Anti-Atherosclerotic Activity of (3R)-5-Hydroxymellein from an Endophytic Fungus Neofusicoccum parvum JS-0968 Derived from Vitex rotundifolia through the Inhibition of Lipoproteins Oxidation and Foam Cell Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. General Experimental Procedures
2.3. Isolation and Cultivation of Fungus Strain
2.4. Extraction and Isolation
2.5. Isolation of LDL and HDL from Human Plasma
2.6. Conjugated Dienes (CD) Formation
2.7. Thiobarbituric Acid Reactive Substances (TBARS) Formation
2.8. Change of Relative Electrophoretic Mobility (REM)
2.9. Determination of Apolipoproteins Modification by SDS-PAGE
2.10. Measurement of UV Absorbance
2.11. Measurement of Protein-Bound Carbonyl Groups
2.12. Measurement of Dichlorofluorescein (DCF) Fluorescence
2.13. RAW 264.7 Cell Culture and Treatment
2.14. Cell Viability Assay
2.15. Measurement of Foam Cell Formation
2.16. Statistical Analyses
3. Results
3.1. Structure Elucidation of (3R)-5-Hydroxymellein
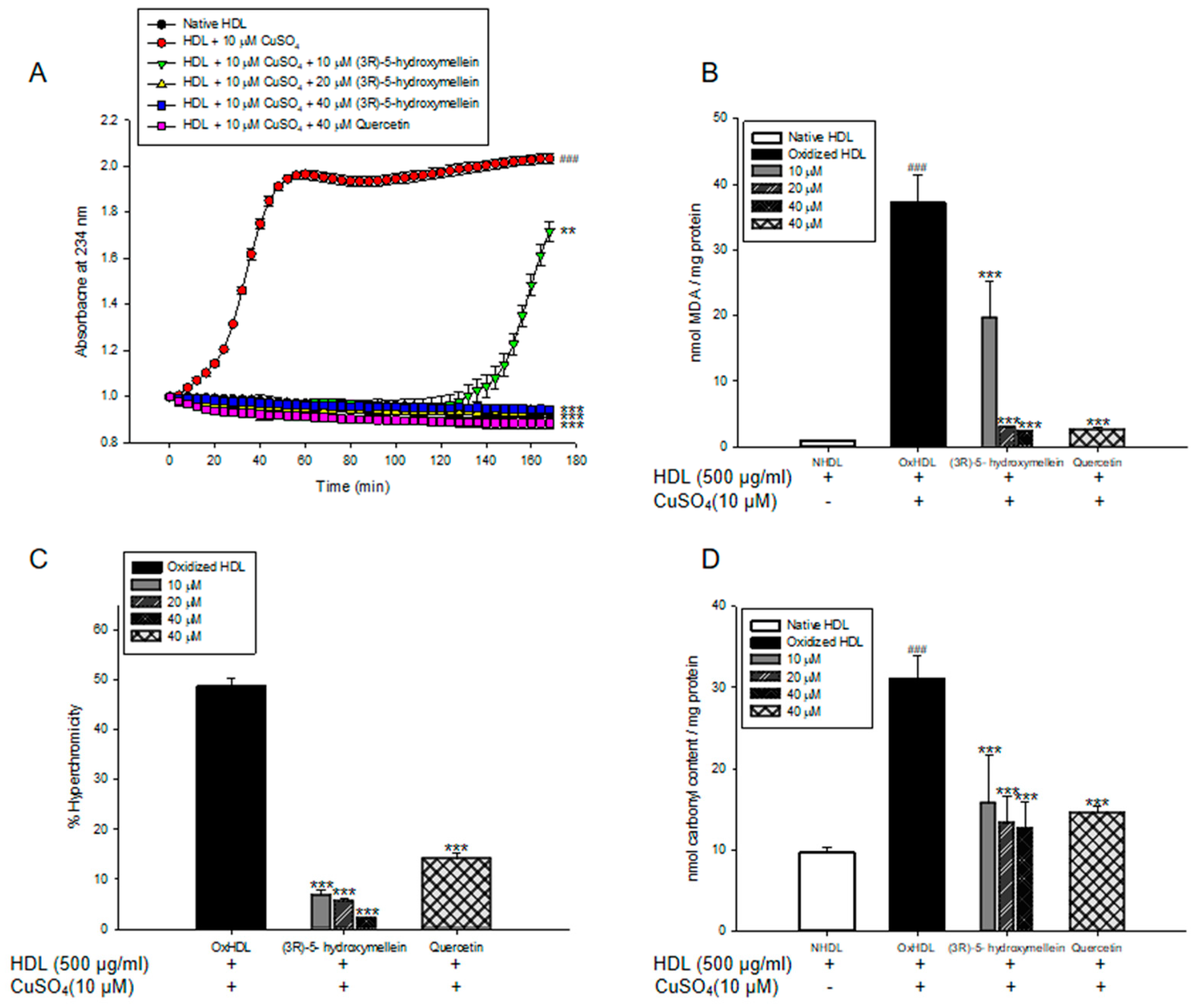
3.2. Effects of (3R)-5-Hydroxymellein on Conjugated Dienes and TBARS Formation from LDL and HDL Oxidation
3.3. Effects of (3R)-5-Hydroxymellein on UV Absorption and Carbonyl Content of Oxidized LDL and HDL
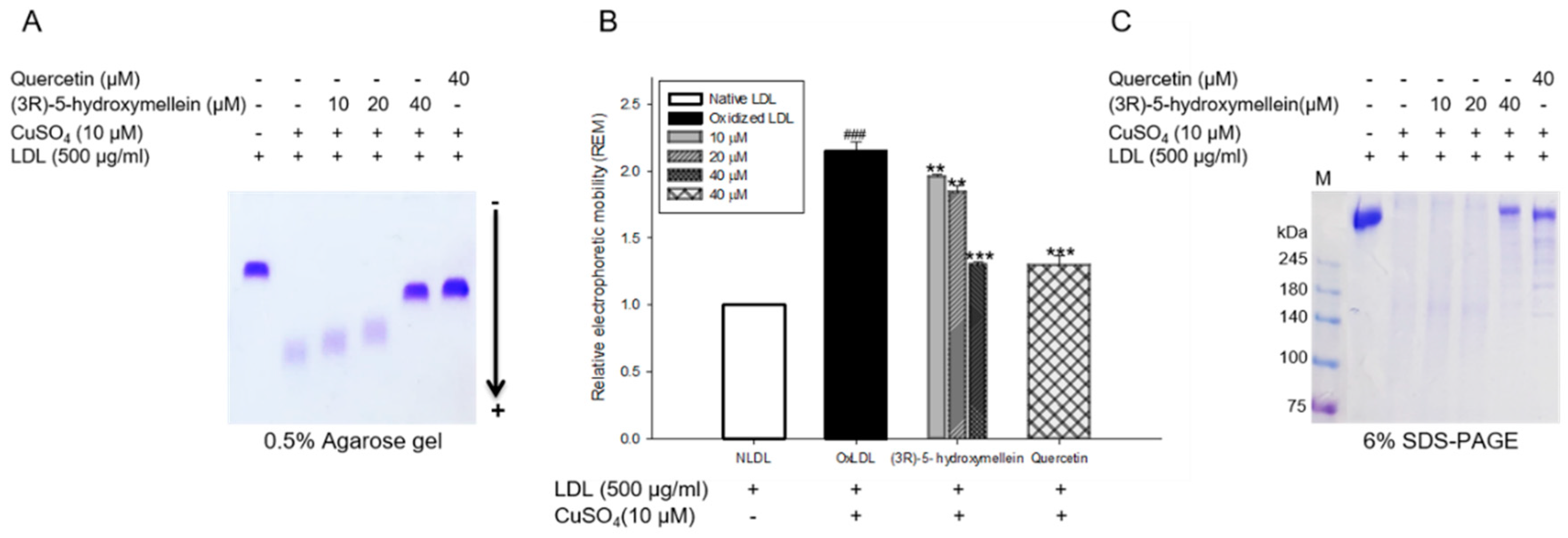
3.4. Effects of (3R)-5-Hydroxymellein on Change of Charge and apoB-100 Degradation on LDL Oxidation
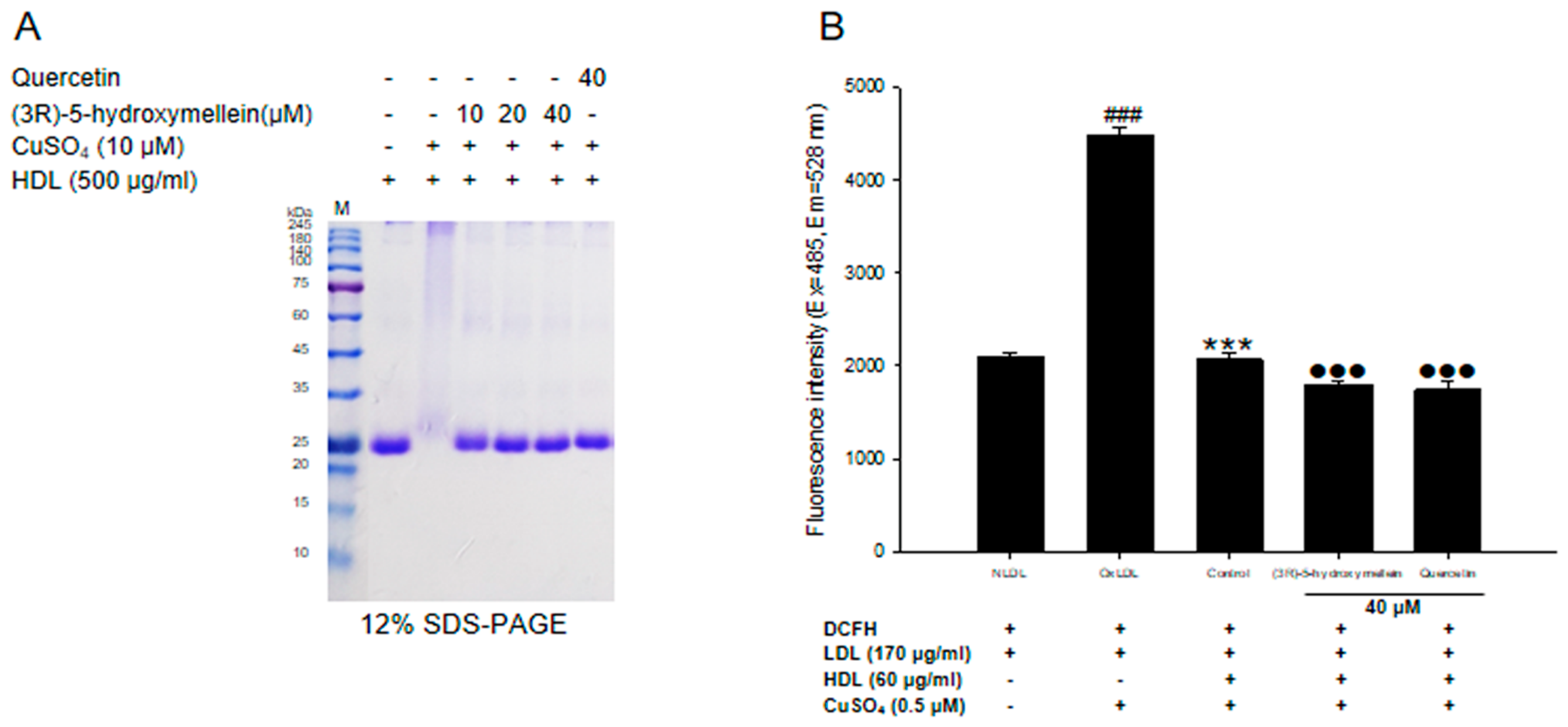
3.5. Effects of (3R)-5-Hydroxymellein on apoA-I Aggregation and anti-LDL Oxidation in oxHDL
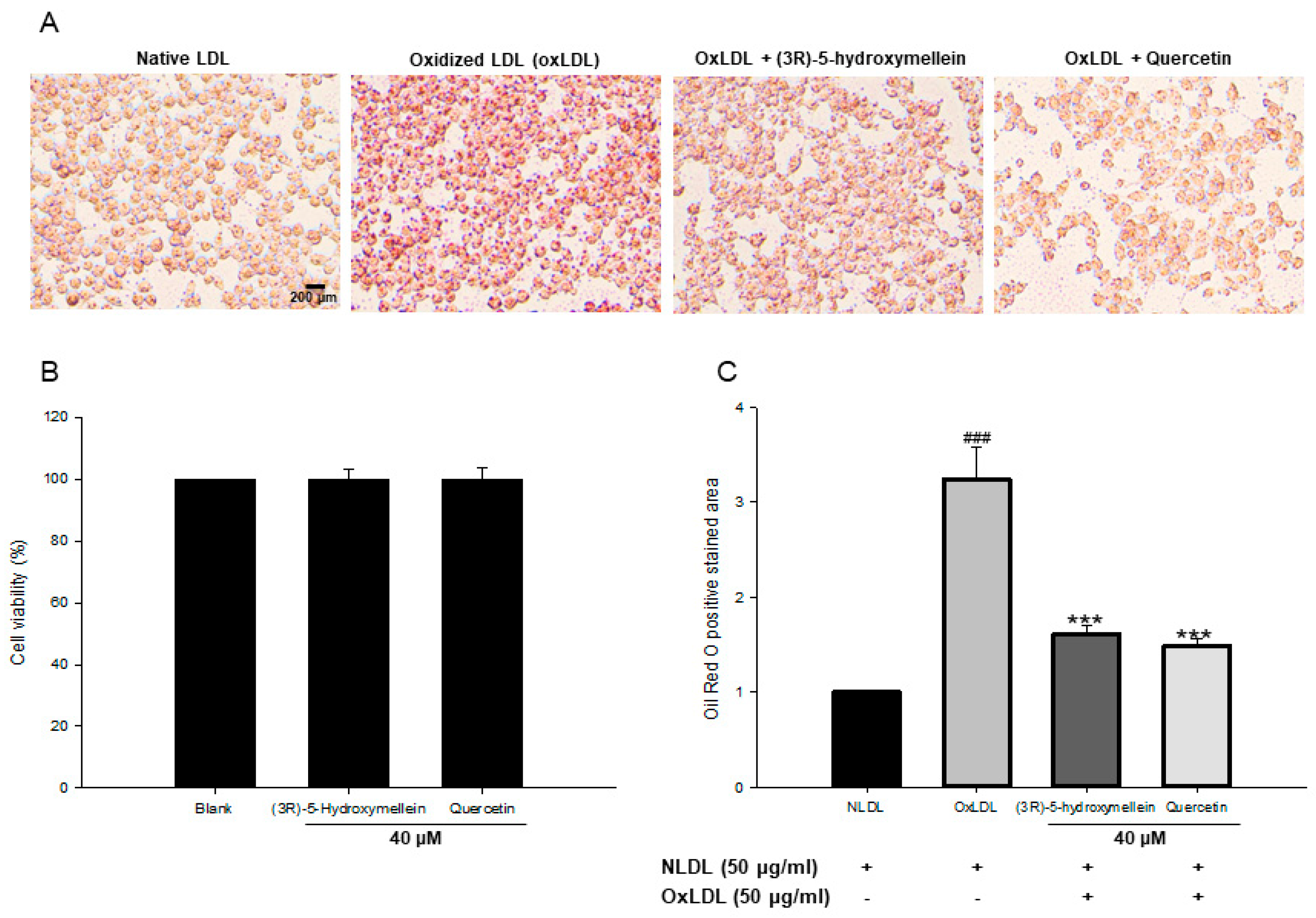
3.6. Effect of (3R)-5-Hydroxymellein on Foam Cell Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar] [PubMed]
- Orekhov, A.N.; Sobenin, I.A. Modified and dysfunctional lipoproteins in atherosclerosis: Effectors or biomarkers? Curr. Med. Chem. 2019, 26, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and reverse cholesterol transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Luna, C.; Carracedo, J.; Ramírez, R. LDL biochemical modifications: A link between atherosclerosis and aging. Food Nutr. Res. 2015, 59, 29240. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Pirillo, A.; Catapano, A.L. Modified HDL: Biological and physiopathological consequences. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 371–386. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Y.; Zhang, L. Antiatherogenic effects of Phyllanthus emblica associated with corilagin and its analogue. Yakugaku Zasshi 2005, 125, 587–591. [Google Scholar] [CrossRef]
- Sun, N.; Wang, H.; Wang, L. Ghrelin inhibits oxLDL-induced inflammation in RAW264.7 mouse macrophages through down-regulation of LOX-1 expression via NF-κB signaling pathway. Cell Mol. Biol. 2016, 62, 57–61. [Google Scholar]
- Feng, J.; Han, J.; Pearce, S.F.; Silverstein, R.L.; Gotto, A.M., Jr.; Hajjar, D.P.; Nicholson, A.C. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J. Lipid Res. 2000, 41, 688–696. [Google Scholar]
- Marchant, C.E.; Law, N.S.; van der Veen, C.; Hardwick, S.J.; Carpenter, K.L.; Mitchinson, M.J. Oxidized low density lipoprotein is cytotoxic to human monocyte-macrophages: Protection with lipophilic antioxidants. FEBS Lett. 1995, 358, 175–178. [Google Scholar] [CrossRef]
- Hessler, J.R.; Robertson, A.L., Jr.; Chisolm, G.M. LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis 1979, 32, 213–329. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar]
- Tsutsui, T.; Tsutamoto, T.; Wada, A.; Maeda, K.; Mabuchi, N.; Hayashi, M.; Ohnishi, M.; Kinoshita, M. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J. Am. Coll. Cardiol. 2002, 39, 957–962. [Google Scholar] [CrossRef]
- Hguxens, M.; Fitó, M.; Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Vinyoles, E.; Fiol, M.; Corella, D.; Arós, F.; Gómez-Gracia, E.; et al. Hypertensive status and lipoprotein oxidation in an elderly population at high cardiovascular risk. Am. J. Hypertens. 2009, 22, 68–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 1986, 256, 2835–2838. [Google Scholar] [CrossRef]
- Oram, J.F.; Yokoyama, S. Apolipoprotein-mediated removal of cellular cholesterol and phospholipids. J. Lipid Res. 1996, 37, 2473–2491. [Google Scholar]
- Navab, M.; Hama, S.Y.; Anantharamaiah, G.M.; Hassan, K.; Hough, G.P.; Watson, A.D.; Reddy, S.T.; Sevanian, A.; Fonarow, G.C.; Fogelman, A.M. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J. Lipid Res. 2000, 41, 1495–1508. [Google Scholar]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Anti-inflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Namiri-Kalantari, R.; Gao, F.; Chattopadhyay, A.; Wheeler, A.A.; Navab, K.D.; Farias Eisner, R.; Reddy, S.T. The dual nature of HDL: Anti-inflammatory and pro-inflammatory. Biofactors 2015, 41, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, X.A. Dysfunctional high-density lipoprotein. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, I.; Fiol, C.; Gracia, V.; Caldú, P. In vitro oxidised HDL exerts a cytotoxic effect on macrophages. Atherosclerosis 1996, 125, 39–46. [Google Scholar] [CrossRef]
- Kotani, K.; Sakane, N.; Ueda, M.; Mashiba, S.; Hayase, Y.; Tsuzaki, K.; Yamada, T.; Remaley, A.T. Oxidized high-density lipoprotein is associated with increased plasma glucose in non-diabetic dyslipidemic subjects. Clin. Chim. Acta 2012, 414, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zu, L.; Chen, Y.; Zheng, X.; Wang, Y.; Pan, B.; Dong, M.; Zhou, E.; Zhao, M.; Zhang, Y.; et al. Myeloperoxidase-oxidized high density lipoprotein impairs atherosclerotic plaque stability by inhibiting smooth muscle cell migration. Lipids Health Dis. 2017, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Niimura, N. Determination of the type of lacquer on East Asian lacquer ware. Int. J. Mass Spectrom. 2009, 284, 93–97. [Google Scholar] [CrossRef]
- Wedge, D.E.; Nagle, D.G. A new 2D-TLC bioautography method for the discovery of novel antifungal agents to control plant pathogens. J. Nat. Prod. 2000, 63, 1050–1054. [Google Scholar] [CrossRef]
- Devys, M.; Barbier, M.; Bousquet, J.-F.; Kollmann, A. Isolation of the (-)-(3R)-5-hydroxymellein from the funfus septoria nodorum. Phytochemistry 1994, 35, 825–826. [Google Scholar] [CrossRef]
- Hussain, H.; Kock, I.; Harrasi, A.A.; Rawahi, A.A.; Abbas, G.; Green, I.R.; Shah, A.; Badshah, A.; Saleem, M.; Draeger, S.; et al. Antimicrobial chemical constituents from endophytic fungus Phoma sp. Asian Pac. J. Trop. Med. 2014, 7, 699–702. [Google Scholar] [CrossRef]
- Zhao, L.; Kim, J.C.; Paik, M.J.; Lee, W.; Hur, J.S. A multifunctional and possible skin UV protectant, (3R)-5-hydroxymellein, produced by an endolichenic fungus isolated from Parmotrema austrosinense. Molecules 2016, 22, 26. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Markwell, M.A.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic. Res. Commun. 1989, 6, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Phillips, M.C. Quantitative measurement of lipoprotein surface charge by agarose gel electrophoresis. J. Lipid Res. 1992, 33, 123–130. [Google Scholar]
- Jairajpuri, D.S.; Fatima, S.; Jairajpuri, Z.S. Glycation induced physicochemical changes in low-density lipoprotein and its role in promoting cholesterol accumulation in macrophages along with antiglycation effect of aminoguanidine. Adv. Biol. Chem. 2015, 5, 203–214. [Google Scholar] [CrossRef]
- Nabi, R.; Alvi, S.S.; Khan, R.H.; Ahmad, S.; Ahmad, S.; Khan, M.S. Antiglycation study of HMG-R inhibitors and tocotrienol against glycated BSA and LDL: A comparative study. Int. J. Biol. Macromol. 2018, 116, 983–992. [Google Scholar] [CrossRef]
- Liggins, J.; Furth, A.J. Role of protein-bound carbonyl groups in the formation of advanced glycation endproducts. Biochim. Biophys. Acta. 1997, 1361, 123–130. [Google Scholar] [CrossRef]
- Augustyniak, E.; Adam, A.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Willetts, R.; Korkmaz, A.; Atalay, M. Validation of protein carbonyl measurement: A multi-centre study. Redox Biol. 2015, 4, 149–157. [Google Scholar] [CrossRef]
- Hillstrom, R.J.; Yacapin-Ammons, A.K.; Lynch, S.M. Vitamin C inhibits lipid oxidation in human HDL. J. Nutr. 2013, 133, 3047–3051. [Google Scholar] [CrossRef]
- Navab, M.; Hama, S.Y.; Hough, G.P.; Subbanagounder, G.; Reddy, S.T.; Fogelman, A.M. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipid. J. Lipid Res. 2001, 42, 1308–1317. [Google Scholar]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Cominacini, L.; Garbin, U.; Pastorino, A.M.; Davoli, A.; Campagnola, M.; De Santis, A.; Pasini, C. Predisposition to LDL oxidation in patients with and without angiographically established coronary artery disease. Atherosclerosis 1993, 99, 63–70. [Google Scholar] [CrossRef]
- Prasad, K.; Kalra, J. Oxygen free radicals and hypercholesterolemic atherosclerosis: Effect of vitamin E. Am. Heart J. 1993, 125, 958–973. [Google Scholar] [CrossRef]
- Ahmad, S.; Akhter, F.; Moinuddin; Shahab, U.; Khan, M.S. Studies on glycation of human low density lipoprotein: A functional insight into physico-chemical analysis. Int. J. Biol. Macromol. 2013, 62, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Ahsan, H.; Ahmad, R. Studies of human hemoglobin modified with peroxynitrite: A cytotoxic metabolite generated in numerous disorders. Int. J. Health Sci. 2018, 12, 30–35. [Google Scholar]
- Alouffi, S.; Faisal, M.; Alatar, A.A.; Ahmad, S. Oxidative Modification of LDL by Various Physicochemical Techniques: Its Probable Role in Diabetes Coupled with CVDs. Biomed. Res. Int. 2018, 2018, 7390612. [Google Scholar] [CrossRef]
- Bagheri, B.; Akbari, N.; Tabiban, S.; Habibi, V.; Mokhberi, V. Serum level of copper in patients with coronary artery disease. Niger. Med. J. 2015, 56, 39–42. [Google Scholar]
- Odetti, P.; Garibaldi, S.; Noberasco, G.; Aragno, I.; Valentini, S.; Traverso, N.; Marinari, U.M. Levels of carbonyl groups in plasma proteins of type 2 diabetes mellitus subjects. Acta. Diabetol. 1999, 36, 179–183. [Google Scholar] [CrossRef]
- Saito, Y.; Noguchi, N. Oxidized lipoprotein as a major vessel cell proliferator in oxidized human serum. PLoS ONE 2016, 11, e0160530. [Google Scholar] [CrossRef]
- Steinbrecher, U.P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J. Biol. Chem. 1987, 262, 3603–3608. [Google Scholar]
- Macri, J.; Adeli, K. Conformational changes in apolipoprotein B modulate intracellular assembly and degradation of ApoB-containing lipoprotein particles in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2982–2994. [Google Scholar] [CrossRef] [PubMed]
- Zmysłowski, A.; Szterk, A. Current knowledge on the mechanism of atherosclerosis and pro-atherosclerotic properties of oxysterols. Lipids Health Dis. 2017, 16, 188. [Google Scholar] [CrossRef] [PubMed]
- Ru, D.; Zhiqing, H.; Lin, Z.; Feng, W.; Feng, Z.; Jiayou, Z.; Yusheng, R.; Min, F.; Chun, L.; Zonggui, W. Oxidized high-density lipoprotein accelerates atherosclerosis progression by inducing the imbalance between treg and teff in LDLR knockout mice. APMIS 2015, 123, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, M.I.; Gong, E.L.; Nichols, A.V. Apolipoprotein-lipid association in oxidatively modified HDL and LDL. Biochim. Biophys. Acta 1994, 1210, 355–360. [Google Scholar] [CrossRef]
- McCall, M.R.; Tang, J.Y.; Bielicki, J.K.; Forte, T.M. Inhibition of lecithin-cholesterol acyltransferase and modification of HDL apolipoproteins by aldehydes. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1599–1606. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Raghavamenon, A.; Garelnabi, M.O.; Santanam, N. Oxidized low-density lipoprotein. Methods Mol. Biol. 2010, 610, 403–417. [Google Scholar]
- Lara-Guzman, O.J.; Tabares-Guevara, J.H.; Leon-Varela, Y.M.; Álvarez, R.M.; Roldan, M.; Sierra, J.A.; Londoño-Londoño, J.A.; Ramirez-Pineda, J.R. Proatherogenic macrophage activities are targeted by the flavonoid quercetin. J. Pharmacol. Exp. Ther. 2012, 343, 296–306. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Kim, S.; Shim, S.H. Anti-Atherosclerotic Activity of (3R)-5-Hydroxymellein from an Endophytic Fungus Neofusicoccum parvum JS-0968 Derived from Vitex rotundifolia through the Inhibition of Lipoproteins Oxidation and Foam Cell Formation. Biomolecules 2020, 10, 715. https://doi.org/10.3390/biom10050715
Kim J-Y, Kim S, Shim SH. Anti-Atherosclerotic Activity of (3R)-5-Hydroxymellein from an Endophytic Fungus Neofusicoccum parvum JS-0968 Derived from Vitex rotundifolia through the Inhibition of Lipoproteins Oxidation and Foam Cell Formation. Biomolecules. 2020; 10(5):715. https://doi.org/10.3390/biom10050715
Chicago/Turabian StyleKim, Jae-Yong, Soonok Kim, and Sang Hee Shim. 2020. "Anti-Atherosclerotic Activity of (3R)-5-Hydroxymellein from an Endophytic Fungus Neofusicoccum parvum JS-0968 Derived from Vitex rotundifolia through the Inhibition of Lipoproteins Oxidation and Foam Cell Formation" Biomolecules 10, no. 5: 715. https://doi.org/10.3390/biom10050715
APA StyleKim, J.-Y., Kim, S., & Shim, S. H. (2020). Anti-Atherosclerotic Activity of (3R)-5-Hydroxymellein from an Endophytic Fungus Neofusicoccum parvum JS-0968 Derived from Vitex rotundifolia through the Inhibition of Lipoproteins Oxidation and Foam Cell Formation. Biomolecules, 10(5), 715. https://doi.org/10.3390/biom10050715




