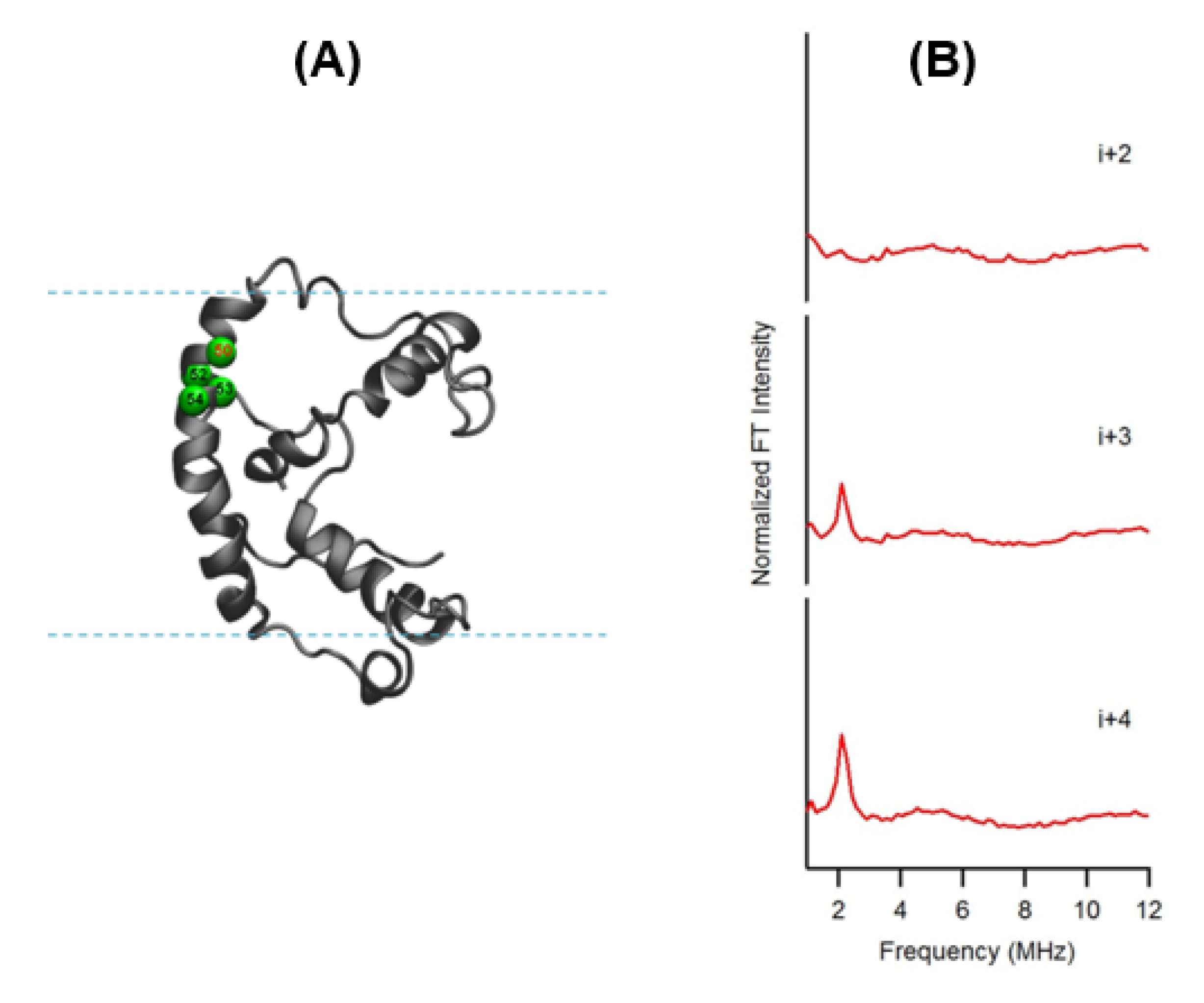
4.2. Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy for Investigating the Local Secondary Structure of Protein/Peptides
4.3. SDSL EPR for Distance Measurement of Membrane Proteins
4.3.1. CW-Dipolar Line Broadening SDSL EPR for Distance Measurement
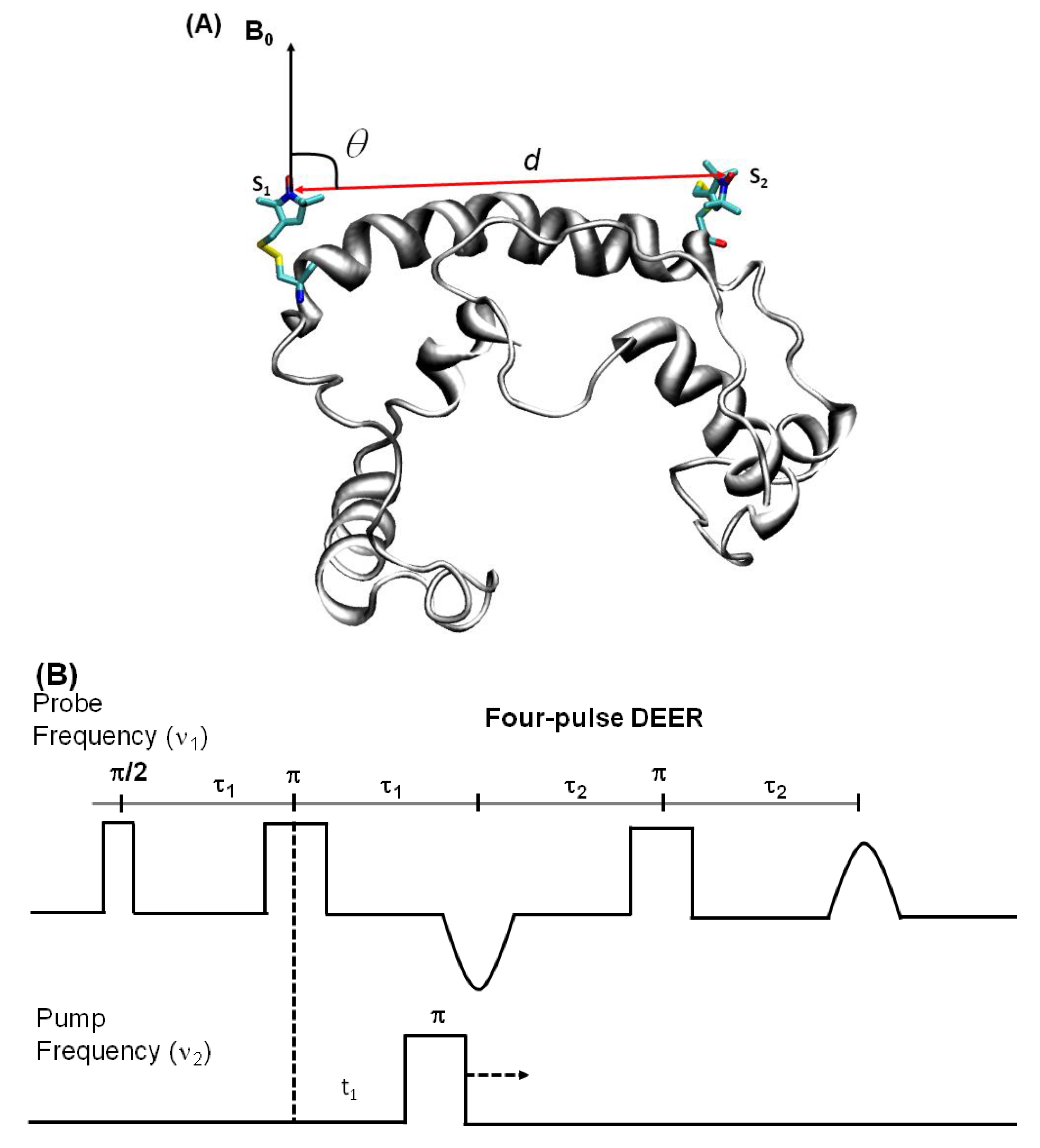
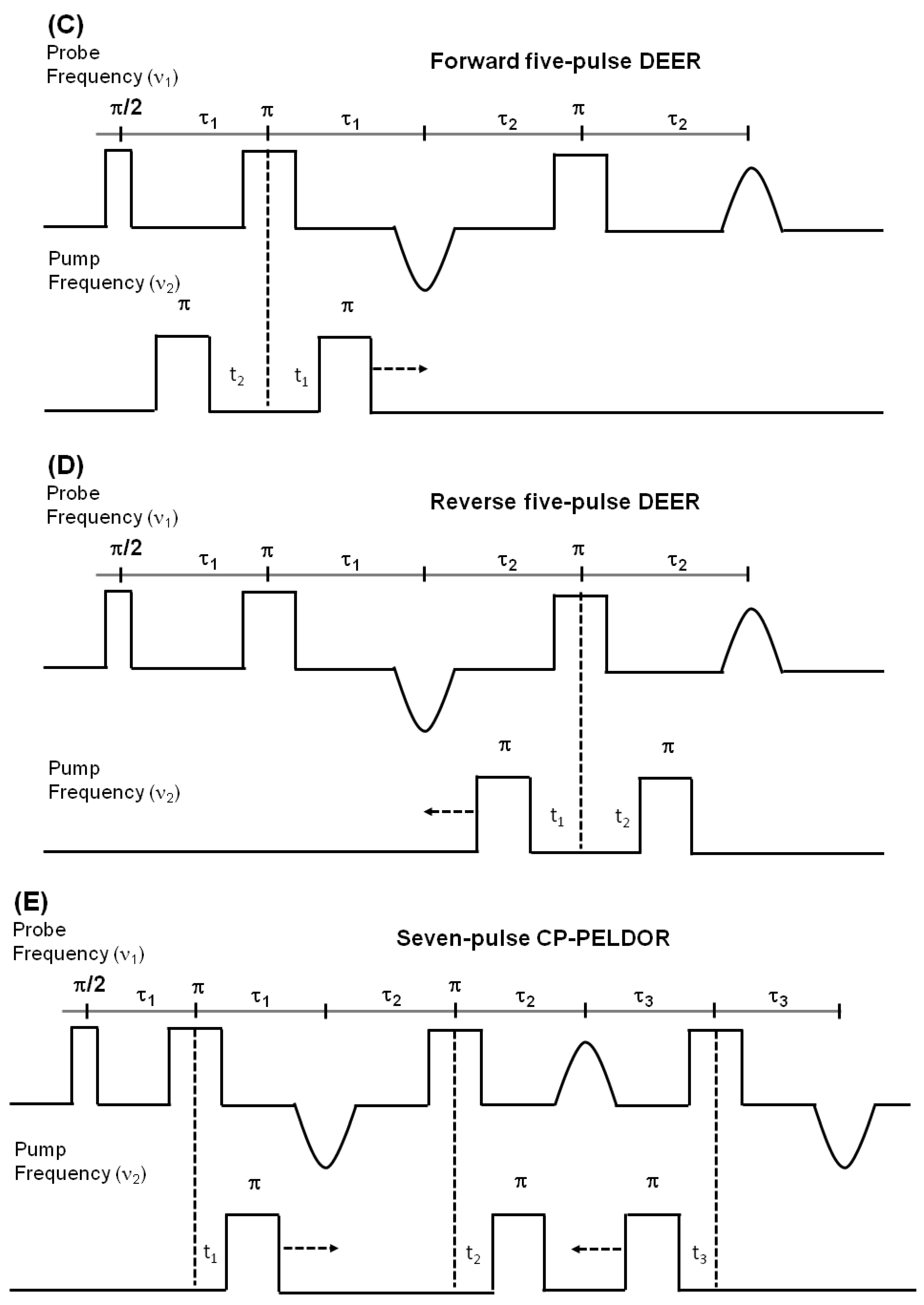
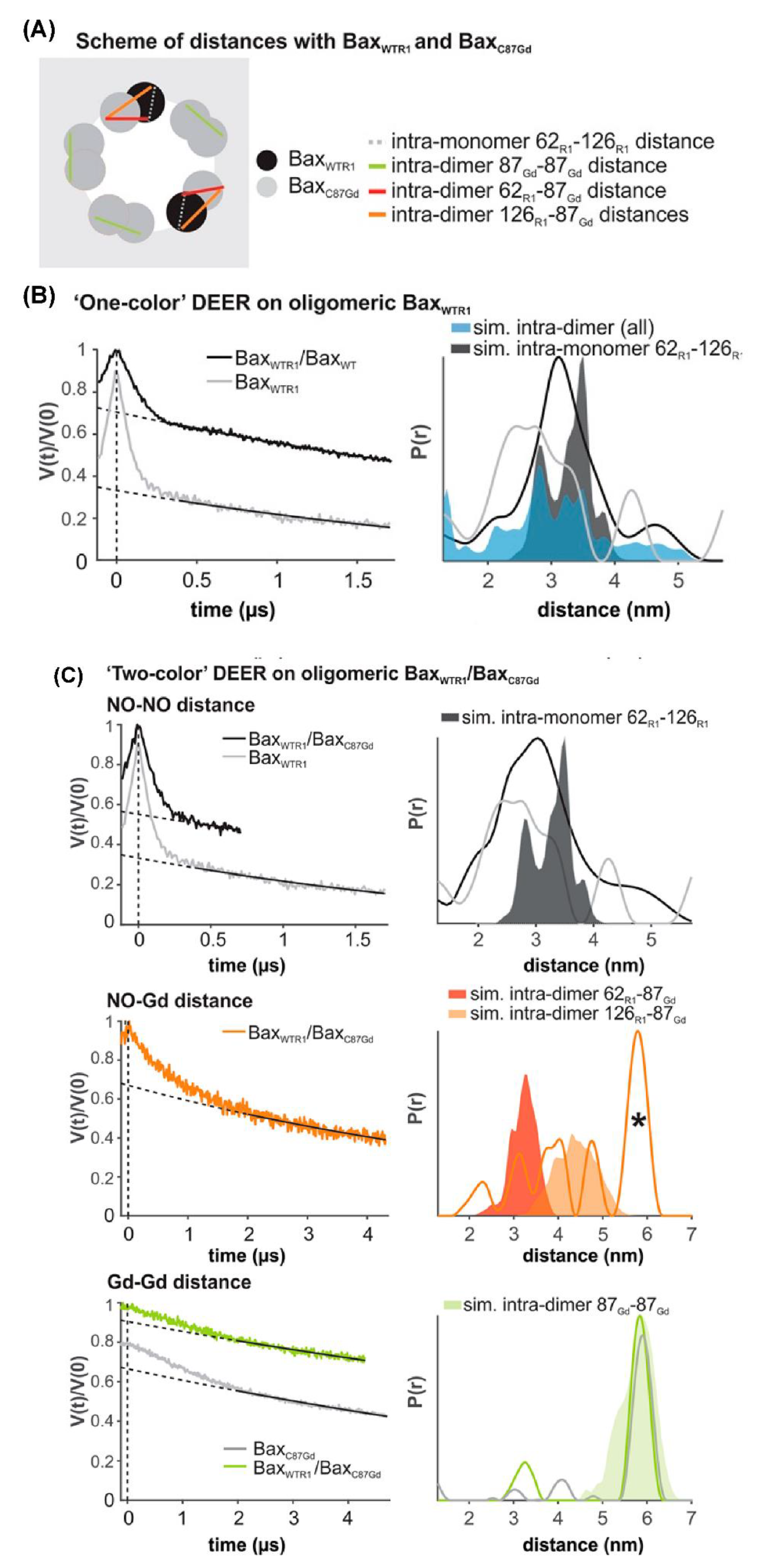
4.3.2. Double Electron Electron Resonance (DEER) Techniques for Distance Measurements
Challenges and Methodological Development in DEER Measurements for Membrane Proteins
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lodish, H.; Berk, A.S.; Zipursky, L.; Matsudaira, P.; Baltimore, D.; James, D. Molecular Cell Biology, 4th ed.; W. H. Freeman and Company: New York, NY, USA, 2000. [Google Scholar]
- Landreh, M.; Robinson, C.V. A new window into the molecular physiology of membrane proteins. J. Physiol. 2015, 593, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Moraes, I.; Evans, G.; Sanchez-Weatherby, J.; Newstead, S.; Stewart, P.D. Membrane protein structure determination—The next generation. Biochim. Biophys. Acta 2014, 1838 1 Pt A, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wallin, E.; Von Heijne, G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998, 7, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.M.; Ulloa-Aguirre, A.; Ito, J.; Janovick, J.A. G protein-coupled receptor trafficking in health and disease: Lessons learned to prepare for therapeutic mutant rescue in vivo. Pharm. Rev. 2007, 59, 225–250. [Google Scholar] [CrossRef]
- Cheung, J.C.; Deber, C.M. Misfolding of the cystic fibrosis transmembrane conductance regulator and disease. Biochemistry 2008, 47, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Von Heijne, G. The membrane protein universe: What’s out there and why bother? J. Intern. Med. 2007, 261, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Poetsch, A.; Wolters, D. Bacterial membrane proteomics. Proteomics 2008, 8, 4100–4122. [Google Scholar] [CrossRef]
- Ramanan, P.; Shabman, R.S.; Brown, C.S.; Amarasinghe, G.K.; Basler, C.F.; Leung, D.W. Filoviral Immune Evasion Mechanisms. Viruses 2011, 3, 1634–1649. [Google Scholar] [CrossRef]
- Engel, A.; Gaub, H.E. Structure and mechanics of membrane proteins. Annu. Rev. Biochem. 2008, 77, 127–148. [Google Scholar] [CrossRef]
- Klug, C.S.; Feix, J.B. Methods and Applications of Site-Directed Spin Labeling EPR Spectroscopy. Methods Cell Biol. 2008, 84, 617–658. [Google Scholar] [PubMed]
- Sahu, I.D.; McCarrick, R.M.; Lorigan, G.A. Use of Electron Paramagnetic Resonance to Solve Biochemical Problems. Biochemistry 2013, 52, 5967–5984. [Google Scholar] [CrossRef]
- Xue, M.M.; Cheng, L.S.; Faustino, I.; Guo, W.L.; Marrink, S.J. Molecular Mechanism of Lipid Nanodisk Formation by Styrene-Maleic Acid Copolymers. Biophys. J. 2018, 115, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, J.; Gilliland, Z.; GaryBhat, T.; Weissig, N.; Shindyalov, S.; Bourne, I.N.; Philip, E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Park, S.H.; Opella, S.J. Membrane protein structure from rotational diffusion. Biochim. Biophys. Acta 2015, 1848 1 Pt B, 229–245. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, C.; Drew, D. Breaking the barriers in membrane protein crystallography. Int. J. Biochem. Cell Biol. 2013, 45, 636–644. [Google Scholar] [CrossRef]
- Liang, B.; Tamm, L.K. NMR as a Tool to Investigate Membrane Protein Structure, Dynamics and Function. Nat. Struct. Mol. Biol. 2016, 23, 468–474. [Google Scholar] [CrossRef]
- Sahu, I.D.; Lorigan, G.A. Site-Directed Spin Labeling EPR for Studying Membrane Proteins. Biomed. Res. Int. 2018, 2018, 3248289. [Google Scholar] [CrossRef]
- Claxton, D.P.; Kazmier, K.; Mishra, S.; McHaourab, H.S. Navigating Membrane Protein Structure, Dynamics, and Energy Landscapes Using Spin Labeling and EPR Spectroscopy. Methods Enzymol. 2015, 564, 349–387. [Google Scholar] [CrossRef]
- Weyanda, S.; Tateb, C.G. Advances in membrane protein crystallography: In situ and in meso data collection. Acta Cryst. 2015, D71, 1226–1227. [Google Scholar]
- Goldie, K.N.; Abeyrathne, P.; Kebbel, F.; Chami, M.; Ringler, P.; Stahlberg, H. Cryo-electron microscopy of membrane proteins. Methods Mol. Biol. 2014, 1117, 325–341. [Google Scholar] [PubMed]
- Loura, L.M.S.; Prieto, M. FRET in membrane biophysics: An overview. Front. Physiol. 2011, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Columbus, L.; Hubbell, W.L. A new spin on protein dynamics. Trends Biochem. Sci. 2002, 27, 288–295. [Google Scholar] [CrossRef]
- Acharya, K.R.; Lloyd, M.D. The advantages and limitations of protein crystal structures. Trends Pharmacol. Sci. 2005, 26, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Wuthrich, K. NMR studies of structure and function of biological macromolecules (Nobel Lecture). J. Biomol. Nmr 2003, 27, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Klare, J.P. Site-Directed Spin Labelingand Electron Paramagnetic Resonance (EPR) Spectroscopy: A Versatile Tool to Study Protein-Protein Interaction; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Schiemann, O.; Prisner, T.F. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q. Rev. Biophys. 2007, 40, 1–53. [Google Scholar] [CrossRef]
- Cheng, Y. Membrane protein structural biology in the era of single particle cryo-EM. Curr. Opin. Struct. Biol. 2018, 52, 58–63. [Google Scholar] [CrossRef]
- Khoshouei, M.; Radjainia, M.; Baumeister, W.; Danev, R. Cryo-EM structure of haemoglobin at 3.2 Å determined with the Volta phase plate. Nat. Commun. 2017, 8, 16099. [Google Scholar] [CrossRef]
- Merk, A.; Bartesaghi, A.; Banerjee, S.; Falconieri, V.; Rao, P.; Davis, M.I.; Pragani, R.; Boxer, M.B.; Earl, L.A.; Milne, J.L.S.; et al. Breaking Cryo-EM Resolution Barriers to Facilitate Drug Discovery. Cell 2016, 165, 1698–1707. [Google Scholar] [CrossRef]
- Sun, J.; MacKinnon, R. Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome. Cell 2017, 169, 1042–1050. [Google Scholar] [CrossRef]
- Sgro, G.G.; Costa, T.R.D. Cryo-EM Grid Preparation of Membrane Protein Samples for Single Particle Analysis. Front. Mol. Biosci. 2018, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Weil, J.A.; Bolton, J.R. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lakshmi, K.V.; Brudvig, G.W. Pulsed electron paramagnetic resonance methods for macromolecular structure determination. Curr. Opin. Struct. Biol. 2001, 11, 523–531. [Google Scholar] [CrossRef]
- Schweiger, A.; Jeschke, G. Principles of Pulse Electron. Paramagnetic Resonance; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Prisner, T.; Rohrer, M.; MacMillan, F. Pulsed EPR spectroscopy: Biological applications. Annu. Rev. Phys. Chem. 2001, 52, 279–313. [Google Scholar] [CrossRef]
- Stone, T.J.; Buckman, T.; Nordio, P.L.; McConnel, H.M. SPIN-LABELED BIOMOLECULES. Proc. Natl. Acad. Sci. USA 1965, 54, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Cornish, V.W.; Benson, D.R.; Altenbach, C.A.; Hideg, K.; Hubbell, W.L.; Schultz, P.G. Site-specific incorporation of biophysical probes into proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 2910–2914. [Google Scholar] [CrossRef]
- Klare, J.P.; Steinhoff, H.-J. Spin labeling EPR. Photosynth. Res. 2009, 102, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, H.J. Multi-frequency EPR spectroscopy studies of the structure and conformational changes of site-directed spin labelled membrane proteins. In Supramolecular Structure and Function 8; Pifat-Mrzljak, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 8, pp. 157–177. [Google Scholar]
- Haugland, M.M.; Anderson, E.A.; Lovett, J.E. Tuning the properties of nitroxide spin labels for use in electron paramagnetic resonance spectroscopy through chemical modification of the nitroxide framework. In Electron Paramagnetic Resonance; Chechik, V., Murphy, D.M., Eds.; Royal Society of Chemistry: London, UK, 2017; Volume 25, pp. 1–34. [Google Scholar]
- Balo, A.R.; Feyrer, H.; Ernst, O.P. Toward Precise Interpretation of DEER-Based Distance Distributions: Insights from Structural Characterization of V1 Spin-Labeled Side Chains. Biochemistry 2016, 55, 5256–5263. [Google Scholar] [CrossRef]
- Sahu, I.D.; MaCarrick, R.M.; Troxel, K.R.; Zhang, R.; Smith, J.H.; Dunagan, M.M.; Swartz, M.S.; Rajan, P.V.; Kroncke, B.M.; Sanders, C.R.; et al. DEER EPR measurement for Membrane Protein Structures via Bifunctional Spin Labels and Lipodisq Nanoparticles. Biochemistry 2013, 52, 6627–6632. [Google Scholar] [CrossRef][Green Version]
- Fleissner, M.R.; Bridges, M.D.; Brooks, E.K.; Cascio, D.; Kálai, T.; Hideg, K.; Hubbell, W.L. Structure and dynamics of a conformationally constrained nitroxide side chain and applications in EPR spectroscopy. Proc. Natl. Acad. Sci. USA 2012, 108, 16241–16246. [Google Scholar] [CrossRef]
- Schreier, S.; Bozelli, J.C., Jr.; Marín, N.; Vieira, R.F.; Nakaie, C.R. The spin label amino acid TOAC and its uses in studies of peptides: Chemical, physicochemical, spectroscopic, and conformational aspects. Biophys. Rev. 2012, 4, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, W.L.; Lopez, C.J.; Altenbach, C.; Yang, Z.Y. Technological advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 2013, 23, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Stoller, S.; Sicoli, G.; Baranova, T.Y.; Bennati, M.; Diederichsen, U. TOPP: A Novel Nitroxide-Labeled Amino Acid for EPR Distance Measurements. Angew. Chem. Int. Ed. 2011, 50, 9743–9746. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.J.; Concilio, M.G.; Heaven, G.; Hollas, M.A. New Developments in Spin Labels for Pulsed Dipolar EPR. Molecules 2014, 19, 16998–17025. [Google Scholar] [CrossRef]
- Roser, P.; Schmidt, M.J.; Drescher, M.; Summerer, D. Site-directed spin labeling of proteins for distance measurements in vitro and in cells. Org. Biomol. Chem. 2016, 14, 5468–5476. [Google Scholar] [CrossRef]
- Sahu, I.D.; Craig, A.F.; Dunagan, M.M.; Troxel, K.R.; Zhang, R.; Meiberg, A.G.; Harmon, C.N.; MaCarrick, R.M.; Kroncke, B.M.; Sanders, C.R.; et al. Probing Structural Dynamics and Topology of the KCNE1 Membrane Protein in Lipid Bilayers via Site-Directed Spin Labeling and Electron Paramagnetic Resonance Spectroscopy. Biochemistry 2015, 54, 6402–6412. [Google Scholar] [CrossRef]
- Kang, C.; Tian, C.; Sonnichsen, F.D.; Smith, J.A.; Meiler, J.; George, A.L.J.; Vanoye, C.G.; Kim, H.J.; Sanders, C.R. Structure of KCNE1 and Implications for How It Modulates the KCNQ1Potassium Channel. Biochemistry 2008, 47, 7999–8006. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-Visual Molecular Dynamics. J. Molec. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Bordignon, E.; Steinhoff, H.J. Membrane protein structure and dynamics studied by site-directed spin-labeling ESR. ESR Spectrosc. Membr. Biophys. 2007, 27, 129–164. [Google Scholar]
- Klug, C.S.; Feix, J.B. SDSL: A survey of biological applications. Biol. Magn. Reson. 2004, 24, 269–308. [Google Scholar]
- Bordignon, E.; Bleicken, S. New limits of sensitivity of site-directed spin labeling electron paramagnetic resonance for membrane proteins. Biochim. Et Biophys. Acta-Biomembr. 2018, 1860, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Sahu, I.D.; Lorigan, G.A. EPR Techniques, Spin Labeling and Spin Trapping. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 315–327. [Google Scholar]
- Wunnicke, D.; Hanelt, I. The Synergetic Effects of Combining Structural Biology and EPR Spectroscopy on Membrane Proteins. Crystals 2017, 7, 117. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. Easyspin: Simulating cw ESR spectra. Biol. Magn. Reson. 2007, 27, 299–321. [Google Scholar]
- Li, Q.; Wanderling, S.; Sompornpisut, P.; Perozo, E. Structural basis of lipid-driven conformational transitions in the KvAP voltage-sensing domain. Nat. Struct. Mol. Biol. 2014, 21, 160–166. [Google Scholar] [CrossRef]
- Sahu, I.D.; Lorigan, G.A. Biophysical EPR Studies Applied to Membrane Proteins. J. Phys. Chem. Biophys. 2015, 5, 188. [Google Scholar] [CrossRef]
- Soria, M.A.; Cervantes, S.A.; Bajakian, T.H.; Siemer, A.B. The Functional Amyloid Orb2A Binds to Lipid Membranes. Biophys. J. 2017, 113, 37–47. [Google Scholar] [CrossRef]
- Victor, K.G.; Cafiso, D.S. Location and dynamics of basic peptides at the membrane interface: Electron paramagnetic resonance spectroscopy of tetramethyl-piperidine-N-oxyl-4-amino-4-carboxylic acid-labeled peptides. Biophys. J. 2001, 81, 2241–2250. [Google Scholar] [CrossRef][Green Version]
- Kim, S.S.; Upshur, M.A.; Saotome, K.; Sahu, I.D.; McCarrick, R.M.; Feix, J.B.; Lorigan, G.A.; Howard, K.P. Cholesterol-Dependent Conformational Exchange of the C-Terminal Domain of the Influenza A M2 Protein. Biochemistry 2015, 54, 7157–7167. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.; Ling, S.; Liu, S.; Xiao, L.; Xin, Y.; Lai, C.; Xiong, Y.; Zhang, L.; Tian, C. CW-EPR studies revealed different motional properties and oligomeric states of the integrin beta(1a) transmembrane domain in detergent micelles or liposomes. Sci. Rep. 2015, 5, 7848. [Google Scholar] [CrossRef]
- Ahammad, T.; Drew, D.L., Jr.; Sahu, I.D.; Serafin, R.A.; Clowes, K.R.; Lorigan, G.A. Continuous Wave Electron Paramagnetic Resonance Spectroscopy Reveals the Structural Topology and Dynamic Properties of Active Pinholin S2168 in a Lipid Bilayer. J. Phys. Chem. B 2019, 123, 8048–8056. [Google Scholar] [CrossRef] [PubMed]
- Budil, D.E.; Lee, S.; Saxena, S.; Freed, J.H. Nonlinear-Least-Squares Analysis of Slow-Motion EPR Spectra in One and Two Dimensions Using a Modified Levenberg–Marquardt Algorithm. J. Magn. Reson. Ser. A 1996, 120, 155–189. [Google Scholar] [CrossRef]
- Freed, J.H. Spin Labelling: Theory and Application; Academic Press: New York, NY, USA, 1976; pp. 53–132. [Google Scholar]
- Sahu, I.D.; Zhang, R.; Dunagan, M.M.; Craig, A.F.; Lorigan, G.A. Characterization of KCNE1 inside lipodisq nanoparticles for EPR spectroscopic studies of membrane proteins. J. Phys. Chem. B 2017, 121, 5112–5321. [Google Scholar] [CrossRef] [PubMed]
- Altenbach, C.; Greenhalgh, D.A.; Khorana, H.G.; Hubbell, W.L. A Collision Gradient Method to Determine the Immersion Depth of Nitroxides in Lipid Bilayers: Application to Spin-Labeled Mutants of Bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 1994, 91, 1667–1671. [Google Scholar] [CrossRef]
- Cortes, D.M.; Cuello, L.G.; Perozo, E. Molecular architecture of full-length KcsA—Role of cytoplasmic domains in ion permeation and activation gating. J. Gen. Physiol. 2001, 117, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.; He, M.M.; Hubbell, W.L.; Kaback, H.R. Site-directed spin labeling demonstrates that transmembrane domain XII in the lactose permease of Escherichia coli is an alpha-helix. Biochemistry 1996, 35, 12915–12918. [Google Scholar] [CrossRef]
- Song, Y.; Hustedt, E.J.; Brandon, S.; Sanders, C.R. Competition between Homodimerization and Cholesterol Binding to the C99 Domain of the Amyloid Precursor Protein. Biochemistry 2013, 52, 5051–5064. [Google Scholar] [CrossRef]
- Perozo, E.; Hubbell, W.L. Transmembrane voltage control in liposomes—The use of bacteriorhodopsin as a light-driven current source. Biophys. J. 1993, 64, A222. [Google Scholar]
- Hubbell, W.L.; Altenbach, C. Investigation of structure and dynamics in membrane-proteins using site-directed spin labeling. Curr. Opin. Struct. Biol. 1994, 4, 566–573. [Google Scholar] [CrossRef]
- Mokdad, A.; Herrick, D.Z.; Kahn, A.K.; Andrews, E.; Kim, M.; Cafiso, D.S. Ligand-Induced Structural Changes in the Escherichia coli Ferric Citrate Transporter Reveal Modes for Regulating Protein-Protein Interactions. J. Mol. Biol. 2012, 423, 818–830. [Google Scholar] [CrossRef][Green Version]
- Hess, J.F.; Budamagunta, M.S.; Aziz, A.; FitzGerald, P.G.; Voss, J.C. Electron paramagnetic resonance analysis of the vimentin tail domain reveals points of order in a largely disordered region and conformational adaptation upon filament assembly. Protein Sci. 2013, 22, 47–55. [Google Scholar] [CrossRef]
- Aziz, A.; Hess, J.F.; Budamagunta, M.S.; Voss, J.C.; FitzGerald, P.G. Site-directed Spin Labeling and Electron Paramagnetic Resonance Determination of Vimentin Head Domain Structure. J. Biol. Chem. 2010, 285, 15278–15285. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.H.; Yang, G.Y.; McHaourab, H.S. Structural basis of energy transduction in the transport cycle of MsbA. Science 2005, 308, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, N.J.; Falke, J.J. Use of EPR power saturation toanalyze the membrane-docking geometries of peripheral proteins: A applications to C2 domains. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 71–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, Y.G.; Thorgeirsson, T.E.; Shin, Y.K. Topology of an amphiphilic mitochondrial signal sequence in the membrane-inserted state: A spin-labeling study. Biochemistry 1994, 33, 14221–14226. [Google Scholar] [CrossRef]
- Klug, C.S.; Su, W.Y.; Feix, J.B. Mapping of the residues involved in a proposed beta-strand located in the ferric enterobactin receptor FepA using site-directed spin-labeling. Biochemistry 1997, 36, 13027–13033. [Google Scholar] [CrossRef]
- Carter, J.D.; Mathias, J.D.; Gomez, E.F.; Ran, Y.; Xu, F.; Galiano, L.; Tran, N.Q.; D’Amore, P.W.; Wright, C.S.; Chakravorty, D.K.; et al. Characterizing Solution Surface Loop Conformational Flexibility of the GM2 Activator Protein. J. Phys. Chem. B 2014, 118, 10607–10617. [Google Scholar] [CrossRef]
- Dixit, G.; Sahu, I.D.; Reynolds, W.D.; Wadsworth, T.M.; Harding, B.D.; Jaycox, C.K.; Dabney-Smith, C.; Sanders, C.R.; Lorigan, G.A. Probing the Dynamics and Structural Topology of the Reconstituted Human KCNQ1 Voltage Sensor Domain (Q1-VSD) in Lipid Bilayers Using Electron Paramagnetic Resonance Spectroscopy. Biochemistry 2019, 58, 965–973. [Google Scholar] [CrossRef]
- Galazzo, L.; Maso, L.; De Rosa, E.; Bortolus, M.; Doni, D.; Acquasaliente, L.; De Filippis, V.; Costantini, P.; Carbonera, D. Identifying conformational changes with site-directed spin labeling reveals that the GTPase domain of HydF is a molecular switch. Sci. Rep. 2017, 7, 1714. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Cardon, T.B.; Laryukhin, M.; Grosser, S.M.; Lorigan, G.A. Determining the topology of integral membrane peptides using EPR spectroscopy. J. Am. Chem. Soc. 2006, 128, 9549–9554. [Google Scholar] [CrossRef]
- Sahu, I.D.; Hustedt, E.J.; Ghimire, H.; Inbaraj, J.J.; McCarrick, R.M.; Lorigan, G.A. CW dipolar broadening EPR spectroscopy and mechanically aligned bilayers used to measure distance and relative orientation between two TOAC spin labels on an antimicrobial peptide. J. Magn. Reson. 2014, 249, 72–79. [Google Scholar] [CrossRef]
- Sahu, I.D.; Mayo, D.J.; Subbaraman, N.; Inbaraj, J.J.; McCarrick, R.M.; Lorigan, G.A. Probing topology and dynamics of the second transmembrane domain (M2δ) of the acetyl choline receptor using magnetically aligned lipid bilayers (bicelles) and EPR spectroscopy. Chem. Phys. Lipids 2017, 206, 9–15. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, J.E.; James, Z.M.; Svensson, B.; Binder, B.P.; Thomas, D.D. A bifunctional spin label reports the structural topology of phospholamban in magnetically-aligned bicelles. J. Magn. Reson. 2016, 262, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mayo, D.J.; Sahu, I.D.; Lorigan, G.A. Assessing topology and surface orientation of an antimicrobial peptide magainin 2 using mechanically aligned bilayers and electron paramagnetic resonance spectroscopy. Chem. Phys. Lipids 2018, 213, 124–130. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J. Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Cieslak, J.A.; Focia, P.J.; Gross, A. Electron Spin-Echo Envelope Modulation (ESEEM) Reveals Water and Phosphate Interactions with the KcsA Potassium Channel. Biochemistry 2010, 49, 1486–1494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Volkov, A.; Dockter, C.; Polyhach, Y.; Paulsen, H.; Jeschke, G. Site-Specific Information on Membrane Protein Folding by Electron Spin Echo Envelope Modulation Spectroscopy. J. Phys. Chem. Lett. 2010, 1, 663–667. [Google Scholar] [CrossRef]
- Dzuba, S.A.; Raap, J. Spin-Echo Electron Paramagnetic Resonance (EPR) Spectroscopy of a Pore-Forming (Lipo)Peptaibol in Model and Bacterial Membranes. Chem. Biodivers. 2013, 10, 864–875. [Google Scholar] [CrossRef]
- Dzuba, S.A. STRuctural studies of biological membranes using ESEEM spectroscopy of spin labels and deuterium substitution. J. Struct. Chem. 2013, 54, S1–S15. [Google Scholar] [CrossRef]
- Zhou, A.; Abu-Baker, S.; Sahu, I.D.; Liu, L.; McCarrick, R.M.; Dabney-Smith, C.; Lorigan, G.A. Determining α-Helical and β-Sheet Secondary Structures via Pulsed Electron Spin Resonance Spectroscopy. Biochemistry 2012, 51, 7417–7419. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Sahu, I.D.; Mayo, D.J.; McCarrick, R.M.; Troxel, K.; Zhou, A.; Shockley, E.; Lorigan, G.A. Enhancement of Electron Spin Echo Envelope Modulation Spectroscopic Methods to Investigate the Secondary Structure of Membrane Proteins. J. Phys. Chem. B 2012, 116, 11041–11045. [Google Scholar] [CrossRef][Green Version]
- Mayo, D.; Zhou, A.; Sahu, I.; McCarrick, R.; Walton, P.; Ring, A.; Troxel, K.; Coey, A.; Hawn, J.; Emwas, A.H.; et al. Probing the structure of membrane proteins with electron spin echo envelope modulation spectroscopy. Protein Sci. 2011, 20, 1100–1104. [Google Scholar] [CrossRef]
- Liu, L.; Mayo, D.J.; Sahu, I.D.; Zhou, A.; Zhang, R.; McCarrick, R.M.; Lorigan, G.A. Determining the Secondary Structure of Membrane Proteins and Peptides Via Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy. Methods Enzymol. 2015, 564, 289–313. [Google Scholar] [PubMed]
- Zhang, R.; Sahu, I.D.; Gibson, K.R.; Muhammad, N.; Bali, A.P.; Comer, R.G.; Liu, L.; Craig, A.F.; McCarrick, R.M.; Dabney-Smith, C.; et al. Development of Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy to probe the Secondary Structure of Recomninant Membrane Proteins in Lipid bilayer. Protein Sci. 2015, 24, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hess, J.; Sahu, I.D.; FitzGerald, P.G.; McCarrick, R.M. Probing the Local Secondary Structure of Human Vimentin with Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy. J. Phys. Chem. B 2016, 120, 12321–12328. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sahu, I.D.; McCarrick, R.M.; Lorigan, G. Probing the Secondary Structure of Membrane Peptides Using 2H-labeled d10 Leucine via Site-Directing Spin-Labeling (SDSL) and Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy. J. Phys. Chem. B 2016, 120, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Bottorf, L.; Rafferty, S.; Sahu, I.D.; McCarrick, R.M.; Lorigan, G.A. Utilizing Electron Spin Echo Envelope Modulation to Distinguish Between the Local Secondary Structures of an α-Helix and an Amphipathic 310-Helical Peptide. J. Phys. Chem. B 2017, 121, 2961–2967. [Google Scholar] [CrossRef]
- Liu, L.; Sahu, I.D.; Bottorf, L.; McCarrick, R.M.; Lorigan, G.A. Investigating the Secondary Structure of Membrane Peptides Utilizing Multiple 2 H-Labeled Hydrophobic Amino Acids via Electron Spin Echo Envelope Modulation (ESEEM) Spectroscopy. Phys. Chem. B 2018, 122, 4388–4396. [Google Scholar] [CrossRef]
- Bottorf, L.; Sahu, I.D.; McCarrick, R.M.; Lorigan, G.A. Utilization of C-13-labeled amino acids to probe the alpha-helical local secondary structure of a membrane peptide using electron spin echo envelope modulation (ESEEM) spectroscopy. Biochim. Et Biophys. Acta Biomembr. 2018, 1860, 1447–1451. [Google Scholar] [CrossRef]
- Colaneri, M.J.; Peisach, J. Electron Spin-Echo Envelope Modulation Studies Of 14N In Biological Systems. In Biomedical EPR, Part A: Free Radicals, Metals, Medicine, and Physiology; Springer: Boston, MA, USA, 2005; Volume 23, pp. 455–491. [Google Scholar]
- Lorigan, G.A.; Britt, R.D.; Kim, J.H.; Hille, R. Electron-spin echo envelope modulation spectroscopy of the molybdenum center of xanthine-oxidase. Biochim. Et Biophys. Acta-Bioenerg. 1994, 1185, 284–294. [Google Scholar] [CrossRef]
- Kubota, T.; Lacroix, J.J.; Bezanilla, F.; Correa, A.M. Probing α-3(10) transitions in a voltage-sensing S4 helix. Biophys. J. 2014, 107, 1117–1128. [Google Scholar] [CrossRef]
- Yu, X.; Lorigan, G.A. Secondary structure, backbone dynamics, and structural topology of phospholamban and its phosphorylated and Arg9Cys-mutated forms in phospholipid bilayers utilizing 13C and 15N solid-state NMR spectroscopy. J. Phys. Chem. B 2014, 118, 2124–2133. [Google Scholar] [CrossRef]
- Hustedt, E.J.; Beth, A.H. Nitroxide spin-spin interactions: Applications to protein structure and dynamics. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Hustedt, E.J.; Smirnov, A.I.; Laub, C.F.; Cobb, C.E.; Beth, A.H. Molecular distances from dipolar coupled spin-labels: The global analysis of multifrequency continuous wave electron paramagnetic resonance data. Biophys. J. 1997, 72, 1861–1877. [Google Scholar] [CrossRef]
- Hustedt, E.J.; Stein, R.A.; Sethaphong, L.; Brandon, S.; Zhou, Z.; DeSensi, S.C. Dipolar coupling between nitroxide spin labels: The development and application of a tether-in-a-cone model. Biophys. J. 2006, 90, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Banham, J.E.; Baker, C.M.; Ceola, S.; Day, I.J.; Grant, G.H.; Groenen, E.J.J.; Rodgers, C.T.; Jeschke, G.; Timmel, C.R. Distance measurements in the borderline region of applicability of CW EPR and DEER: A model study on a homologous series of spin-labelled peptides. J. Magn. Reson. 2008, 191, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Rabenstein, M.D.; Shin, Y.K. Determination of the distance between 2 spin labels attached to a macromolecule. Proc. Natl. Acad. Sci. USA 1995, 92, 8239–8243. [Google Scholar] [CrossRef]
- Czogalla, A.; Pieciul, A.; Jezierski, A.; Sikorski, A.F. Attaching a spin to a protein—Site-directed spin labeling in structural biology. Acta Biochim. Pol. 2007, 54, 235–244. [Google Scholar] [CrossRef]
- Czogalla, A.; Jaszewski, A.R.; Diakowski, W.; Bok, E.; Jezierski, A.; Sikorski, A.F. Structural insight into an ankyrin-sensitive lipid-binding site of erythroid beta-spectrin. Mol. Membr. Biol. 2007, 24, 215–224. [Google Scholar] [CrossRef]
- Essen, L.O.; Siegert, R.; Lehmann, W.D.; Oesterhelt, D. Lipid patches in membrane protein oligomers: Crystal structure of the bacteriorhodopsin-lipid complex. Proc. Natl. Acad. Sci. USA 1998, 95, 11673–11678. [Google Scholar] [CrossRef]
- Ling, S.; Wang, W.; Yu, L.; Peng, J.; Cai, X.; Xiong, Y.; Hayati, Z.; Zhang, L.; Zhang, Z.; Song, L.; et al. Structure of an E. coli integral membrane sulfurtransferase and its structural transition upon SCN- binding defined by EPR-based hybrid method. Sci. Rep. 2016, 6, 20025. [Google Scholar] [CrossRef]
- Ghimire, H.; Hustedt, E.J.; Sahu, I.D.; Inbaraj, J.J.; McCarrick, R.; Mayo, D.J.; Benedikt, M.R.; Lee, R.T.; Grosser, S.M.; Lorigan, G.A. Distance Measurements on a Dual-Labeled TOAC AChR M2δ Peptide in Mechanically Aligned DMPC Bilayers via Dipolar Broadening CW-EPR Spectroscopy. J. Phys. Chem. B 2012, 116, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, I.; Wunnicke, D.; Muller-Trimbusch, M.; Vor der Bruggen, M.; Kraus, I.; Bakker, E.P.; Steinhoff, H.J. Membrane Region M-2C2 in Subunit KtrB of the K+ Uptake System KtrAB from Vibrio alginolyticus Forms a Flexible Gate Controlling K+ Flux AN ELECTRON PARAMAGNETIC RESONANCE STUDY. J. Biol. Chem. 2010, 285, 28210–28219. [Google Scholar] [CrossRef] [PubMed]
- Sahu, I.D.; Craig, A.F.; Dunagum, M.M.; McCarrick, R.M.; Lorigan, G.A. Characterization of bifunctional spin labels for investigating the structural and dynamic properties of membrane proteins using EPR spectroscopy. J. Phys. Chem. B 2017, 121, 9185–9195. [Google Scholar] [CrossRef]
- Steinhoff, H.J. Inter- and intra-molecular distances determined by EPR spectroscopy and site-directed spin labeling reveal protein-protein and protein-oligonucleotide interaction. Biol. Chem. 2004, 385, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Wegener, A.A.; Klare, J.P.; Engelhard, M.; Steinhoff, H.J. Structural insights into the early steps of receptor-transducer signal transfer in archaeal phototaxis. Embo. J. 2001, 20, 5312–5319. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, F.; Drescher, M.; Rutters-Meijneke, T.; Holt, A.; Rijkers, D.T.S.; Killian, J.A.; Huber, M. Aggregation of Transmembrane Peptides Studied by Spin-Label EPR. J. Phys. Chem. B 2009, 113, 12257–12264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, L.; Wang, W.; Ling, S.L.; He, Y.; Xiao, L.; Wu, K.Q.; Zhang, L.H.; Tian, C.L. Distance measurement between two flexible sites in proteins in high viscosity medium at physiological temperature using continuous wave EPR. Protein Cell 2014, 5, 334–337. [Google Scholar] [CrossRef][Green Version]
- Stone, K.M.; Voska, J.; Kinnebrew, M.; Pavlova, A.; Junk, M.J.N.; Han, S.G. Structural Insight into Proteorhodopsin Oligomers. Biophys. J. 2013, 104, 472–481. [Google Scholar] [CrossRef]
- Majsnerowska, M.; Hanelt, I.; Wunnicke, D.; Schafer, L.V.; Steinhoff, H.J.; Slotboom, D.J. Substrate-Induced Conformational Changes in the S-Component ThiT from an Energy Coupling Factor Transporter. Structure 2013, 21, 861–867. [Google Scholar] [CrossRef]
- Jeschke, G.; Polyhach, Y. Distance measurements on spin-labelled biomacromolecules by pulsed electron paramagnetic resonance. Phys. Chem. Chem. Phys. 2007, 9, 1895–1910. [Google Scholar] [CrossRef]
- Borbat, P.P.; McHaourab, H.S.; Freed, J.H. Protein structure determination using long-distance constraints from double-quantum coherence ESR: Study of T4 lysozyme. J. Am. Chem. Soc. 2002, 124, 5304–5314. [Google Scholar] [CrossRef]
- Jeschke, G. DEER Distance Measurements on Proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Pannier, M.; Veit, S.; Godt, A.; Jeschke, G.; Spiess, H.W. Dead-time free measurement of dipole-dipole interactions between electron spins. J. Magn. Reson. 2000, 142, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, O.; Piton, N.; Mu, Y.G.; Stock, G.; Engels, J.W.; Prisner, T.F. A PELDOR-based nanometer distance ruler for oligonucleotides. J. Am. Chem. Soc. 2004, 126, 5722–5729. [Google Scholar] [CrossRef] [PubMed]
- Milov, A.D.; Tsvetkov, Y.D.; Formaggio, F.; Crisma, M.; Toniolo, C.; Raap, J. Self-assembling properties of membrane-modifying peptides studied by PELDOR and CW-ESR spectroscopies. J. Am. Chem. Soc. 2000, 122, 3843–3848. [Google Scholar] [CrossRef]
- Hilger, D.; Jung, H.; Padan, E.; Wegener, C.; Vogel, K.P.; Steinhoff, H.J.; Jeschke, G. Assessing oligomerization of membrane proteins by four-pulse DEER: pH-dependent dimerization of NhaA Na+/H+ antiporter of E-coli. Biophys. J. 2005, 89, 1328–1338. [Google Scholar] [CrossRef]
- Banham, J.E.; Timmel, C.R.; Abbott, R.J.M.; Lea, S.M.; Jeschke, G. The characterization of weak protein-protein interactions: Evidence from DEER for the trimerization of a von Willebrand Factor A domain in solution. Angew. Chem. 2006, 45, 1058–1061. [Google Scholar] [CrossRef]
- Sahu, I.D.; Kroncke, B.M.; Zhang, R.; Dunagan, M.M.; Smith, H.J.; Craig, A.; McCarrick, R.M.; Sanders, C.R.; Lorigan, G.A. Structural Investigation of the Transmembrane Domain of KCNE1 in Proteoliposomes. Biochemistry 2014, 53, 6392–6401. [Google Scholar] [CrossRef]
- Meyer, V.; Swanson, M.A.; Clouston, L.J.; Boratynski, P.J.; Stein, R.A.; McHaourab, H.S.; Rajca, A.; Eaton, S.S.; Eaton, G.R. Room-Temperature Distance Measurements of Immobilized Spin-Labeled Protein by DEER/PELDOR. Biophys. J. 2015, 108, 1213–1219. [Google Scholar] [CrossRef][Green Version]
- Feintuch, A.; Otting, G.; Goldfarb, D. Gd3+ Spin Labeling for Measuring Distances in Biomacromolecules: Why and How? Methods Enzymol. 2015, 563, 415–457. [Google Scholar] [CrossRef]
- Jassoy, J.J.; Berndhauser, A.; Duthie, F.; Kuhn, S.P.; Hagelueken, G.; Schiemann, O. Versatile Trityl Spin Labels for Nanometer Distance Measurements on Biomolecules In Vitro and within Cells. Angew. Chem. Int. Ed. 2017, 56, 177–181. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Ji, M.; Cunningham, T.F.; Saxena, S. Cu2+ as an ESR Probe of Protein Structure and Function. Methods Enzymol. 2015, 563, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Tormyshev, V.M.; Rogozhnikova, O.Y.; Akhmetzyanov, D.; Bagryanskaya, E.G.; Prisner, T.F. Selective High-Resolution Detection of Membrane Protein-Ligand Interaction in Native Membranes Using Trityl-Nitroxide PELDOR. Angew. Chem. Int. Ed. 2016, 55, 11538–11542. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G.; Chechik, V.; Ionita, P.; Godt, A.; Zimmermann, H.; Banham, J.; Timmel, C.R.; Hilger, D.; Jung, H. DeerAnalysis2006—A comprehensive software package for analyzing pulsed ELDOR data. Appl. Magn. Reson. 2006, 30, 473–498. [Google Scholar] [CrossRef]
- Tait, C.E.; Stoll, S. Coherent pump pulses in Double Electron Electron Resonance spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 18470–18485. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Jaumann, E.A.; Sikora, A.; Barth, K.; Prisner, T.F.; Cafiso, D.S. In situ observation of conformational dynamics and protein ligand-substrate interactions in outer-membrane proteins with DEER/PELDOR spectroscopy. Nat. Protoc. 2019, 14, 2344–2369. [Google Scholar] [CrossRef] [PubMed]
- Borbat, P.P.; Georgieva, E.R.; Freed, J.H. Improved Sensitivity for Long-Distance Measurements in Biomolecules: Five-Pulse Double Electron-Electron Resonance. J. Phys. Chem. Lett. 2013, 4, 170–175. [Google Scholar] [CrossRef]
- Spindler, P.E.; Waclawska, I.; Endeward, B.; Plackrneyer, J.; Ziegler, C.; Prisner, T.F. Carr-Purcell Pulsed Electron Double Resonance with Shaped Inversion Pulses. J. Phys. Chem. Lett. 2015, 6, 4331–4335. [Google Scholar] [CrossRef]
- Zou, P.; McHaourab, H.S. Increased Sensitivity and Extended Range of Distance Measurements in Spin-Labeled Membrane Proteins: Q-Band Double Electron-Electron Resonance and Nanoscale Bilayers. Biophys. J. 2010, 98, L18–L20. [Google Scholar] [CrossRef]
- McHaourab, H.S.; Steed, P.R.; Kazmier, K. Toward the fourth dimension of membrane protein structure: Insight into dynamics from spin-labeling EPR spectroscopy. Structure 2011, 19, 1549–1561. [Google Scholar] [CrossRef]
- Zou, P.; Bortolus, M.; McHaourab, H.S. Conformational Cycle of the ABC Transporter MsbA in Liposomes: Detailed Analysis Using Double Electron-Electron Resonance Spectroscopy. J. Mol. Biol. 2009, 393, 586–597. [Google Scholar] [CrossRef]
- Endeward, B.; Butterwick, J.A.; MacKinnon, R.; Prisner, T.F. Pulsed Electron-Electron Double-Resonance Determination of Spin-Label Distances and Orientations on the Tetrameric Potassium Ion Channel KcsA. J. Am. Chem. Soc. 2009, 131, 15246–15250. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.R.; Ramlall, T.F.; Borbat, P.P.; Freed, J.H.; Eliezer, D. Membrane-bound alpha-synuclein forms an extended helix: Long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J. Am. Chem. Soc. 2008, 130, 12856–12857. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ellena, J.F.; Kim, M.; Cafiso, D.S. Substrate-dependent unfolding of the energy coupling motif of a membrane transport protein determined by double electron-electron resonance. Biochemistry 2006, 45, 10847–10854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polyhach, Y.; Bordignon, E.; Tschaggelar, R.; Gandra, S.; Godt, A.; Jeschke, G. High sensitivity and versatility of the DEER experiment on nitroxide radical pairs at Q-band frequencies. Phys. Chem. Chem. Phys. 2012, 14, 10762–10773. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.F.; Putterman, M.R.; Desai, A.; Horne, W.S.; Saxena, S. The Double-Histidine Cu2+-Binding Motif: A Highly Rigid, Site-Specific Spin Probe for Electron Spin Resonance Distance Measurements. Angew. Chem. Int. Ed. 2015, 54, 6330–6334. [Google Scholar] [CrossRef]
- Li, C.C.; Hung, C.L.; Yeh, P.S.; Li, C.E.; Chiang, Y.W. Doubly spin-labeled nanodiscs to improve structural determination of membrane proteins by ESR. Rsc. Adv. 2019, 9, 9014–9021. [Google Scholar] [CrossRef]
- Georgieva, E.R. Nanoscale lipid membrane mimetics in spin-labeling and electron paramagnetic resonance spectroscopy studies of protein structure and function. Nanotechnol. Rev. 2017, 6, 75–92. [Google Scholar] [CrossRef]
- Jao, C.C.; Hegde, B.G.; Chen, J.; Haworth, I.S.; Langen, R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc. Natl. Acad. Sci. USA 2008, 105, 19666–19671. [Google Scholar] [CrossRef]
- Milikisiyants, S.; Wang, S.L.; Munro, R.A.; Donohue, M.; Ward, M.E.; Bolton, D.; Brown, L.S.; Smirnova, T.I.; Ladizhansky, V.; Smirnov, A.I. Oligomeric Structure of Anabaena Sensory Rhodopsin in a Lipid Bilayer Environment by Combining Solid-State NMR and Long-range DEER Constraints. J. Mol. Biol. 2017, 429, 1903–1920. [Google Scholar] [CrossRef]
- Shen, R.; Han, W.; Fiorin, G.; Islam, S.M.; Schulten, K.; Roux, B. Structural Refinement of Proteins by Restrained Molecular Dynamics Simulations with Non-interacting Molecular Fragments. Plos Comput. Biol. 2015, 11, e1004368. [Google Scholar] [CrossRef]
- Vicente, E.F.; Sahu, I.D.; Costa-Filho, A.J.; Cilli, E.M.; Lorigan, G.A. Conformational changes of the HsDHODH N-terminal Microdomain via DEER Spectroscopy. J. Phys. Chem. B 2015, 119, 8693–8697. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.R.; Borbat, P.P.; Norman, H.D.; Freed, J.H. Mechanism of influenza A M2 transmembrane domain assembly in lipid membranes. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Kim, S.; Matthews, G.F.; Erreger, K.; Galli, A.; Cobb, C.E.; Hustedt, E.J.; Beth, A.H. Structural Arrangement of the Intracellular Ca2+ Binding Domains of the Cardiac Na+/Ca2+ Exchanger (NCX1.1) EFFECTS OF Ca2+ BINDING. J. Biol. Chem. 2013, 288, 4194–4207. [Google Scholar] [CrossRef]
- Hilger, D.; Polyhach, Y.; Jung, H.; Jeschke, G. Backbone Structure of Transmembrane Domain IX of the Na+/Proline Transporter PutP of Escherichia coli. Biophys. J. 2009, 96, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kroncke, B.M.; Van Horn, W.D.; Smith, J.; Kang, C.B.; Welch, R.C.; Song, Y.L.; Nannemann, D.P.; Taylor, K.C.; Sisco, N.J.; George, A.L.; et al. Structural basis for KCNE3 modulation of potassium recycling in epithelia. Sci. Adv. 2016, 2, e1501228. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.J.; Song, Y.; Van Horn, W.D.; Hustedt, E.J.; Schafer, J.M.; Hadziselimovic, A.; Beel, A.J.; Sanders, C.R. The Amyloid Precursor Protein Has a Flexible Transmembrane Domain and Binds Cholesterol. Science 2012, 336, 1168–1171. [Google Scholar] [CrossRef]
- Mullen, A.; Hall, J.; Diegel, J.; Hassan, I.; Fey, A.; MacMillan, F. Membrane transporters studied by EPR spectroscopy: Structure determination and elucidation of functional dynamics. Biochem. Soc. Trans. 2016, 44, 905–915. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Ramelot, T.A.; McCarrick, R.M.; Ni, S.; Feldmann, E.A.; Cort, J.R.; Wang, H.; Ciccosanti, C.; Jiang, M.; Janjua, H.; et al. Combining NMR and EPR Methods for Homodimer Protein Structure Determination. J. Am. Chem. Soc. 2010, 132, 11910–11913. [Google Scholar] [CrossRef]
- Basak, S.; Schmandt, N.; Gicheru, Y.; Chakrapani, S. Crystal structure and dynamics of a lipid induced potential desensitized-state of a pentameric ligand-gated channel. Elife 2017, 6, e23886. [Google Scholar] [CrossRef]
- Herneisen, A.L.; Sahu, I.D.; McCarrick, R.M.; Feix, J.B.; Lorigan, G.A.; Howard, K.P. A Budding-Defective M2 Mutant Exhibits Reduced Membrane Interaction, Insensitivity to Cholesterol, and Perturbed Interdomain Coupling. Biochemistry 2017, 56, 5955–5963. [Google Scholar] [CrossRef]
- Kumar, P.; Van Son, M.; Zheng, T.T.; Valdink, D.; Raap, J.; Kros, A.; Huber, M. Coiled-coil formation of the membrane-fusion K/E peptides viewed by electron paramagnetic resonance. PLoS ONE 2018, 13, e0191197. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.M.; Baber, J.L.; Ghirlando, R.; Aniana, A.; Bax, A.; Roche, J. Insights into the Conformation of the Membrane Proximal Regions Critical to the Trimerization of the HIV-1 gp41 Ectodomain Bound to Dodecyl Phosphocholine Micelles. PLoS ONE 2016, 11, e0160597. [Google Scholar] [CrossRef] [PubMed]
- Puljung, M.C.; DeBerg, H.A.; Zagotta, W.N.; Stoll, S. Double electron-electron resonance reveals cAMP-induced conformational change in HCN channels. Proc. Natl. Acad. Sci. USA 2014, 111, 9816–9821. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.; Stein, R.A.; Masureel, M.; Roth, A.; Mishra, S.; Dawaliby, R.; Konijnenberg, A.; Sobott, F.; Govaerts, C.; McHaourab, H.S. Lipids modulate the conformational dynamics of a secondary multidrug transporter. Nat. Struct. Mol. Biol. 2016, 23, 744–745. [Google Scholar] [CrossRef]
- Dalmas, O.; Hyde, H.C.; Hulse, R.E.; Perozo, E. Symmetry-Constrained Analysis of Pulsed Double Electron-Electron Resonance (DEER) Spectroscopy Reveals the Dynamic Nature of the KcsA Activation Gate. J. Am. Chem. Soc. 2012, 134, 16360–16369. [Google Scholar] [CrossRef]
- Merten, J.A.; Schultz, K.M.; Klug, C.S. Concentration-dependent oligomerization and oligomeric arrangement of LptA. Protein Sci. 2012, 21, 211–218. [Google Scholar] [CrossRef]
- Bracher, S.; Hilger, D.; Guerin, K.; Polyhach, Y.; Jeschke, G.; Krafczyk, R.; Giacomelli, G.; Jung, H. Comparison of the functional properties of trimeric and monomeric CaiT of Escherichia coli. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Teucher, M.; Zhang, H.; Bader, V.; Winklhofer, K.F.; Garcia-Saez, A.J.; Rajca, A.; Bleicken, S.; Bordignon, E. A new perspective on membrane-embedded Bax oligomers using DEER and bioresistant orthogonal spin labels. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Joseph, B.; Sikora, A.; Bordignon, E.; Jeschke, G.; Cafiso, D.S.; Prisner, T.F. Distance Measurement on an Endogenous Membrane Transporter in E-coli Cells and Native Membranes Using EPR Spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 6196–6199. [Google Scholar] [CrossRef]
- Joseph, B.; Sikora, A.; Cafiso, D.S. Ligand Induced Conformational Changes of a Membrane Transporter in E-coli Cells Observed with DEER/PELDOR. J. Am. Chem. Soc. 2016, 138, 1844–1847. [Google Scholar] [CrossRef]
- Riederer, E.A.; Focke, P.J.; Georgieva, E.R.; Akyuz, N.; Matulef, K.; Borbat, P.P.; Freed, J.H.; Blanchard, S.C.; Boudker, O.; Valiyaveetil, F.I. A Facile approach forte in vitro assembly of multimeric membrane transport proteins. Elife 2018, 7, e36478. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.R.; Borbat, P.P.; Ginter, C.; Freed, J.H.; Boudker, O. Conformational ensemble of the sodium-coupled aspartate transporter. Nat. Struct. Mol. Biol. 2013, 20, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Bleicken, S.; Jeschke, G.; Stegmueller, C.; Salvador-Gallego, R.; Garcia-Saez, A.J.; Bordignon, E. Structural Model of Active Bax at the Membrane. Mol. Cell 2014, 56, 496–505. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, I.D.; Lorigan, G.A. Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins. Biomolecules 2020, 10, 763. https://doi.org/10.3390/biom10050763
Sahu ID, Lorigan GA. Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins. Biomolecules. 2020; 10(5):763. https://doi.org/10.3390/biom10050763
Chicago/Turabian StyleSahu, Indra D., and Gary A. Lorigan. 2020. "Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins" Biomolecules 10, no. 5: 763. https://doi.org/10.3390/biom10050763
APA StyleSahu, I. D., & Lorigan, G. A. (2020). Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins. Biomolecules, 10(5), 763. https://doi.org/10.3390/biom10050763



