Combined Yeast Cultivation and Pectin Hydrolysis as an Effective Method of Producing Prebiotic Animal Feed from Sugar Beet Pulp
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biological Material Screened for SBP Fermentation
2.3. Yeast Screening
2.3.1. Cell Concentration of Yeast Strains
2.3.2. Utilization of Monosaccharides
2.3.3. Protein Concentration
2.3.4. Antagonistic Properties of Yeast Metabolites against Lactic Acid Bacteria
2.4. SSF Process
2.5. High Performance Anion Exchange Chromatography
2.6. In Vitro Fermentability of POS
2.7. Adhesion of Lactic Acid Bacteria (LAB) or Pathogens to Human Gut Epithelial Cells
2.7.1. Bacterial Strains
2.7.2. Caco-2 Cell Culture
2.7.3. Adherence Assay
2.8. Statistical Analysis
3. Results and Discussion
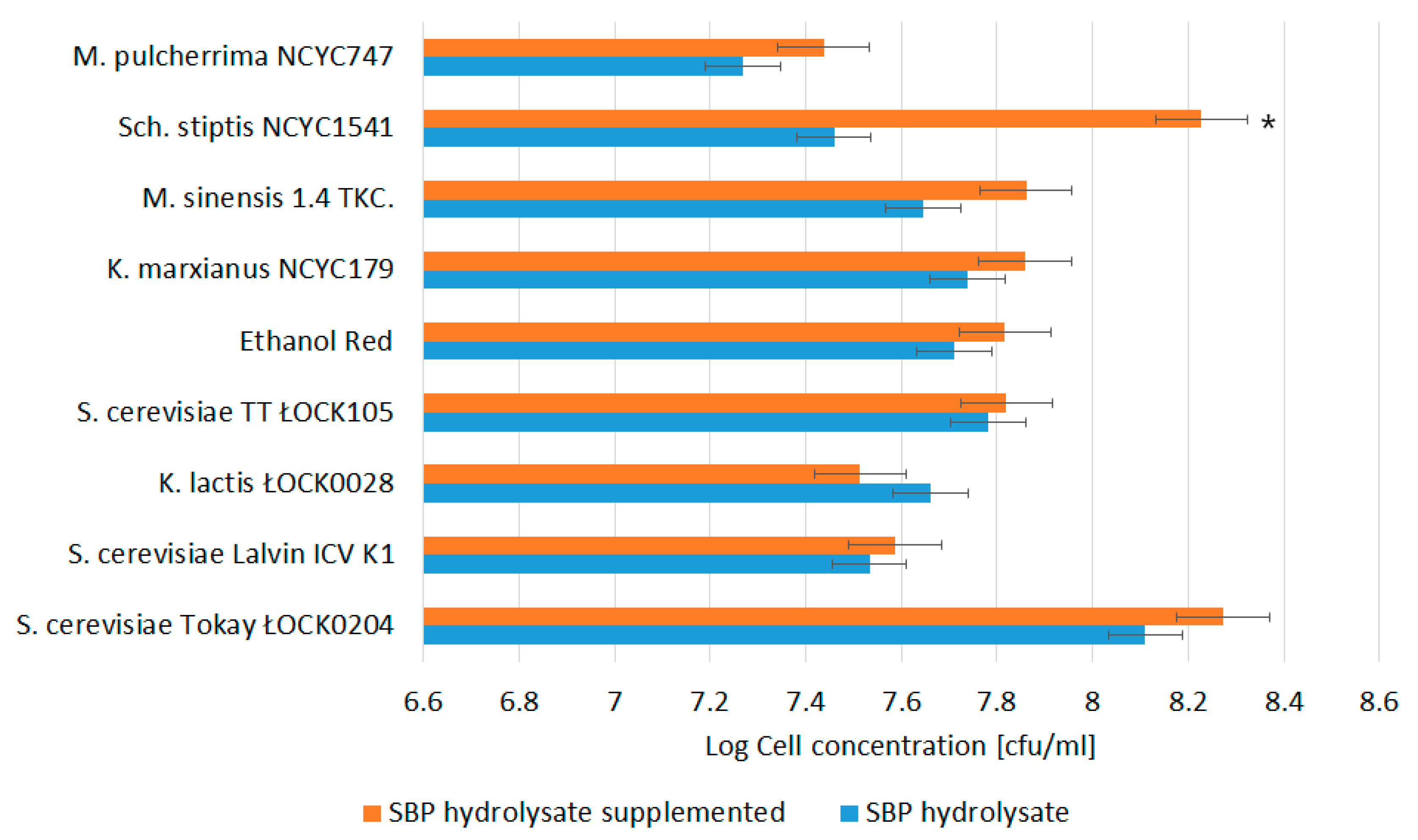
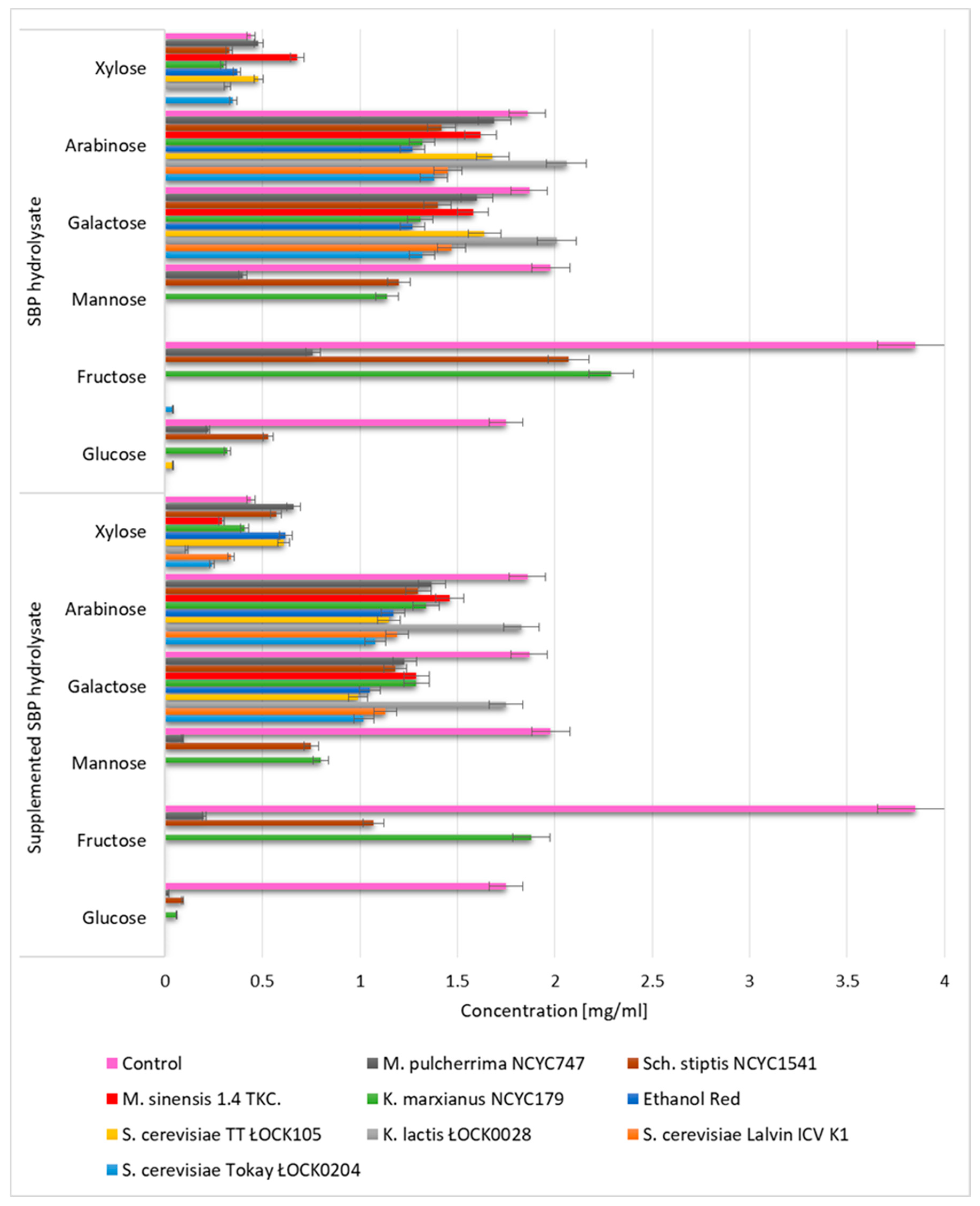
3.1. Biomass Productivity and Profile of Monosaccharides in SBP Hydrolysates utilized by Yeast
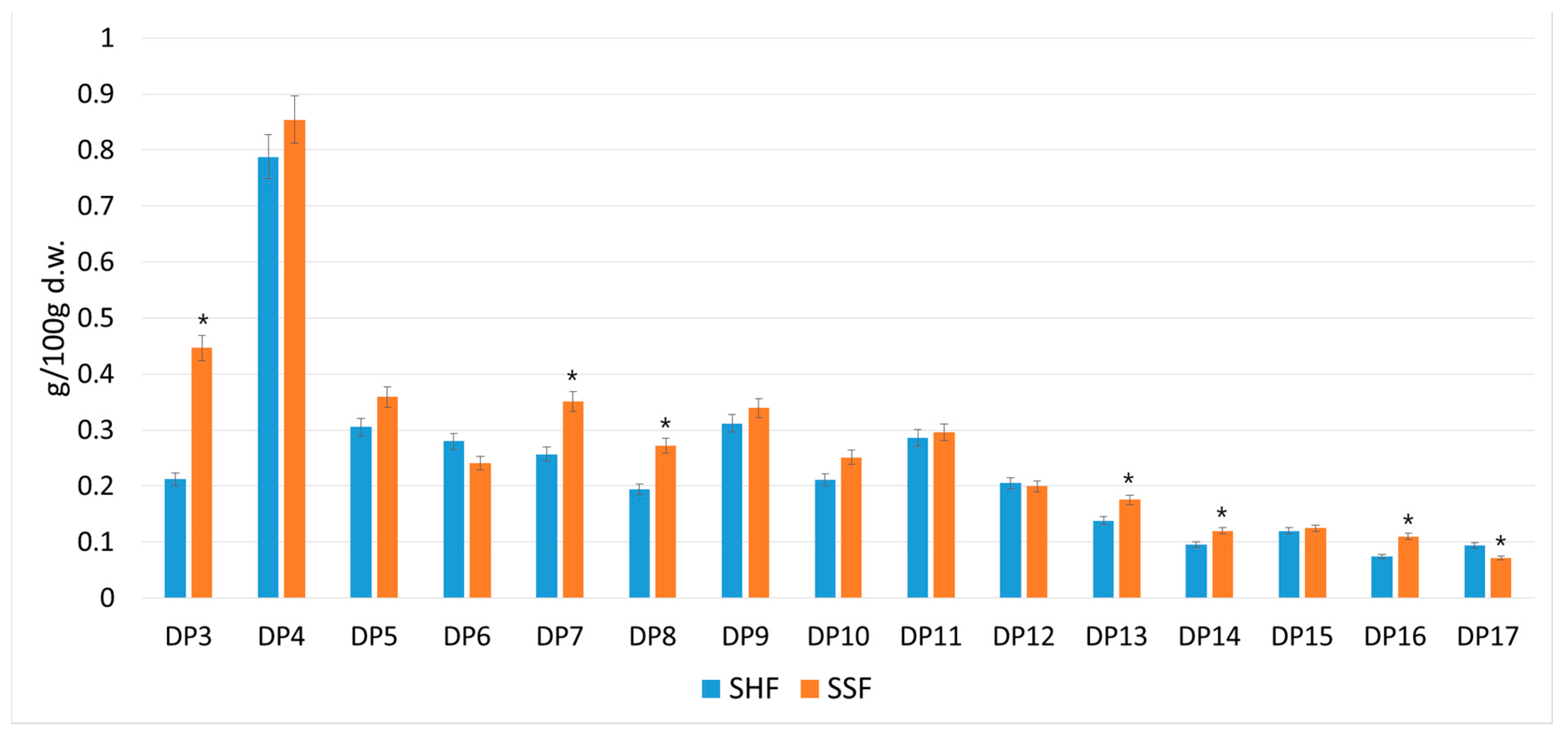
3.2. Yield of Oligosaccharides with Different Degrees of Polymerization Obtained by Separate or Simultaneous Enzymatic Hydrolysis and Production of SCP from Sugar Beet Pulp
3.3. Influence of SBP Animal Feed Component on the Growth of Selected Lactic Acid Bacteria (LAB) or Pathogens
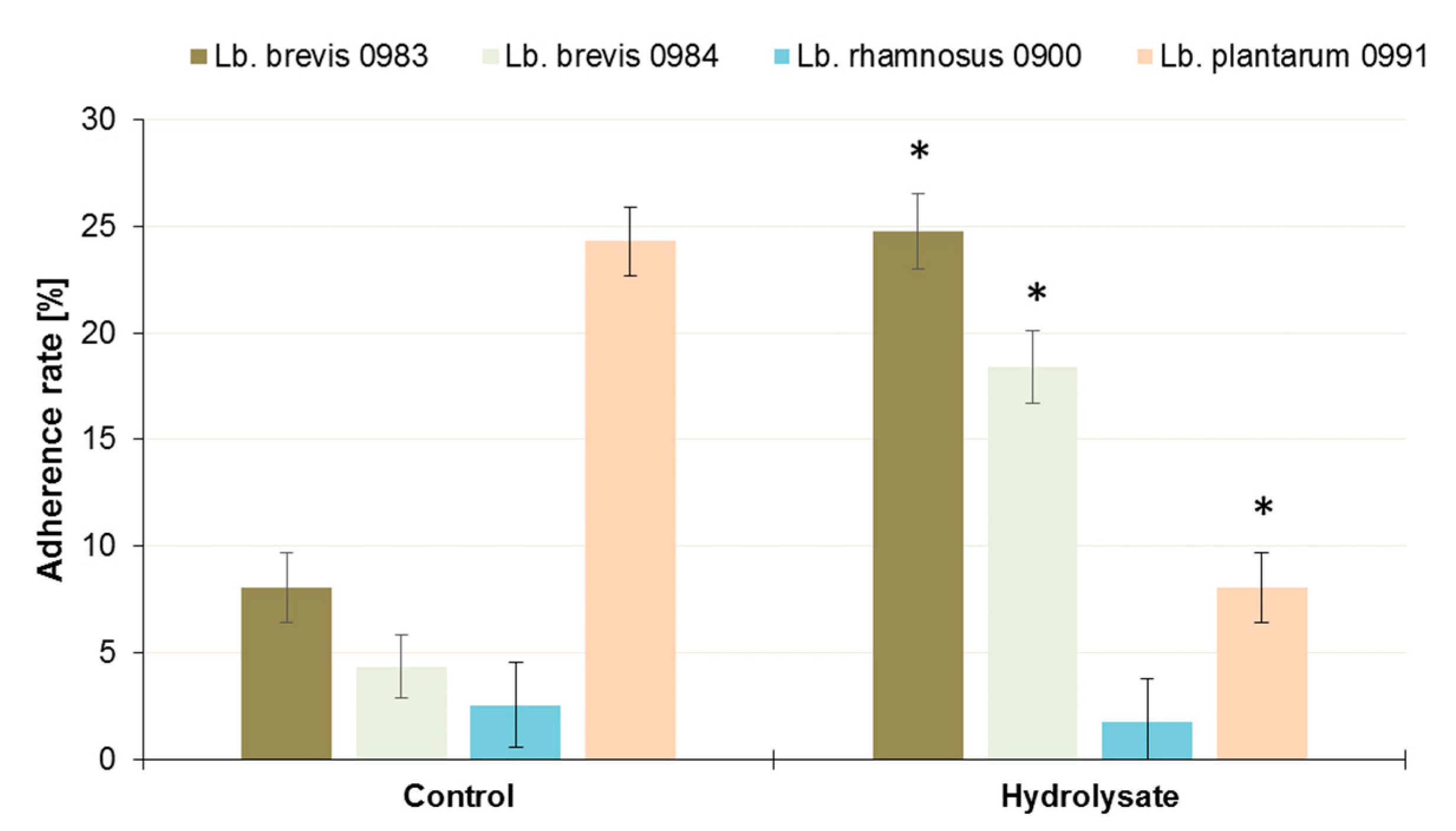
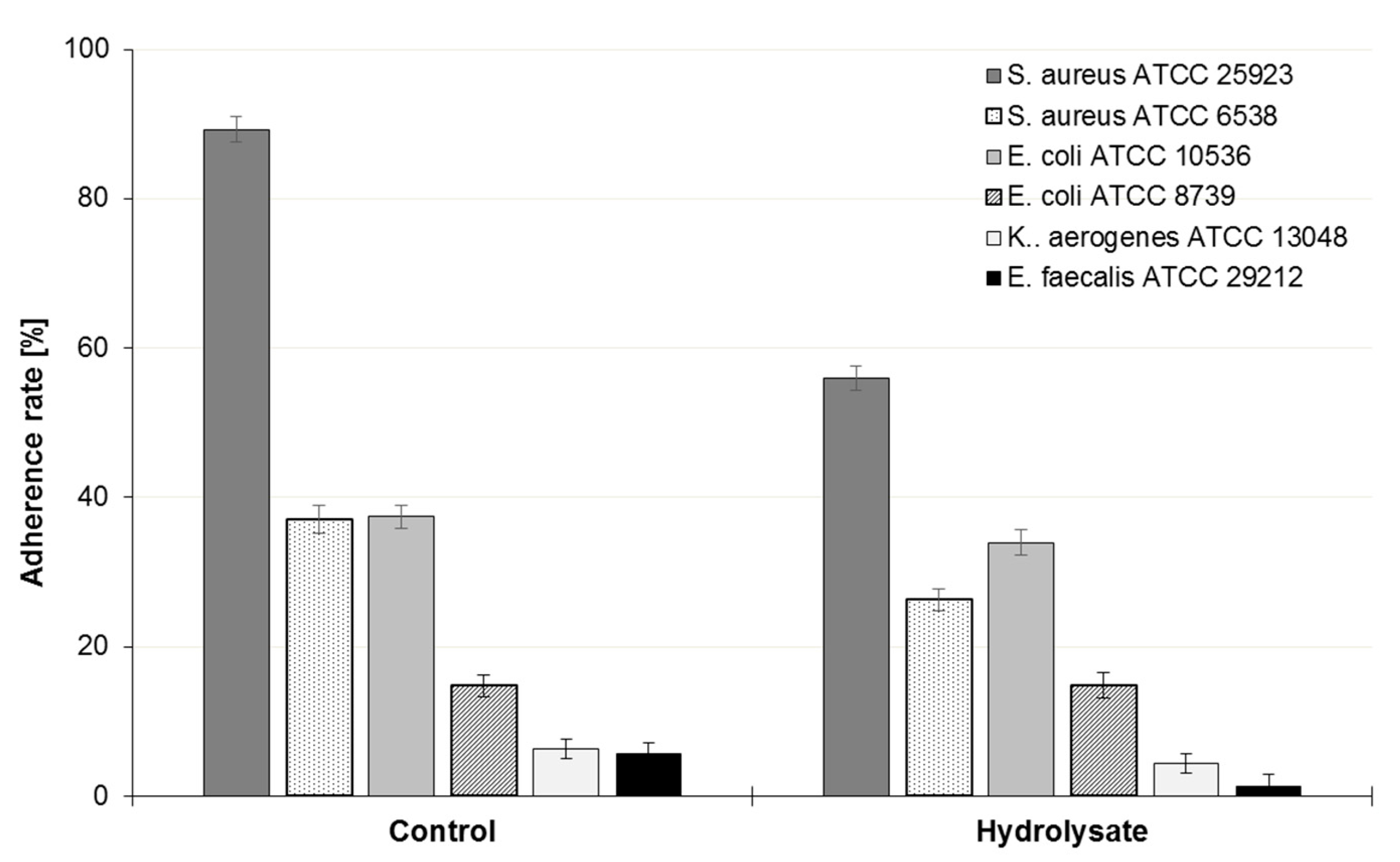
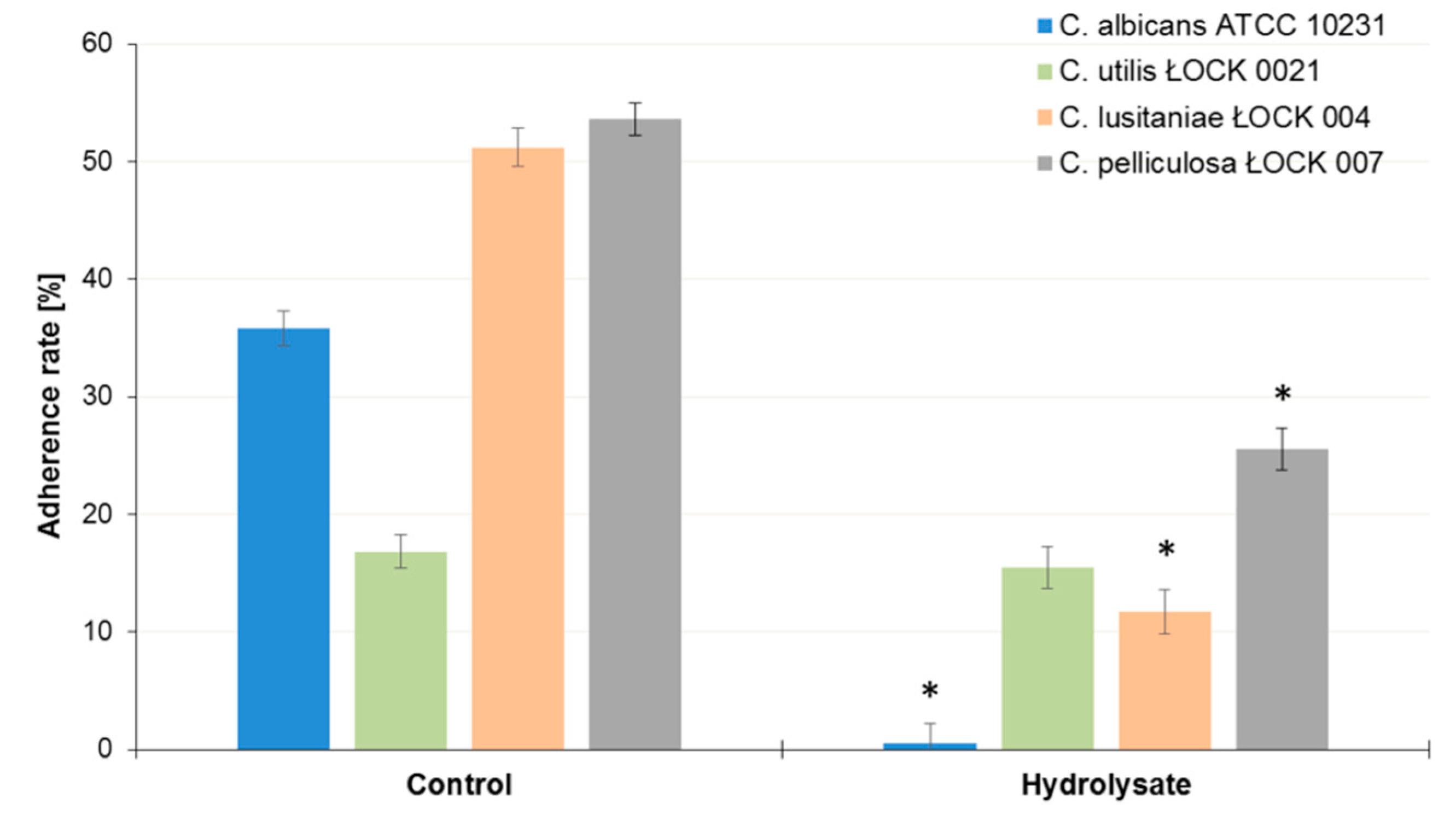
3.4. Influence of the SBP Animal Feed Component on the Adhesion of Lactic Acid Bacteria (LAB) or Pathogens to Caco-2 Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salak-Johnson, J.L.; McGlone, J.J. Making sense of apparently conflicting data: Stress and immunity in swine and cattle. J. Anim. Sci. 2014, 85, 81–88. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [PubMed]
- Rosmini, M.R.; Sequeira, G.J.; Guerrero-Legarreta, I.; Martí, L.E.; Dalla-Santina, R.; Frizzo, L.; Bonazza, J.C. Producción de probióticos para animales de abasto: importancia del uso de la microbiota intestinal indígena. Rev. Mex. Ing. Quim. 2004, 3, 181–191. [Google Scholar]
- Signorini, M.L.; Soto, L.P.; Zbrun, M.V.; Sequeira, G.J.; Rosmini, M.R.; Frizzo, L.S. Impact of probiotic administration on the health and fecal microbiota of young calves: A meta-analysis of randomized controlled trials of lactic acid bacteria. Res. Vet. Sci. 2012, 93, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: production, characterization and health benefits. Crit. Rev. Biotech. 2016, 36, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: a review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Garthoff, J.A.; Heemskerk, S.; Hempenius, R.A.; Lina, B.A.; Krul, C.A.; Koeman, J.H.; Speijers, G.J. Safety evaluation of pectin-derived acidic oligosaccharides (pAOS): genotoxicity and sub-chronic studies. Regul. Toxicol. Pharmacol. 2010, 57, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Gomez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic-oligosaccharides: manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Leijdekkers, A.G.M.; Bink, J.P.M.; Geuthes, S.; Schols, H.A.; Gruppen, H. Enzymatic saccharification of sugarbeet pulp for the production of galacturonic acid and arabinose; a study on the impact of the formation of recalcitrant oligosaccharides. Bioresour. Technol. 2013, 128, 518–525. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, S.; Gullón, B.; Moreira, M.T. Environmental assessment of biorefinery processes for the valorization of lignocellulosic wastes into oligosaccharides. J. Clean. Prod. 2018, 172, 4066–4073. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [PubMed]
- Taherzadeh, M.J.; Karimi, K. Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: a review. Bioresources 2007, 2, 707–738. [Google Scholar]
- Wilkowska, A.; Nowak, A.; Chrobot-Antczak, A.; Motyl, I.; Czyżowska, A.; Paliwoda, A. Structurally different pectic oligosaccharides produced from apple pomace and their biological activity in vitro. Foods 2019, 8, 365. [Google Scholar]
- Bonnin, E.; Clavurier, K.; Daniel, S.; Kauppinen, S.; Mikkelsen, J.D.M.; Thibault, J.-F. Pectin acetylesterases from Aspergillus are able to deacetylate homogalacturonan as well as rhamnogalacturonan. Carbohydr. Polym. 2008, 74, 411–418. [Google Scholar]
- Patelski, P.; Berłowska, J.; Dziugan, P.; Pielech-Przybylska, K.; Balcerek, M.; Dziekońska, U.; Kalinowska, H. Utilisation of sugar beet bagasse for the biosynthesis of yeast SCP. J. Food Engin. 2015, 167, 32–37. [Google Scholar]
- Alexandre, H.; Guilloux-Benatier, M. Yeast autolysis in sparkling wine – a review. Aust. J. Grape Wine R. 2006, 12, 119–127. [Google Scholar]
- Berlowska, J.; Cieciura, W.; Borowski, S.; Dudkiewicz, M.; Binczarski, M.; Witonska, I.; Otlewska, A.; Kregiel, D. Simultaneous saccharification and fermentation of sugar beet pulp with mixed bacterial cultures for lactic acid and propylene glycol production. Molecules 2016, 21. [Google Scholar]
- Berlowska, J.; Cieciura-Włoch, W.; Kalinowska, H.; Kregiel, D.; Borowski, S.; Pawlikowska, E.; Binczarski, M.; Witonska, I. Enzymatic conversion of sugar beet pulp: A comparison of simultaneous saccharification and fermentation and separate hydrolysis and fermentation for lactic acid production. Food Technol. Biotechnol. 2018, 56, 188–196. [Google Scholar]
- Quigley, J.D.; Kost, C.J.; Wolfe, T.A. Effects of spraydried animal plasma in milk replacers or additives containing serum and oligosaccharides on growth and health of calves. J. Dairy Sci. 2002, 85, 413–421. [Google Scholar]
- Grand, E.; Respondek, F.; Martineau, C.; Detilleux, J.; Bertrand, G. Effects of short-chain fructooligosaccharides on growth performance of preruminant veal calves. J. Dairy Sci. 2016, 96, 1094–1101. [Google Scholar]
- Heinrichs, A.J.; Jones, C.M.; Heinrichs, B.S. Effects of mannan oligosaccharide or antibiotics in neonatal diets on health and growth of dairy calves. J. Dairy Sci. 2003, 86, 4064–4069. [Google Scholar] [PubMed]
- Ghosh, S.; Mehla, R.K. Influence of dietary supplementation of prebiotics (mannanoligosaccharide) on the performance of crossbred calves. Trop. Anim. Health. Prod. 2012, 44, 617–622. [Google Scholar] [PubMed]
- Moreira dos Anjosa, C.; Dias Goisa, F.; Moreira dos Anjosa, C.; de Souza Rochab, V.; de Sá e Castrob, D.E.; Bezerra Allamana, I.; Lessa Silvaa, F.; de Oliveira Carvalhob, P.L.; Meneghettia, C.; Costac, L.B. Effects of dietary beta-glucans, glucomannans and mannan oligosaccharides or chlorohydroxyquinoline on the performance, diarrhea, hematological parameters, organ weight and intestinal health of weanling pigs. Livestock Science 2019, 223, 39–46. [Google Scholar]
- Uyeno, Y.; Kawashima, K.; Hasunuma, T.; Wakimoto, W.; Noda, M.; Nagashima, S.; Akiyama, K.; Tabata, M.; Kushibiki, S. Effects of cellooligosaccharide or a combination of cellooligosaccharide and live Clostridium butyricum culture on performance and intestinal ecology in holstein calves fed milk or milk replacer. Livest. Sci. 2013, 153, 88–93. [Google Scholar]
- Jiao, L.F.; Song, Z.H.; Ke, Y.L.; Xiao, K.; Hu, C.H.; Shi, B. Cello-oligasaccharides influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim. Feed Sci. Technol. 2014, 195, 85–91. [Google Scholar]
- Masanetz, S.; Preißinger, W.; Meyer, H.H.D.; Pfaffl, M.W. Effects of the prebiotics inulin and lactulose on intestinal immunology and hematology of preruminant calves. Animal 2011, 5, 1099–1106. [Google Scholar]
- Fleige, S.; Preißinger, W.; Meyer, H.H.D.; Pfaffl, M.W. Effect of lactulose on growth performance and intestinal morphology of pre-ruminant calves using a milk replacer containing Enterococcus faecium. Animal 2007, 1, 367–373. [Google Scholar]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar]
- Al-Saiady, M.Y. Effect of probiotic bacteria on immunoglobulin g concentration and other blood components of newborn calves. J. Anim. Vet. Adv. 2010, 9, 604–609. [Google Scholar]
- Chiofalo, V.; Liotta, L.; Chiofalo, B. Effects of the administration of Lactobacilli on body growth and on the metabolic profile in growing Maltese goat kids. Reprod. Nutr. Dev. 2004, 44, 449–457. [Google Scholar]
- Casey, P.G.; Gardiner, G.E.; Casey, G.; Bradshaw, B.; Lawlor, P.G.; Lynch, P.B.; Leonard, F.C.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; et al. A 5-strain probiotic combination reduces pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica serovar Typhimurium. Appl. Environ. Microb. 2007, 73, 1858–1863. [Google Scholar]


| Yeast Strain | Protein Content [mg/mL] |
|---|---|
| S. cerevisiae Tokay ŁOCK0204 | 0.41 ± 0.07a |
| S. cerevisiae Ethanol Red | 0.16 ± 0.02b |
| S. cerevisiae Lalvin ICV K1 | 0.29 ± 0.06b |
| M. pulcherrima NCYC747 | 0.22 ± 0.02b |
| M. sinensis 1.4 TKC. | 0.20 ± 0.09b |
| Sch. stiptis NCYC1541 | 0.09 ± 0.01c |
| K. marxianus NCYC179 | 0.11 ± 0.07c |
| K. lactis ŁOCK0028 | 0.05c |
| Strain | Stimulation (+) or Inhibition Rate (-) [%] |
|---|---|
| Lb. brevis 0983 | +206.8 |
| Lb. brevis 0984 | +324.8 |
| Lb. rhamnosus 0900 | −30.7 |
| Lb. plantarum 0991 | −66.9 |
| E. coli ATCC 8739 | −0.4 |
| E. coli ATCC 10536 | −9.3 |
| S. aureus ATCC 6538 | −30.0 |
| S. aureus ATCC 25923 | −37.3 |
| K. aerogenes ATCC 13048 | −31.2 |
| E. faecalis ATCC 29212 | −77.8 |
| C. albicans ATCC 10231 | −98.5 |
| C. lusitaniae ŁOCK 0004 | −77.1 |
| C. utilis ŁOCK 0021 | −7.8 |
| C. pelliculosa ŁOCK 0007 | −52.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkowska, A.; Berlowska, J.; Nowak, A.; Motyl, I.; Antczak-Chrobot, A.; Wojtczak, M.; Kunicka-Styczyńska, A.; Binczarski, M.; Dziugan, P. Combined Yeast Cultivation and Pectin Hydrolysis as an Effective Method of Producing Prebiotic Animal Feed from Sugar Beet Pulp. Biomolecules 2020, 10, 724. https://doi.org/10.3390/biom10050724
Wilkowska A, Berlowska J, Nowak A, Motyl I, Antczak-Chrobot A, Wojtczak M, Kunicka-Styczyńska A, Binczarski M, Dziugan P. Combined Yeast Cultivation and Pectin Hydrolysis as an Effective Method of Producing Prebiotic Animal Feed from Sugar Beet Pulp. Biomolecules. 2020; 10(5):724. https://doi.org/10.3390/biom10050724
Chicago/Turabian StyleWilkowska, Agnieszka, Joanna Berlowska, Adriana Nowak, Ilona Motyl, Aneta Antczak-Chrobot, Maciej Wojtczak, Alina Kunicka-Styczyńska, Michał Binczarski, and Piotr Dziugan. 2020. "Combined Yeast Cultivation and Pectin Hydrolysis as an Effective Method of Producing Prebiotic Animal Feed from Sugar Beet Pulp" Biomolecules 10, no. 5: 724. https://doi.org/10.3390/biom10050724
APA StyleWilkowska, A., Berlowska, J., Nowak, A., Motyl, I., Antczak-Chrobot, A., Wojtczak, M., Kunicka-Styczyńska, A., Binczarski, M., & Dziugan, P. (2020). Combined Yeast Cultivation and Pectin Hydrolysis as an Effective Method of Producing Prebiotic Animal Feed from Sugar Beet Pulp. Biomolecules, 10(5), 724. https://doi.org/10.3390/biom10050724









