Dynamic Expansion and Functional Evolutionary Profiles of Plant Conservative Gene Family SBP-Box in Twenty Two Flowering Plants and the Origin of miR156
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. SBP Gene Identification and Characterization
2.3. Phylogenetic Analyses and Detection Orthologous Genes
2.4. MIR156 Prediction
2.5. MiR156 Target Prediction
2.6. Gene Duplication and Synteny Analyses and Estimations of Synonymous (Ks) and Nonsynonymous (Ka) Substitutions per Site and Their Ratio
2.7. Conservative Analysis
2.8. Upstream Regulatory Element Analyses
2.9. Tissue Expression and Stress Response
3. Results
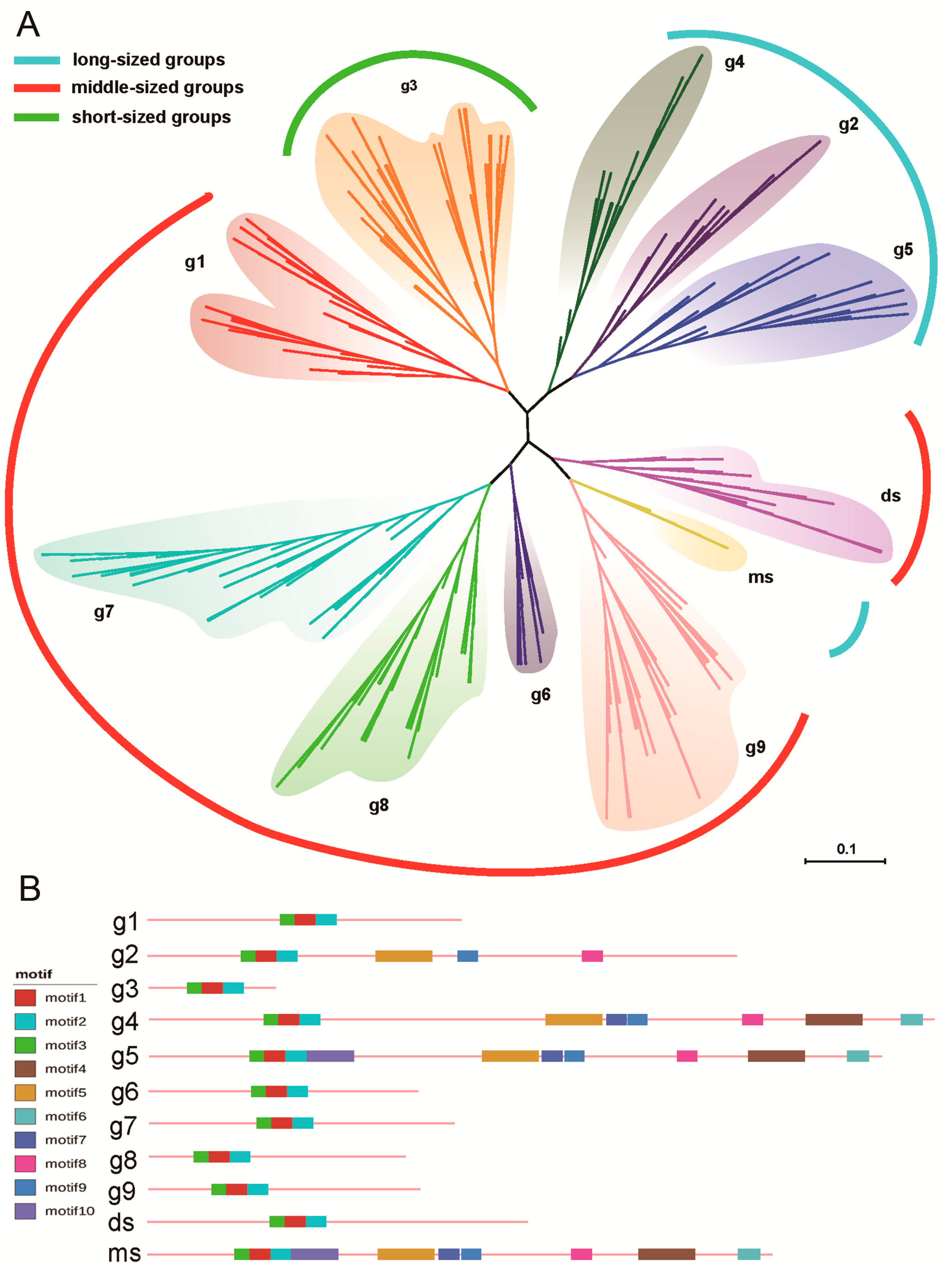
3.1. The SBP-Box Gene Family Is Diverse in Angiosperms
3.2. Short and Moderate-Length SBPs Are Typical Targets of miR156
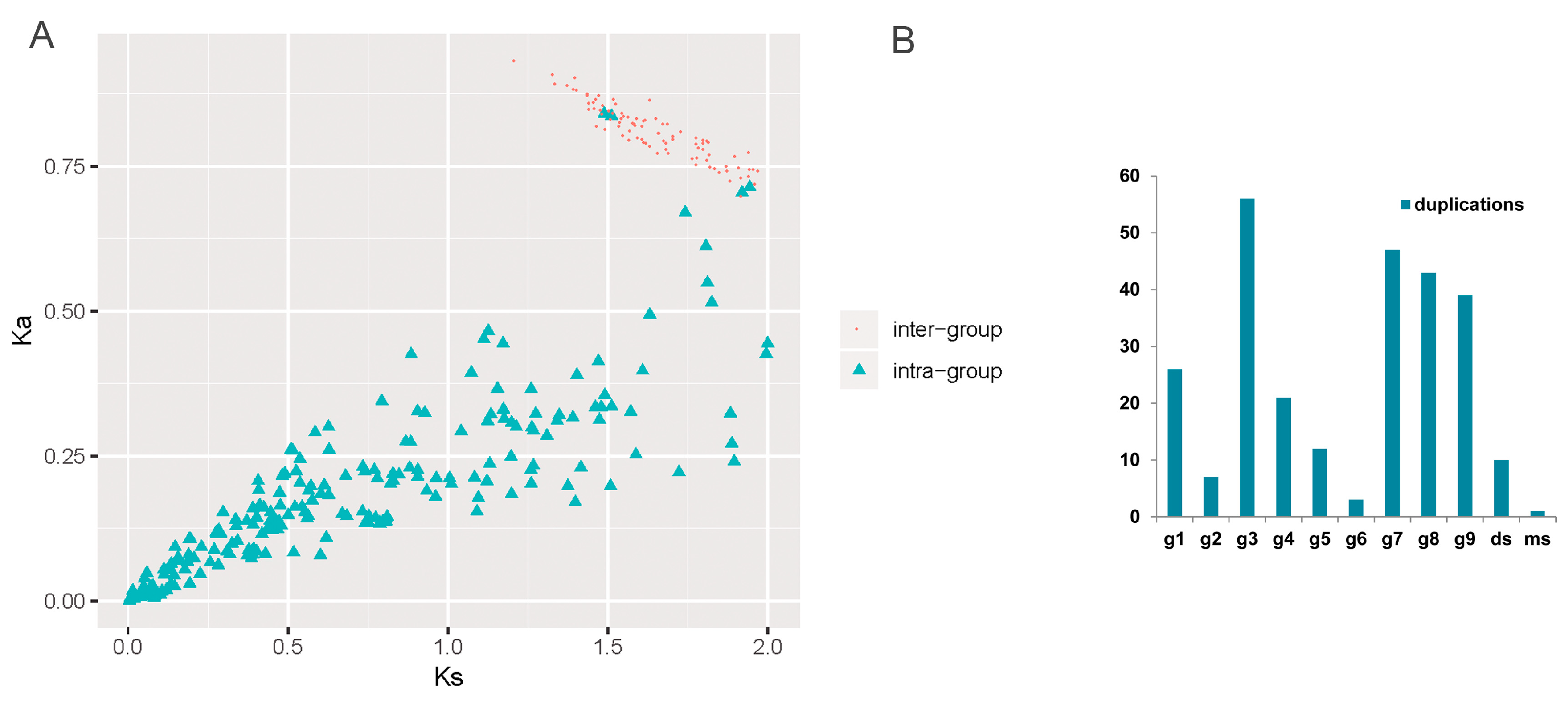
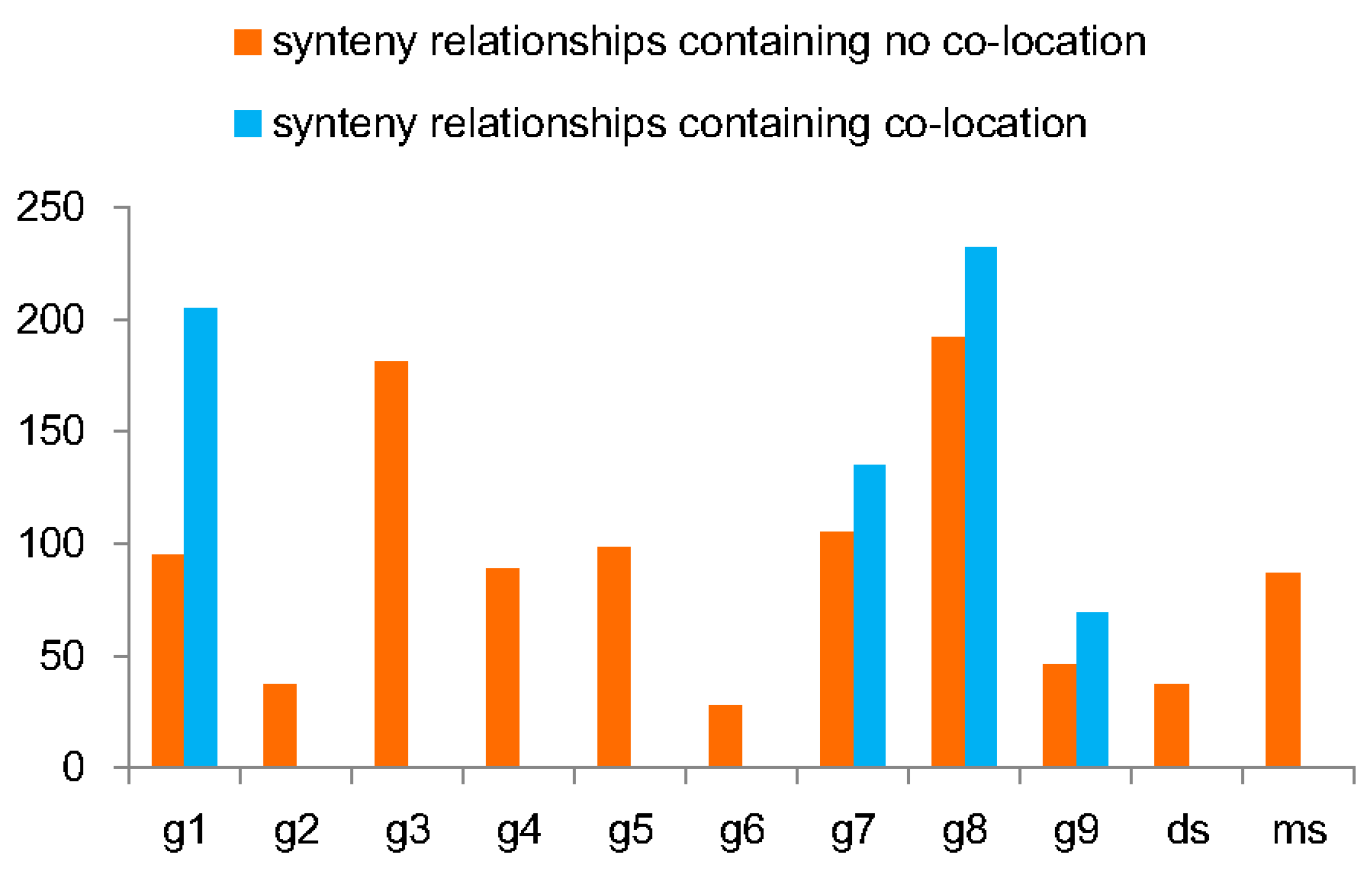
3.3. Evolutionary Profiles of SBP Genes in Typical Ancient WGD Events
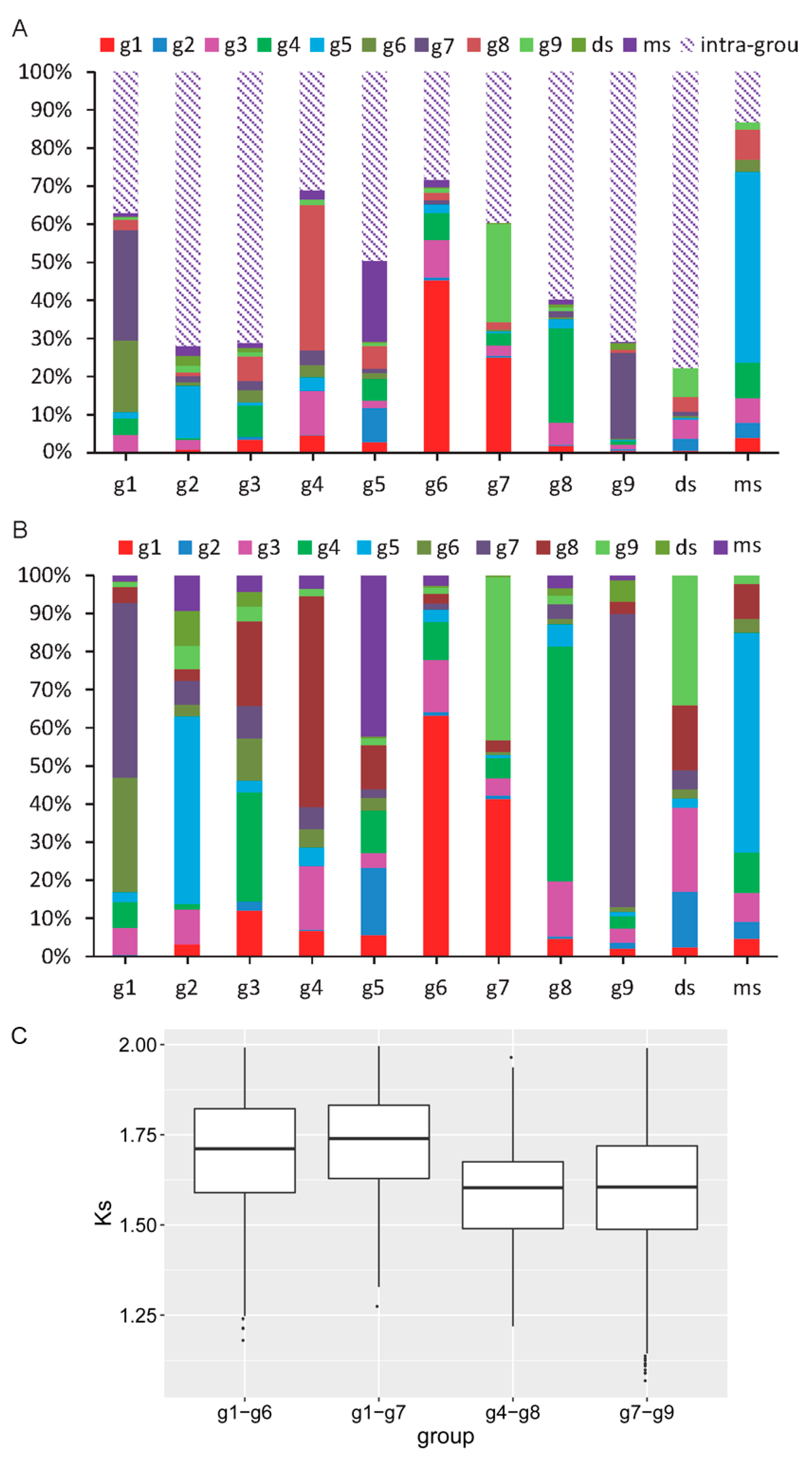
3.4. Expansion Profile Describing the Dynamic Evolutionary Histories
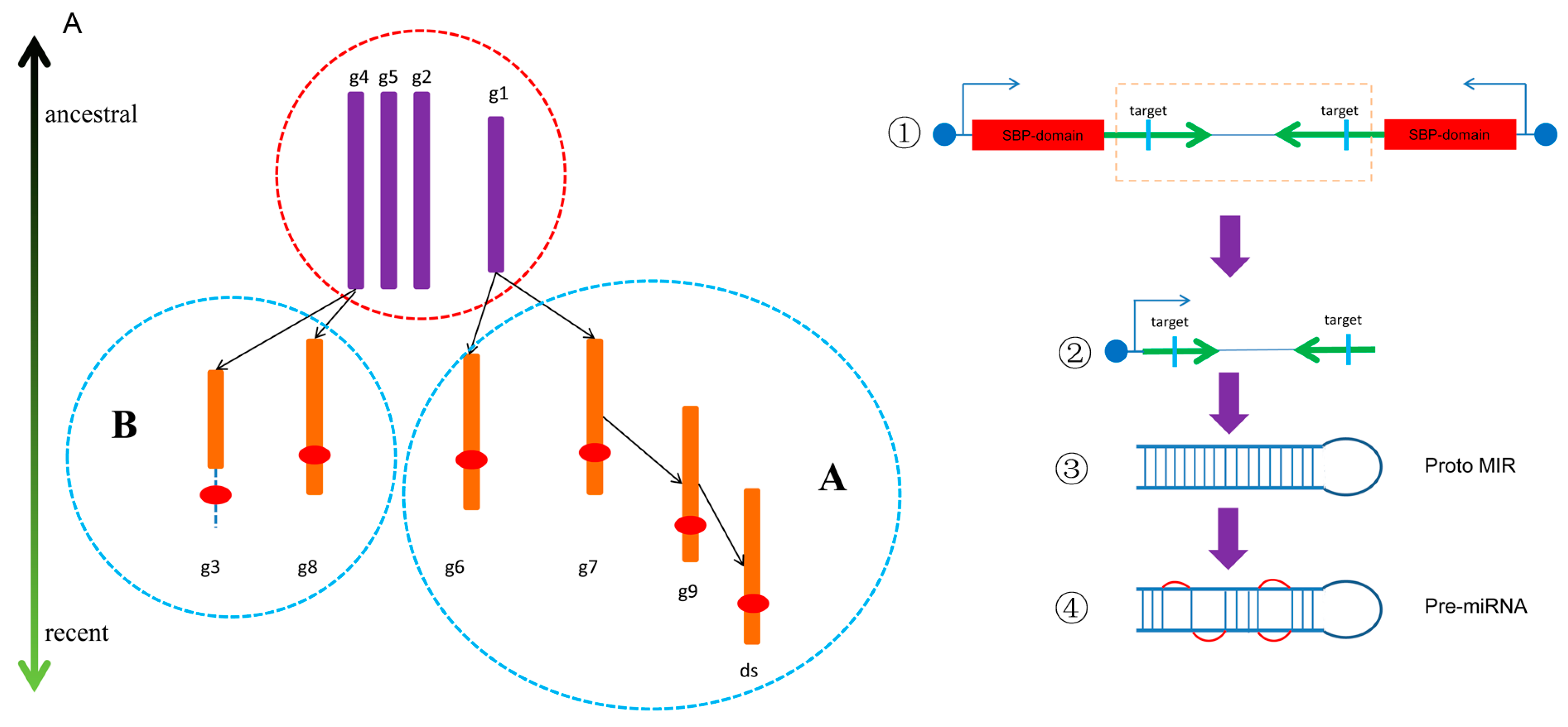
3.5. Coevolutionary Profile of miR156 and SBP Showing the Origin of miR156
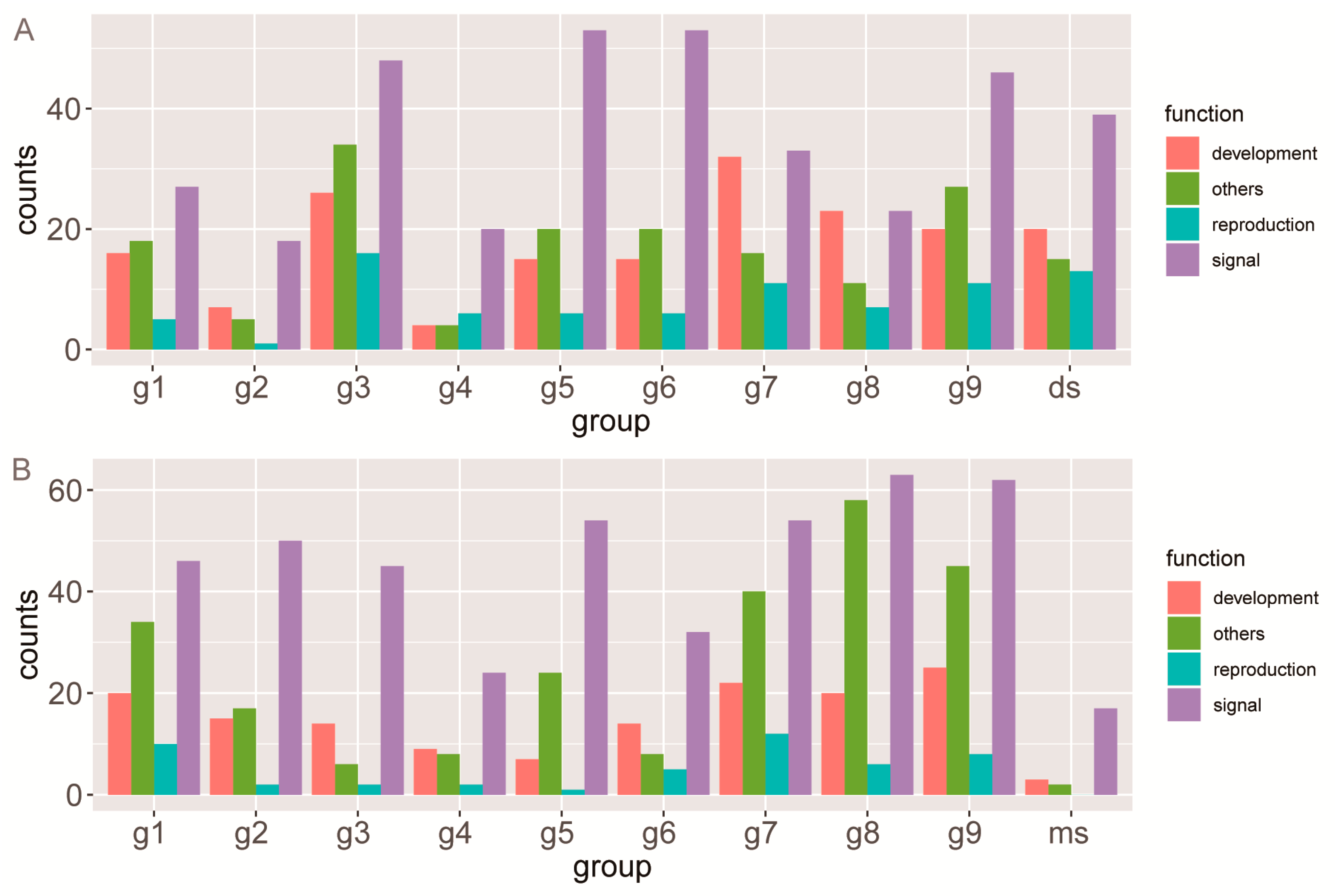
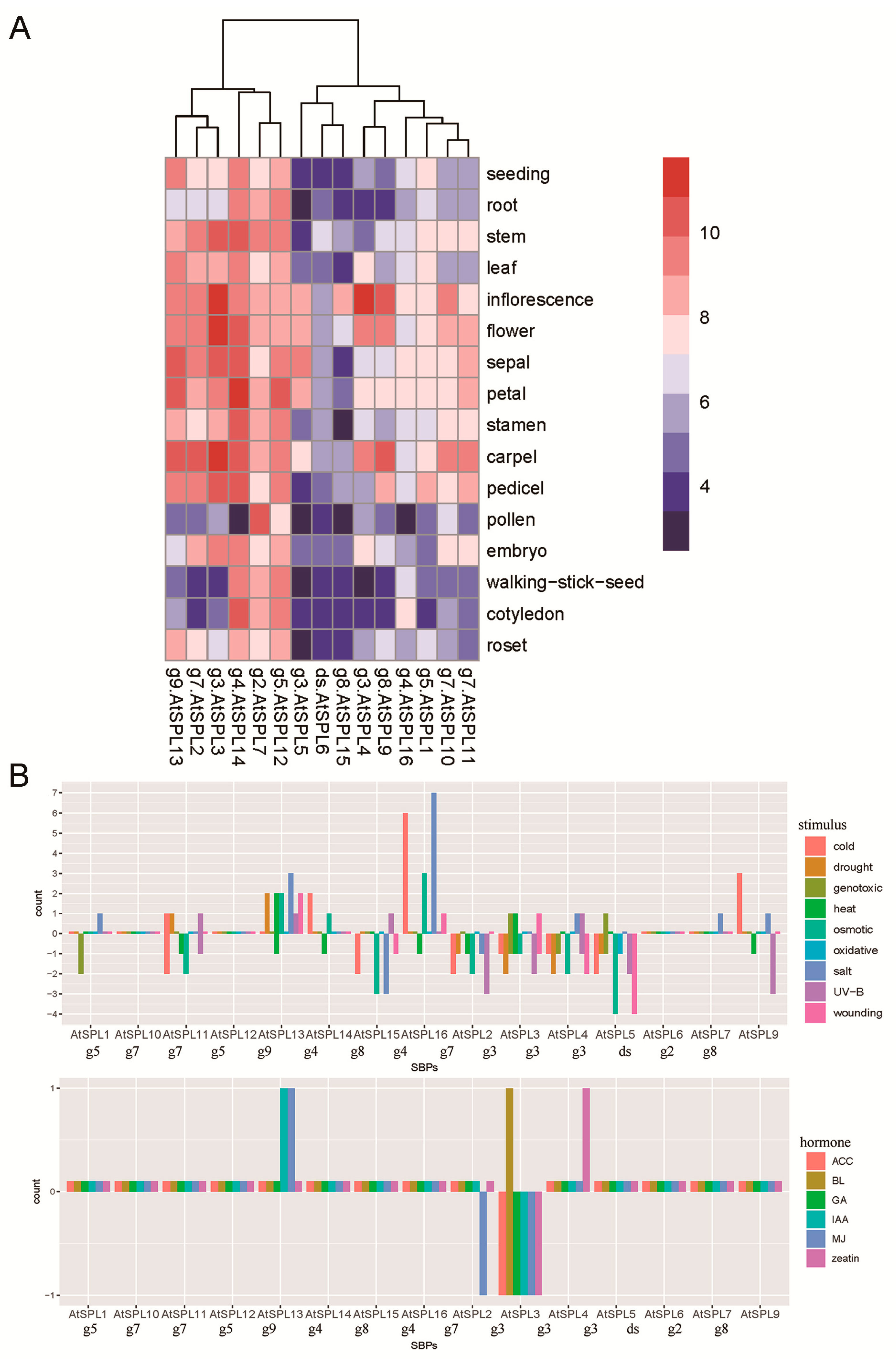
3.6. Functional Evolutionary Profile Indicating the Functional Differentiation of Each Group
4. Discussion
4.1. A Consistent Classification Standard Is Helpful for Exploring Conservative Gene Families in Plants
4.2. Promotion of the Evolution of Angiosperms by the Differential Expansion of the SBP-Box Gene Family during Typical Ancient WGDs
4.3. Short and Moderate-Length SBPs as Important Regulators of Growth and Reproduction in Angiosperms
4.4. Dynamic Evolutionary Process Analyses Reveal the Expansion Process of Different Groups
4.5. MiR156 May Originate From Face-to-Face Tandem Duplication of SBP Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crane, P.R.; Friis, E.M.; Pedersen, K.R. The origin and early diversification of angiosperms. Nature 1995, 374, 27–33. [Google Scholar] [CrossRef]
- Magallón, S.; Castillo, A. Angiosperm diversification through time. Am. J. Bot. 2009, 96, 349–365. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. The origin and diversification of angiosperms. Am. J. Bot. 2004, 91, 1614–1626. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Mizutani, M.; Ohta, D. Diversification of P450 genes during land plant evolution. Annu. Rev. Plant Biol. 2010, 61, 291–315. [Google Scholar] [CrossRef]
- Morant, M.; Bak, S.; Møller, B.L.; Werck-Reichhart, D. Plant cytochromes p450: Tools for pharmacology, plant protection and phytoremediation. Curr. Opin. Biotech. 2003, 14, 151–162. [Google Scholar] [CrossRef]
- Oh, S.-K.; Baek, K.-H.; Park, J.M.; Yi, S.Y.; Yu, S.H.; Kamoun, S.; Choi, D. Capsicum annuum WRKY protein CaWRKY1 is a negative regulator of pathogen defense. New Phytol. 2008, 177, 977–989. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Rinerson, C.I.; Scully, E.D.; Palmer, N.A.; Donze-Reiner, T.; Rabara, R.C.; Tripathi, P.; Shen, Q.J.; Sattler, S.E.; Rohila, J.S.; Sarath, G.; et al. The WRKY transcription factor family and senescence in switchgrass. BMC Genom. 2015, 16, 912. [Google Scholar] [CrossRef]
- Becker, A.; Theißen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 2004, 29, 464–489. [Google Scholar] [CrossRef]
- Klein, J.; Saedler, H.; Huijser, P. A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity genes QUAMOSA. Mol. Gen. Genet. 1996, 250, 7–16. [Google Scholar]
- Guo, A.-Y.; Zhu, Q.-H.; Gu, X.; Ge, S.; Yang, J.; Luo, J. Genome-wide identification and evolutionary analysis of the plant specific SBP-box transcription factor family. Gene 2008, 418, 1–8. [Google Scholar] [CrossRef]
- Riese, M.; Höhmann, S.; Saedler, H.; Münster, T.; Huijser, P. Comparative analysis of the SBP-box gene families in P. patens and seed plants. Gene 2007, 401, 28–37. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Gu, S.; Hu, Z.; Xu, H.; Xu, C. Comparative study of SBP-box gene family in Arabidopsis and rice. Gene 2008, 407, 1–11. [Google Scholar] [CrossRef]
- Cardon, G.; Höhmann, S.; Klein, J.; Nettesheim, K.; Saedler, H.; Huijser, P. Molecular characterisation of the Arabidopsis SBP-box genes. Gene 1999, 237, 91–104. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Sang, S.; Liu, C. Genome-wide identification, phylogeny, and expression analysis of the SBP-box gene family in Euphorbiaceae. BMC Genom. 2019, 20, 912. [Google Scholar] [CrossRef]
- Jung, J.-H.; Lee, H.-J.; Ryu, J.Y.; Park, C.-M. SPL3/4/5 integrate developmental aging and photoperiodic signals into the FT-FD module in Arabidopsis flowering. Mol. Plant 2016, 9, 1647–1659. [Google Scholar] [CrossRef]
- Shikata, M.; Koyama, T.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis SBP-box genes SPL10, SPL11 and SPL2 control morphological change in association with shoot maturation in the reproductive phase. Plant Cell Physiol. 2009, 50, 2133–2145. [Google Scholar] [CrossRef]
- De Bodt, S.; Maere, S.; Van de Peer, Y. Genome duplication and the origin of angiosperms. Trends Ecol. Evol. 2005, 20, 591–597. [Google Scholar] [CrossRef]
- Guo, H.; Lee, T.-H.; Wang, X.; Paterson, A.H. Function relaxation followed by diversifying selection after whole-genome duplication in flowering plants. Plant Physiol. 2013, 162, 769. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. Ancient WGD events as drivers of key innovations in angiosperms. Curr. Opin. Plant Biol. 2016, 30, 159–165. [Google Scholar] [CrossRef]
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Tomsho, L.P.; Hu, Y.; Liang, H.; Soltis, P.S.; et al. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef]
- Jaillon, O.; Aury, J.-M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar]
- Jiao, Y.; Leebens-Mack, J.; Ayyampalayam, S.; Bowers, J.E.; McKain, M.R.; McNeal, J.; Rolf, M.; Ruzicka, D.R.; Wafula, E.; Wickett, N.J.; et al. A genome triplication associated with early diversification of the core eudicots. Genome Biol. 2012, 13, R3. [Google Scholar] [CrossRef]
- Tang, H.; Bowers, J.E.; Wang, X.; Paterson, A.H. Angiosperm genome comparisons reveal early polyploidy in the monocot lineage. Proc. Natl. Acad. Sci. USA 2010, 107, 472. [Google Scholar] [CrossRef]
- Vekemans, D.; Proost, S.; Vanneste, K.; Coenen, H.; Viaene, T.; Ruelens, P.; Maere, S.; Van de Peer, Y.; Geuten, K. Gamma paleohexaploidy in the stem lineage of core eudicots: Significance for MADS-box gene and species diversification. Mol. Biol. Evol. 2012, 29, 3793–3806. [Google Scholar] [CrossRef]
- Jiang, W.-K.; Liu, Y.-L.; Xia, E.-H.; Gao, L.-Z. Prevalent role of gene features in determining evolutionary fates of whole-genome duplication duplicated genes in flowering plants. Plant Physiol. 2013, 161, 1844–1861. [Google Scholar] [CrossRef]
- Kassahn, K.S.; Dang, V.T.; Wilkins, S.J.; Perkins, A.C.; Ragan, M.A. Evolution of gene function and regulatory control after whole-genome duplication: Comparative analyses in vertebrates. Genome Res. 2009, 19, 1404–1418. [Google Scholar] [CrossRef]
- Rensing, S.A. Gene duplication as a driver of plant morphogenetic evolution. Curr. Opin. Plant Biol. 2014, 17, 43–48. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, X.; Paterson, A.H. Different patterns of gene structure divergence following gene duplication in Arabidopsis. BMC Genom. 2013, 14, 652. [Google Scholar] [CrossRef]
- Amborella Genome Project. The Amborella genome and the evolution of flowering plants. Science 2013, 342, 1241089. [Google Scholar] [CrossRef]
- Wu, S.; Han, B.; Jiao, Y. Genetic contribution of paleopolyploidy to adaptive evolution in angiosperms. Mol. Plant 2019, 13, 59–71. [Google Scholar] [CrossRef]
- Ncbi Resource Coordinators. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2015, 43, D6–D17. [Google Scholar] [CrossRef]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M.; et al. The Arabidopsis information resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2011, 40, D1202–D1210. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. Planttfdb 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRbase: MicroRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef]
- Ghahramani, Z. An introduction to hidden markov models and bayesian networks. Hidden Markov Models World Sci. 2001, 45, 9–41. [Google Scholar]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. Blast+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2018, 47, D427–D432. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the smart protein domain annotation resource. Nucleic Acids Res. 2017, 46, D493–D496. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2014, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. Meme suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Swofford, D.L. Paup*: Phylogenetic analysis using parsimony (and other methods), version 4.0.B10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Emms, D.M.; Kelly, S. Orthofinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Patanun, O.; Lertpanyasampatha, M.; Sojikul, P.; Viboonjun, U.; Narangajavana, J. Computational identification of microRNAs and their targets in cassava (Manihot esculenta crantz.). Mol. Biotechnol. 2013, 53, 257–269. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. Weblogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Hurst, L.D. The ka/ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. Kaks_calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinf. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhao, X.-Q.; Wang, J.; Wong, G.K.-S.; Yu, J. Kaks_calculator: Calculating ka and ks through model selection and model averaging. Genom. Proteom. Bioinf. 2006, 4, 259–263. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps, version 1.0.12; Raivo Kolde, 2019. [Google Scholar]
- Yu, G. Clusterprofiler: Universal enrichment tool for functional and comparative study. BioRxiv 2018, 256784. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. Clusterprofiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Schaefer, D.G.; Zrÿd, J.-P. The moss Physcomitrella patens, now and then. Plant Physiol. 2001, 127, 1430–1438. [Google Scholar] [CrossRef]
- Castruita, M.; Casero, D.; Karpowicz, S.J.; Kropat, J.; Vieler, A.; Hsieh, S.I.; Yan, W.; Cokus, S.; Loo, J.A.; Benning, C.; et al. Systems biology approach in Chlamydomonas; reveals connections between copper nutrition and multiple metabolic steps. Plant Cell 2011, 23, 1273. [Google Scholar] [CrossRef]
- Kropat, J.; Tottey, S.; Birkenbihl, R.P.; Depège, N.; Huijser, P.; Merchant, S. A regulator of nutritional copper signaling in Chlamydomonas; is an SBP domain protein that recognizes the GTAC core of copper response element. Proc. Natl. Acad. Sci. USA 2005, 102, 18730. [Google Scholar] [CrossRef]
- Stuessy, T.F. A transitional-combinational theory for the origin of angiosperms. Taxon 2004, 53, 3–16. [Google Scholar] [CrossRef]
- Chao, L.-M.; Liu, Y.-Q.; Chen, D.-Y.; Xue, X.-Y.; Mao, Y.-B.; Chen, X.-Y. Arabidopsis transcription factors SPL1 and SPL12 confer plant thermotolerance at reproductive stage. Mol. Plant 2017, 10, 735–748. [Google Scholar] [CrossRef]
- Wang, J.-W.; Czech, B.; Weigel, D. MiR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef]
- Schwarz, S.; Grande, A.V.; Bujdoso, N.; Saedler, H.; Huijser, P. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol. Biol. 2008, 67, 183–195. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H. The miR156/SPL module, a regulatory hub and versatile toolbox, gears up crops for enhanced agronomic traits. Mol. Plant 2015, 8, 677–688. [Google Scholar] [CrossRef]
- Wang, J.-W.; Schwab, R.; Czech, B.; Mica, E.; Weigel, D. Dual Effects of miR156-Targeted SPL Genes and CYP78A5/KLUH on Plastochron Length and Organ Sizein Arabidopsis thaliana. Plant Cell 2008, 20, 1231. [Google Scholar] [CrossRef]
- Khan, S.-A.; Li, M.-Z.; Wang, S.-M.; Yin, H.-J. Revisiting the role of plant transcription factors in the battle against abiotic stress. Int. J. Mol. Sci. 2018, 19, 1634. [Google Scholar] [CrossRef]
- Xing, S.; Salinas, M.; Garcia-Molina, A.; Höhmann, S.; Berndtgen, R.; Huijser, P. SPL8 and miR156-targeted SPL genes redundantly regulate Arabidopsis gynoecium differential patterning. Plant J. 2013, 75, 566–577. [Google Scholar] [CrossRef]
- Mena, M.; Ambrose, B.A.; Meeley, R.B.; Briggs, S.P.; Yanofsky, M.F.; Schmidt, R.J. Diversification of c-function activity in maize flower development. Science 1996, 274, 1537–1540. [Google Scholar] [CrossRef]
- Cho, S.H.; Coruh, C.; Axtell, M.J. miR156 and miR390 Regulate tasiRNA Accumulation and Developmental Timing in Physcomitrella patens. Plant Cell 2012, 24, 4837–4849. [Google Scholar] [CrossRef]
- Unte, U.S.; Sorensen, A.-M.; Pesaresi, P.; Gandikota, M.; Leister, D.; Saedler, H.; Huijser, P. SPL8, an SBP-box gene that affects pollen sac development in Arabidopsis. Plant Cell 2003, 15, 1009. [Google Scholar] [CrossRef]
- Xing, S.; Quodt, V.; Chandler, J.; Höhmann, S.; Berndtgen, R.; Huijser, P. SPL8 acts together with the brassinosteroid-signaling component bim1 in controlling Arabidopsis thaliana male fertility. Plants 2013, 2, 416–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Schwarz, S.; Saedler, H.; Huijser, P. SPL8, a local regulator in a subset of gibberellin-mediated developmental processes in Arabidopsis. Plant Mol. Biol. 2007, 63, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Soltis, D.E.; Soltis, P.S.; Zanis, M.J.; Suh, Y. Phylogenetic relationships among early-diverging eudicots based on four genes: Were the eudicots ancestrally woody? Mol. Phylogenet. Evol. 2004, 31, 16–30. [Google Scholar] [CrossRef]
- Allen, E.; Xie, Z.; Gustafson, A.M.; Sung, G.-H.; Spatafora, J.W.; Carrington, J.C. Evolution of microRNA genes by inverted duplication of target gene sequences in Arabidopsis thaliana. Nat. Genet. 2004, 36, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Fenselau de Felippes, F.; Schneeberger, K.; Dezulian, T.; Huson, D.H.; Weigel, D. Evolution of Arabidopsis thaliana microRNAs from random sequences. RNA 2008, 14, 2455–2459. [Google Scholar] [CrossRef]
- Piriyapongsa, J.; Jordan, I.K. Dual coding of siRNAs and miRNAs by plant transposable elements. RNA 2008, 14, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Xia, J.; Jin, Y. Domestication of transposable elements into microRNA genes in plants. PLoS ONE 2011, 6, e19212. [Google Scholar] [CrossRef]

| Organism | Source | Number | Nomenclature |
|---|---|---|---|
| Arabidopsis thaliana | PlantTFDB | 16 | AtSPL |
| Brassica rapa | This research | 29 | BrSBP |
| Camelina sativa | This research | 45 | CsSBP |
| Phaseolus vulgaris | This research | 22 | PvSBP |
| Vigna angularis | This research | 20 | VaSBP |
| Cajanus cajan | This research | 23 | CcSBP |
| Medicago truncatula | This research | 17 | MtSBP |
| Solanum lycopersicum | This research | 16 | SlSBP |
| Capsicum annuum | This research | 15 | CaSBP |
| Nicotiana tabacum | This research | 40 | NtSBP |
| Prunus persica | This research | 16 | PpSBP |
| Malus domestica | This research | 34 | MdSBP |
| Prunus mume | This research | 17 | PmSBP |
| Brachypodium distachyon | This research | 17 | BdSBP |
| Sorghum bicolor | This research | 19 | SbSBP |
| Panicum hallii | This research | 19 | PhSBP |
| Oryza sativa | PlantTFDB | 19 | OsSPL |
| Setaria italica | This research | 11 | SiSBP |
| Zea mays | This research | 32 | ZmSBP |
| Ananas comosus | This research | 17 | AcSBP |
| Elaeis guineensis | This research | 24 | EgSBP |
| Musa acuminata | This research | 54 | MaSBP |
| Group | Species Number | Sequence Number | Average Number | Average Length (AA) | Average Molecular Weight | Average Isoelectric Point |
|---|---|---|---|---|---|---|
| g1-eudicot | 13 | 22 | 1.69 | 306.9091 | 34,068.8 | 8.913333 |
| g1-monocot | 9 | 35 | 3.89 | 405.4857 | 43,528.37 | 8.200286 |
| g2-eudicot | 12 | 18 | 1.5 | 722.4444 | 80,763.75 | 6.218889 |
| g2-monocot | 9 | 13 | 1.44 | 859.6923 | 95,320.8 | 5.746154 |
| g3-eudicot | 13 | 70 | 5.38 | 167.0857 | 19,179.53 | 8.482571 |
| g3-monocot | 9 | 15 | 1.67 | 203.2 | 21,441.35 | 9.813333 |
| g4-eudicot | 11 | 20 | 1.82 | 1031.25 | 114,075.8 | 8.3985 |
| g4-monocot | 8 | 12 | 1.5 | 1029.75 | 112,982.2 | 7.616667 |
| g5-eudicot | 13 | 35 | 2.69 | 987.7429 | 109,648.9 | 6.420571 |
| g5-monocot | 8 | 15 | 1.875 | 983 | 108,311.9 | 5.984 |
| g6-eudicot | 7 | 12 | 1.71 | 352.5 | 39,079.3 | 8.4025 |
| g6-monocot | 8 | 10 | 1.25 | 436.9 | 46,449.51 | 8.838 |
| g7-eudicot | 13 | 36 | 2.77 | 422.0833 | 46,785.88 | 8.303889 |
| g7-monocot | 9 | 34 | 3.78 | 426.6765 | 46,448.93 | 8.961471 |
| g8-eudicot | 13 | 29 | 2.23 | 363.1034 | 39,381.31 | 8.907143 |
| g8-monocot | 9 | 30 | 3.33 | 382.1 | 40,155.08 | 8.927667 |
| g9-eudicot | 13 | 32 | 2.46 | 368.1563 | 40,526.01 | 8.3625 |
| g9-monocot | 8 | 42 | 5.25 | 396.4286 | 42,237.75 | 7.825714 |
| ds | 13 | 36 | 2.77 | 496.0278 | 54,836.9 | 7.570833 |
| ms | 6 | 7 | 1.17 | 879.2857 | 98,183.74 | 6.792857 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Gao, X.; Zhang, X.; Liu, C. Dynamic Expansion and Functional Evolutionary Profiles of Plant Conservative Gene Family SBP-Box in Twenty Two Flowering Plants and the Origin of miR156. Biomolecules 2020, 10, 757. https://doi.org/10.3390/biom10050757
Li J, Gao X, Zhang X, Liu C. Dynamic Expansion and Functional Evolutionary Profiles of Plant Conservative Gene Family SBP-Box in Twenty Two Flowering Plants and the Origin of miR156. Biomolecules. 2020; 10(5):757. https://doi.org/10.3390/biom10050757
Chicago/Turabian StyleLi, Jing, Xiaoyang Gao, Xuan Zhang, and Changning Liu. 2020. "Dynamic Expansion and Functional Evolutionary Profiles of Plant Conservative Gene Family SBP-Box in Twenty Two Flowering Plants and the Origin of miR156" Biomolecules 10, no. 5: 757. https://doi.org/10.3390/biom10050757
APA StyleLi, J., Gao, X., Zhang, X., & Liu, C. (2020). Dynamic Expansion and Functional Evolutionary Profiles of Plant Conservative Gene Family SBP-Box in Twenty Two Flowering Plants and the Origin of miR156. Biomolecules, 10(5), 757. https://doi.org/10.3390/biom10050757





