A Fluorescence-Based Method to Measure ADP/ATP Exchange of Recombinant Adenine Nucleotide Translocase in Liposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cloning, Isolation and Reconstitution of Murine ANT1 and UCP1
2.3. SDS–PAGE and Silver Staining
2.4. Preparation of Unilamellar (Proteo-) Liposomes
2.5. Calibration of Fluorescence Intensity of MgGrTM
2.6. Fluorescence-Based ADP/ATP Exchange Rate Measurements
2.7. Radioactivity-Based Exchange Rate Measurements
2.8. Statistical Analysis
3. Results
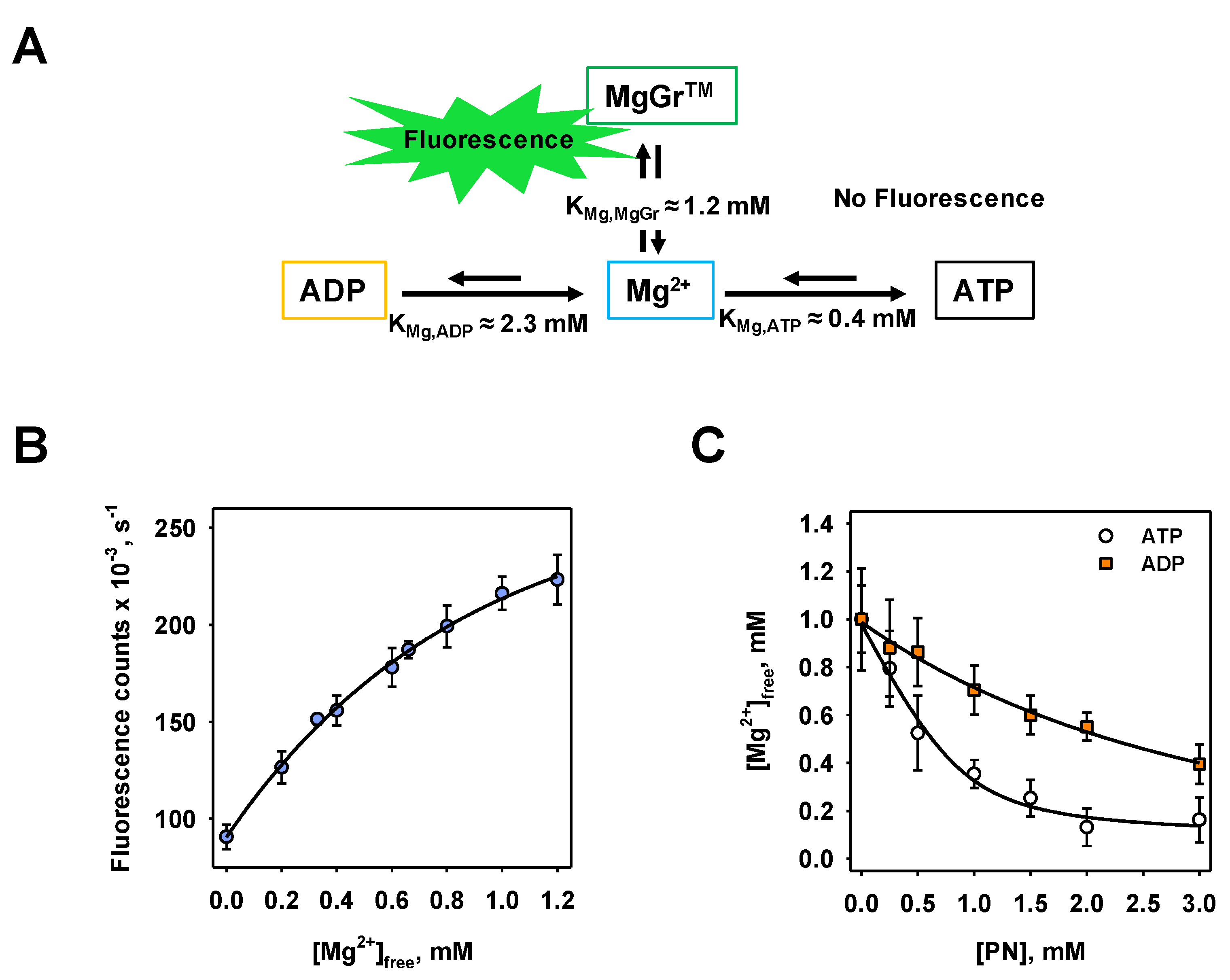
3.1. Calibration of MgGrTM Fluorescence Intensity to ADP/ATP Concentration and Determination of Related Binding Affinities
3.2. Conversion of MgGrTM Fluorescence Intensity to ADP/ATP Exchange Rate Values
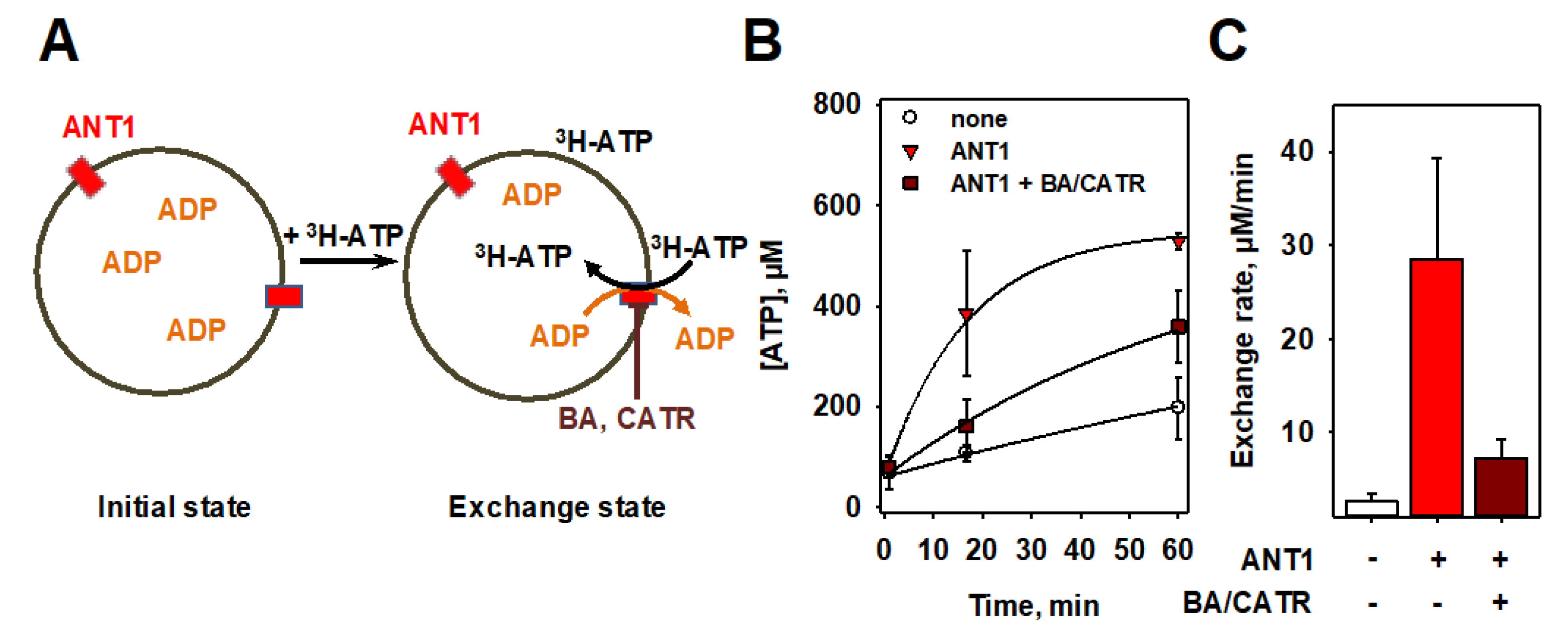
3.3. Measurements of ADP/ATP Exchange Using Radioactivity Assay
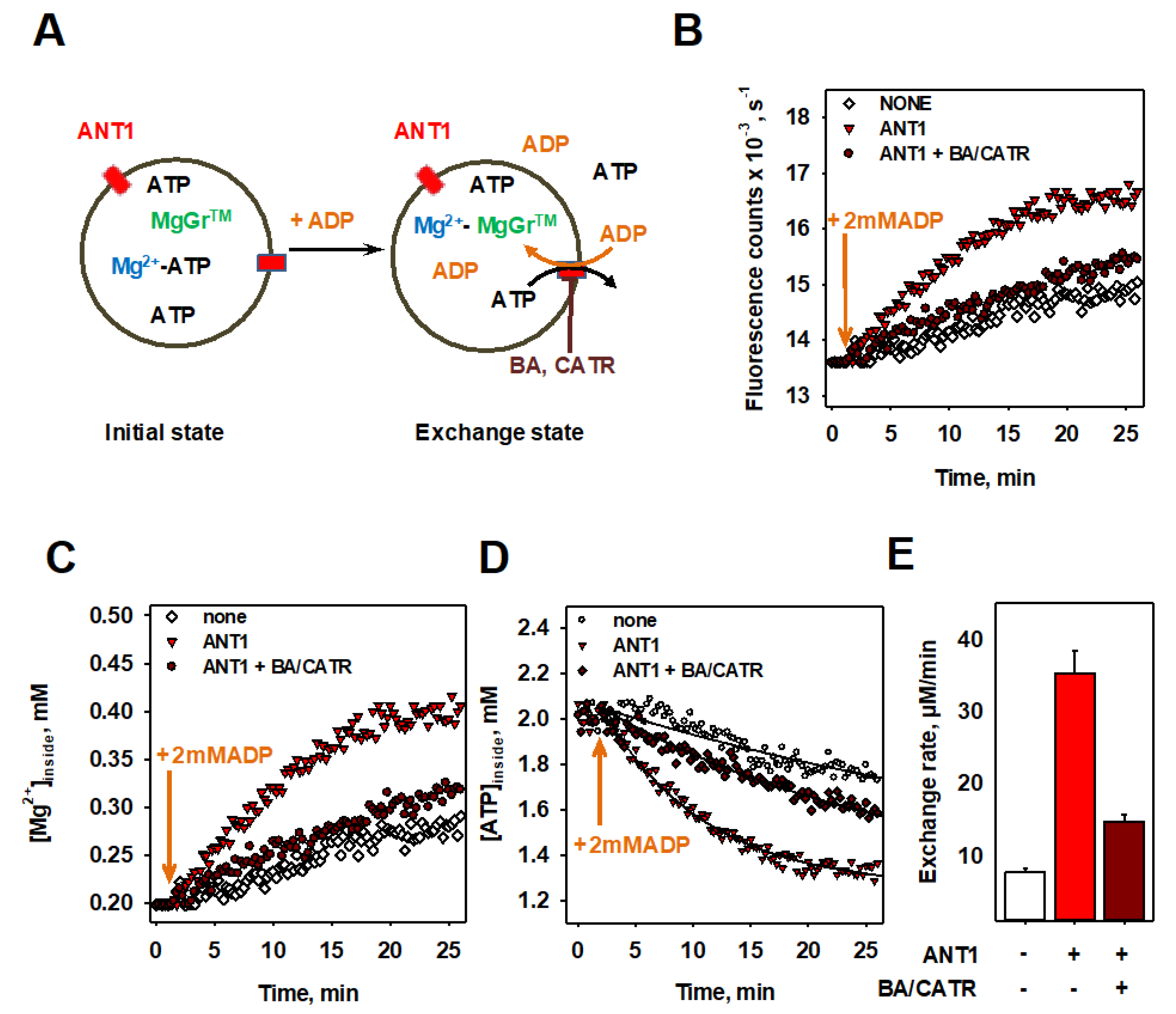
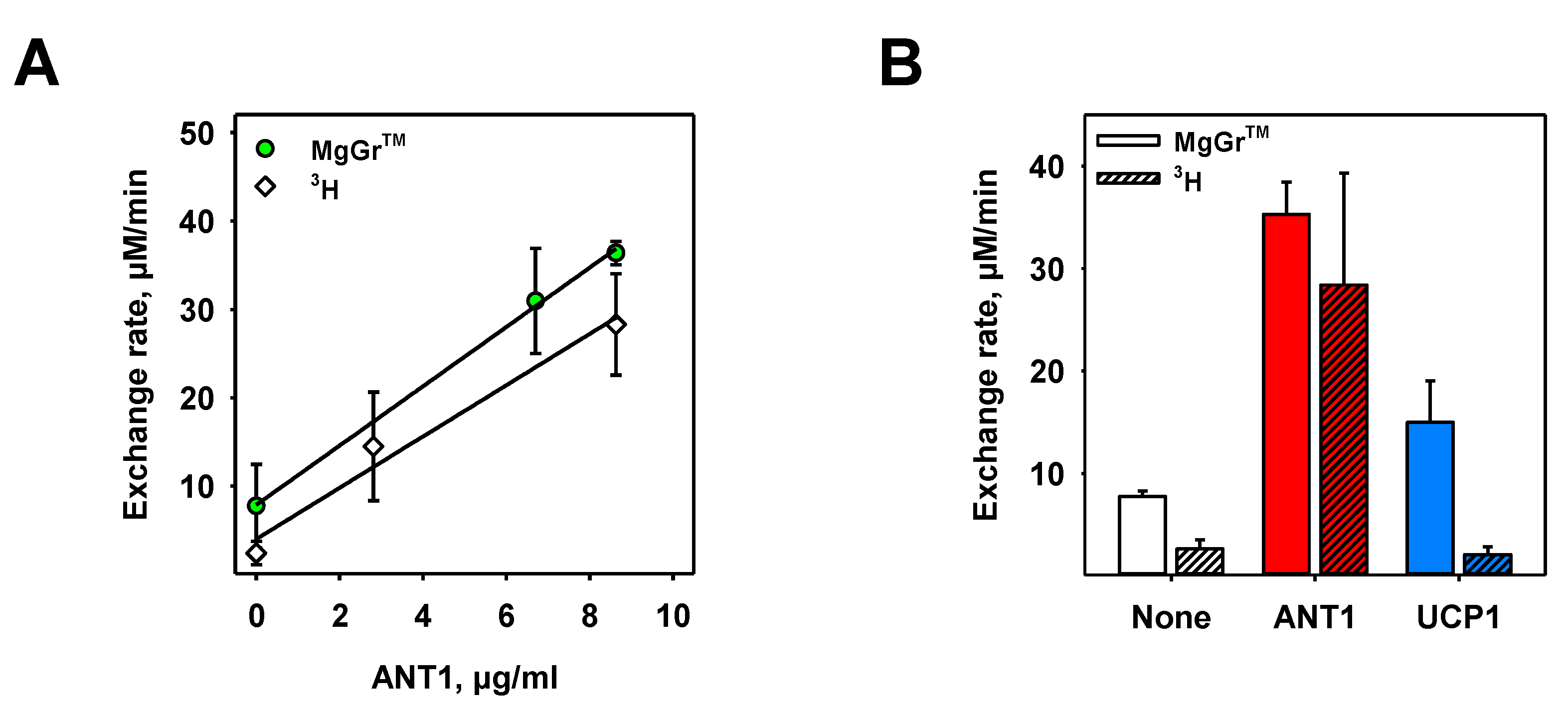
3.4. Proof of Specificity of ANT1-Mediated ADP/ATP Exchange Rate Measured in Both Assays
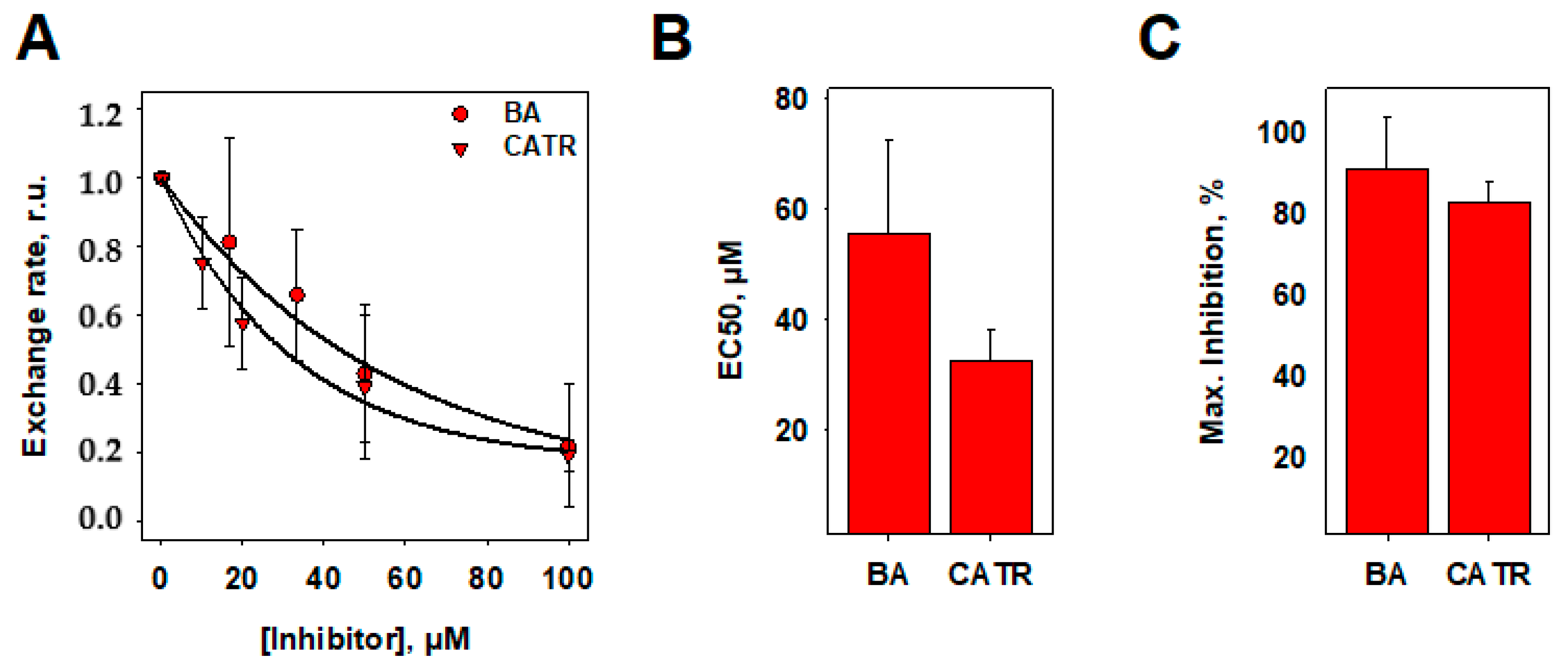
3.5. Inhibition of ANT1-Mediated ADP/ATP Exchange
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palmieri, F. The mitochondrial transporter family (SLC25): Physiological and pathological implications. Pflügers Archiv. 2004, 447, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Monné, M. Discoveries, metabolic roles and diseases of mitochondrial carriers: A review. Biochim. Biophys. Acta Bioenerg. 2016, 1863, 2362–2378. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Himms-Hagen, J.; Harper, M.-E. Physiological role of UCP3 may be export of fatty acids from mitochondria when fatty acid oxidation predominates: An hypothesis. Exp. Boil. Med. 2001, 226, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Pohl, E.E.; Rupprecht, A.; Macher, G.; Hilse, K.E. Important Trends in UCP3 Investigation. Front. Physiol. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Chinopoulos, C.; Vajda, S.; Csanády, L.; Mándi, M.; Mathe, K.; Ádám-Vizi, V. A Novel Kinetic Assay of Mitochondrial ATP-ADP Exchange Rate Mediated by the ANT. Biophys. J. 2009, 96, 2490–2504. [Google Scholar] [CrossRef]
- Yamanaka, R.; Tabata, S.; Shindo, Y.; Hotta, K.; Suzuki, K.; Soga, T.; Oka, K. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016, 6, 30027. [Google Scholar] [CrossRef]
- Aprille, J.R. Mechanism and regulation of the mitochondrial ATP-Mg/P(i) carrier. J. Bioenerg. Biomembr. 1993, 25, 473–481. [Google Scholar] [CrossRef]
- Kraemer, R.; Klingenberg, M. Electrophoretic control of reconstituted adenine nucleotide translocation. Biochemistry 1982, 21, 1082–1089. [Google Scholar] [CrossRef]
- Heidkämper, D.; Müller, V.; Nelson, D.R.; Klingenberg, M. Probing the Role of Positive Residues in the ADP/ATP Carrier from Yeast. The Effect of Six Arginine Mutations on Transport and the Four ATP versus ADP Exchange Modes†. Biochemistry 1996, 35, 16144–16152. [Google Scholar] [CrossRef]
- King, M.; Kerr, M.; Crichton, P.G.; Springett, R.; Kunji, E.R.S. Formation of a cytoplasmic salt bridge network in the matrix state is a fundamental step in the transport mechanism of the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta Bioenerg. 2015, 1857, 14–22. [Google Scholar] [CrossRef]
- Rupprecht, A.; Sokolenko, E.A.; Beck, V.; Ninnemann, O.; Jaburek, M.; Trimbuch, T.; Klishin, S.S.; Jezek, P.; Skulachev, V.P.; Pohl, E.E. Role of the Transmembrane Potential in the Membrane Proton Leak. Biophys. J. 2010, 98, 1503–1511. [Google Scholar] [CrossRef]
- Macher, G.; Koehler, M.; Rupprecht, A.; Kreiter, J.; Hinterdorfer, P.; Pohl, E.E. Inhibition of mitochondrial UCP1 and UCP3 by purine nucleotides and phosphate. Biochim. Biophys. Acta Biomembr. 2018, 1860, 664–672. [Google Scholar] [CrossRef]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Chinopoulos, C.; Kiss, G.; Kawamata, H.; Starkov, A.A. Measurement of ADP-ATP exchange in relation to mitochondrial transmembrane potential and oxygen consumption. Methods Enzymol. 2014, 542, 333–348. [Google Scholar] [CrossRef]
- Block, S.; Boulay, F.; Brandolin, G.; Dupont, Y.; Lauquin, G.J.; Vignais, P.V. Fluorescent probes of the mitochondrial ADP/ATP carrier protein. Methods Enzymol. 1986, 125, 639–649. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Hackenbrock, C.R. Continuous measurement of adenosine triphosphate with firefly luciferase luminescence. Methods Enzymol. 1979, 56, 530–544. [Google Scholar] [CrossRef]
- Williamson, J.R.; Corkey, B. [23] Assay of citric acid cycle intermediates and related compounds—Update with tissue metabolite levels and Intracellular Distribution. Methods Enzymol. 1979, 55, 200–222. [Google Scholar] [CrossRef]
- Passarella, S.; Ostuni, A.; Atlante, A.; Quagliariello, E. Increase in the adp/atp exchange in rat liver mitochondria irradiated in vitro by helium-neon laser. Biochem. Biophys. Res. Commun. 1988, 156, 978–986. [Google Scholar] [CrossRef]
- Deluca, M. Firefly luciferase. Adv. Enzymol. Relat. Areas Mol. Biol. 1976, 44, 37–68. [Google Scholar]
- Asimakis, G.K.; Aprille, J.R. In vitro alteration of the size of the liver mitochondrial adenine nucleotide pool: Correlation with respiratory functions. Arch. Biochem. Biophys. 1980, 203, 307–316. [Google Scholar] [CrossRef]
- Mifsud, J.; Ravaud, S.; Krammer, E.-M.; Chipot, C.; Kunji, E.R.S.; Pebay-Peyroula, E.; Dehez, F. The substrate specificity of the human ADP/ATP carrier AAC1. Mol. Membr. Boil. 2012, 30, 160–168. [Google Scholar] [CrossRef]
- Graue, C.; Klingenberg, M. Studies of the ADP/ATP carrier of mitochondria with fluorescent ADP analogue formycin diphosphate. Biochim. Biophys. Acta Bioenerg. 1979, 546, 539–550. [Google Scholar] [CrossRef]
- Mayer, I.; Dahms, A.S.; Riezler, W.; Klingenberg, M. Interaction of fluorescent adenine nucleotide derivatives with the ADP/ATP carrier in mitochondria. 1. Comparison of various 3′-O-ester adenine nucleotide derivatives. Biochemistry 1984, 23, 2436–2442. [Google Scholar] [CrossRef]
- Klingenberg, M.; Mayer, I.; Dahms, A.S. Interaction of fluorescent adenine nucleotide derivatives with the ADP/ATP carrier in mitochondria. 2. [5-(Dimethylamino)-1-naphthoyl] adenine nucleotides as probes for the transition between c and m states of the ADP/ATP carrier. Biochemistry 1984, 23, 2442–2449. [Google Scholar] [CrossRef]
- Klingenberg, M. The ADP-ATP Carrier in Mitochondrial Membranes. In The Enzymes of Biological Membranes; Springer: Berlin, Germany, 1976; pp. 383–438. [Google Scholar]
- Klingenberg, M. Substrate-Carrier Interaction and the Catalytic Translocation Cycle of the ADP, ATP Carrier. In Structural and Functional Aspects of Enzyme Catalysis; Springer: Berlin, Germany, 1981; pp. 202–212. [Google Scholar]
- Weidemann, M.J.; Erdelt, H.; Klingenberg, M. Adenine Nucleotide Translocation of Mitochondria. Identification of Carrier Sites. J. Boil. Inorg. Chem. 1970, 16, 313–335. [Google Scholar] [CrossRef]
- Klingenberg, M.; Mayer, I.; Appel, M. Interaction of fluorescent 3′-[1, 5-(dimethylamino) naphthoyl] adenine nucleotides with the solubilized ADP/ATP carrier. Biochemistry 1985, 24, 3650–3659. [Google Scholar] [CrossRef]
- Klingenberg, M.; Grebe, K.; Appel, M. Temperature dependence of ADP/ATP translocation in mitochondria. J. Boil. Inorg. Chem. 1982, 126, 263–269. [Google Scholar] [CrossRef]
- Brandolin, G.; Marty, I.; Vignais, P.V. Kinetics of nucleotide transport in rat heart mitochondria studied by a rapid filtration technique. Biochemistry 1990, 29, 9720–9727. [Google Scholar] [CrossRef]
- Kraemer, R.; Klingenberg, M. Modulation of the reconstituted adenine nucleotide exchange by membrane potential. Biochemistry 1980, 19, 556–560. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Bondareva, T.; Dedukhova, V.; Mokhova, E.N.; Skulachev, V.; Volkov, N. Carboxyatractylate inhibits the uncoupling effect of free fatty acids. FEBS Lett. 1988, 226, 265–269. [Google Scholar] [CrossRef]
- Aguirre, E.; Cadenas, S. GDP and carboxyatractylate inhibit 4-hydroxynonenal-activated proton conductance to differing degrees in mitochondria from skeletal muscle and heart. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Samartsev, V.; Smirnov, A.V.; Zeldi, I.P.; Markova, O.V.; Mokhova, E.N.; Skulachev, V.P. Involvement of aspartate/glutamate antiporter in fatty acid-induced uncoupling of liver mitochondria. Biochim. Biophys. Acta Bioenerg. 1997, 1319, 251–257. [Google Scholar] [CrossRef]
- Wieckowski, M.R.; Wojtczak, L. Involvement of the Dicarboxylate Carrier in the Protonophoric Action of Long-Chain Fatty Acids in Mitochondria. Biochem. Biophys. Res. Commun. 1997, 232, 414–417. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreiter, J.; Beitz, E.; Pohl, E.E. A Fluorescence-Based Method to Measure ADP/ATP Exchange of Recombinant Adenine Nucleotide Translocase in Liposomes. Biomolecules 2020, 10, 685. https://doi.org/10.3390/biom10050685
Kreiter J, Beitz E, Pohl EE. A Fluorescence-Based Method to Measure ADP/ATP Exchange of Recombinant Adenine Nucleotide Translocase in Liposomes. Biomolecules. 2020; 10(5):685. https://doi.org/10.3390/biom10050685
Chicago/Turabian StyleKreiter, Jürgen, Eric Beitz, and Elena E. Pohl. 2020. "A Fluorescence-Based Method to Measure ADP/ATP Exchange of Recombinant Adenine Nucleotide Translocase in Liposomes" Biomolecules 10, no. 5: 685. https://doi.org/10.3390/biom10050685
APA StyleKreiter, J., Beitz, E., & Pohl, E. E. (2020). A Fluorescence-Based Method to Measure ADP/ATP Exchange of Recombinant Adenine Nucleotide Translocase in Liposomes. Biomolecules, 10(5), 685. https://doi.org/10.3390/biom10050685





