Upregulation of Sortilin, a Lysosomal Sorting Receptor, Corresponds with Reduced Bioavailability of Latent TGFβ in Mucolipidosis II Cells
Abstract
1. Introduction
2. Materials and Methods
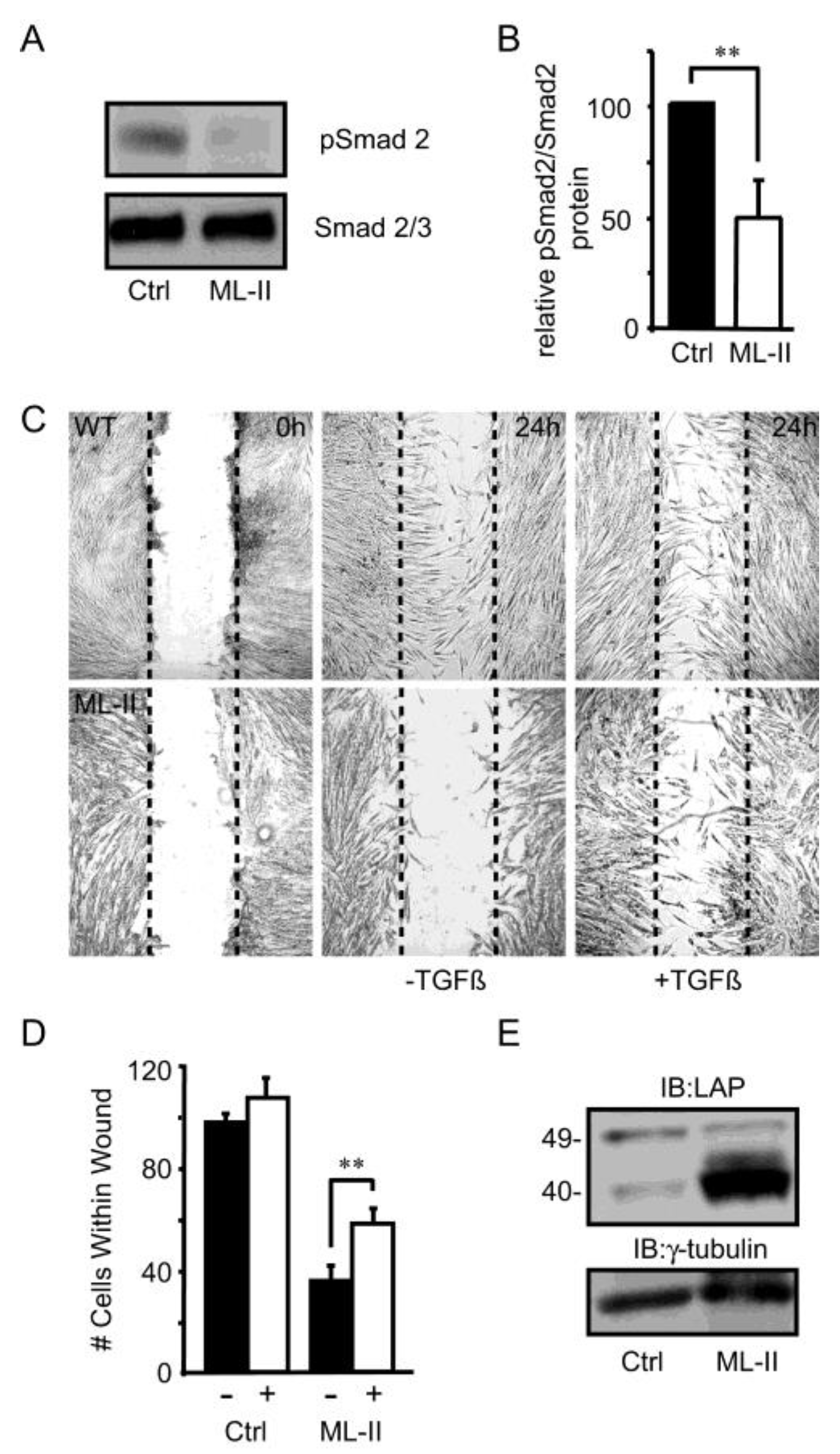
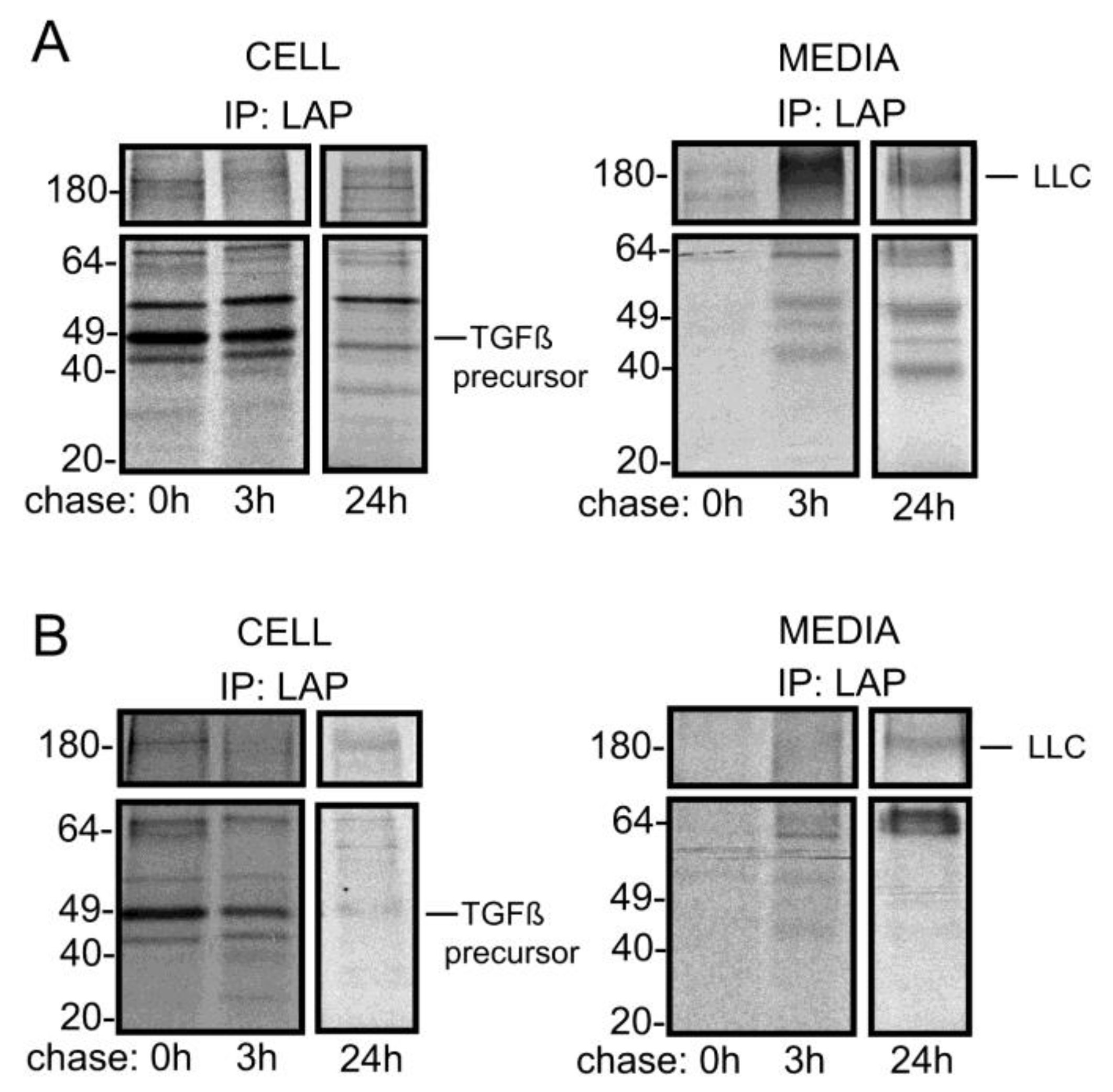
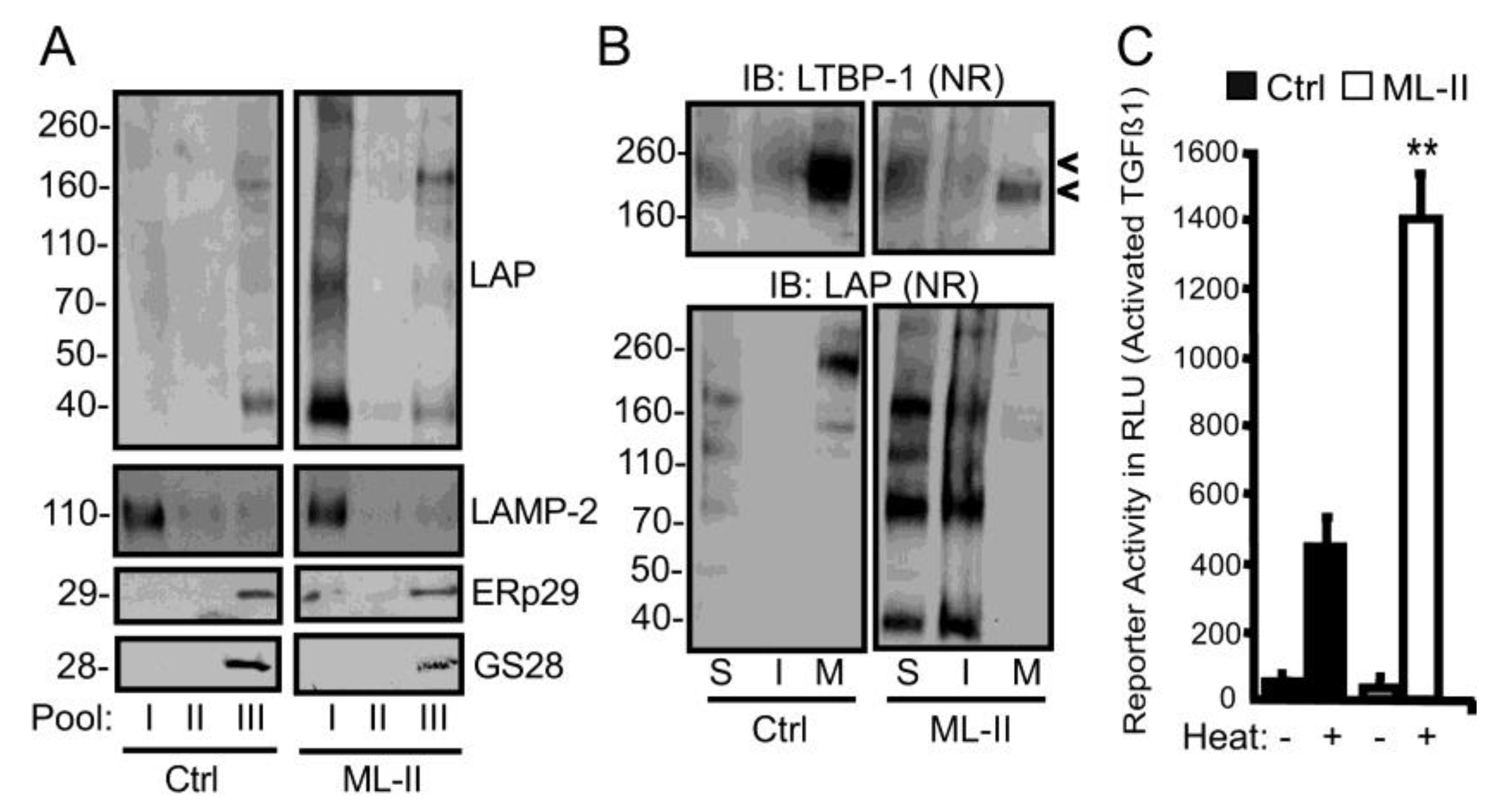
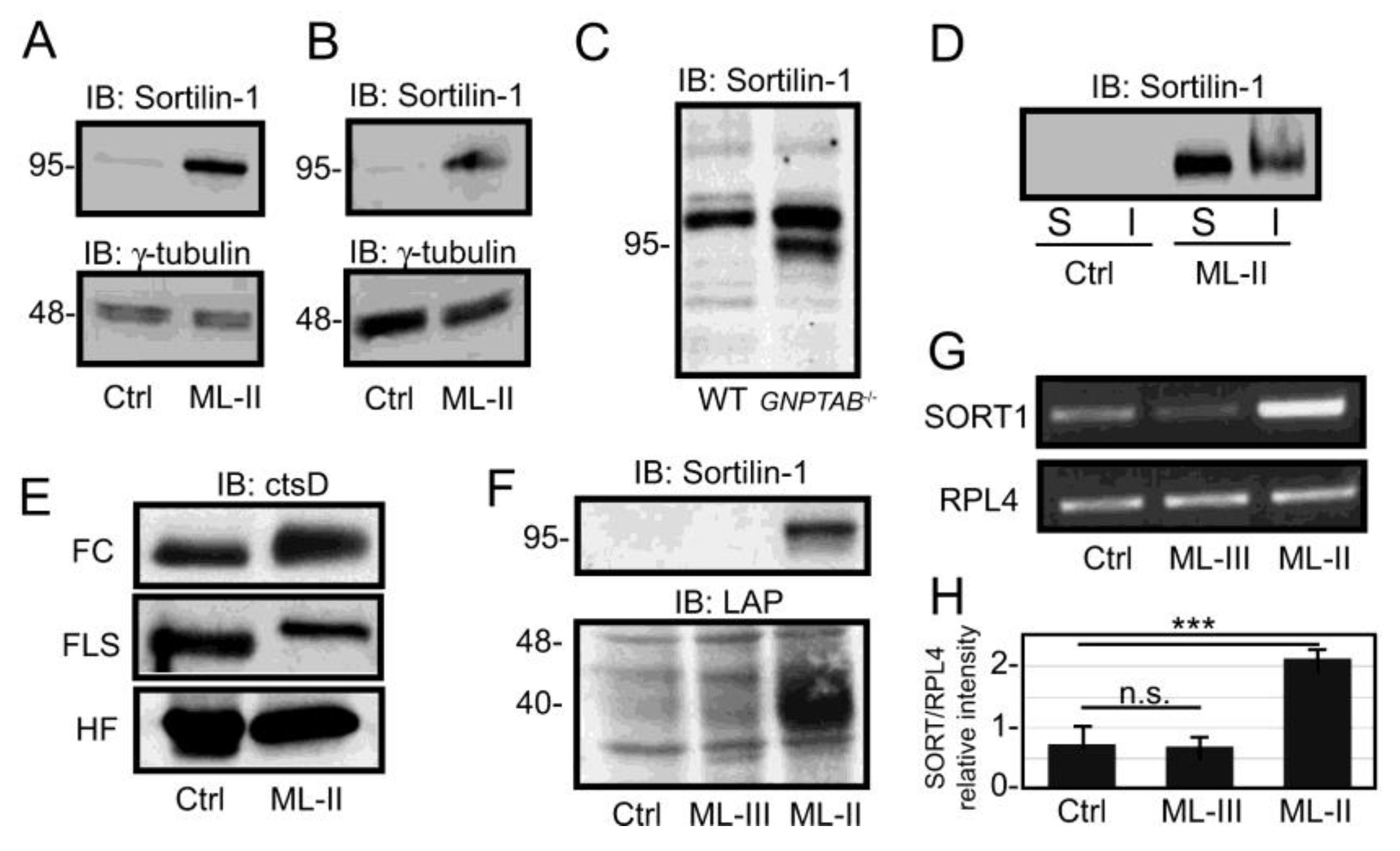
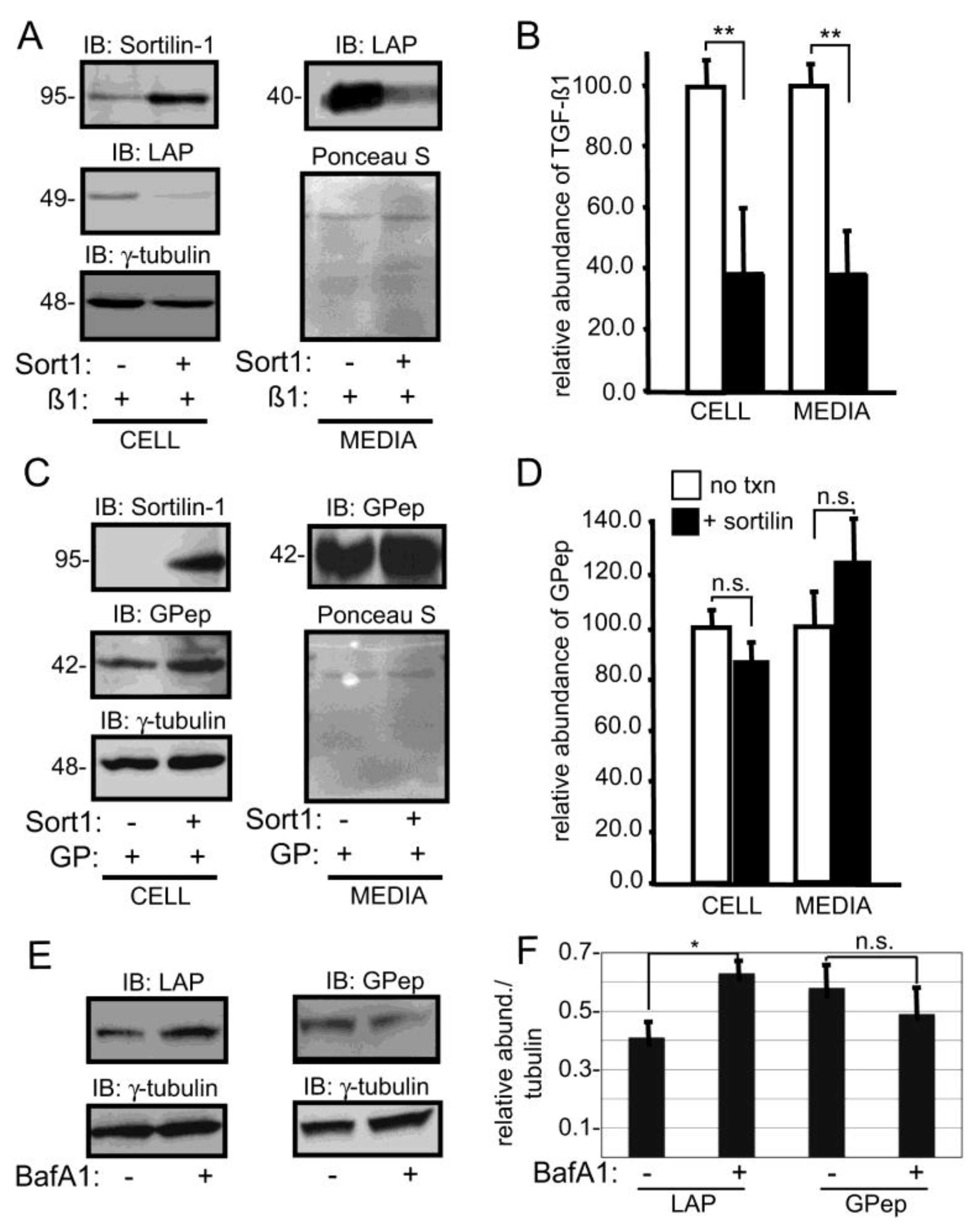
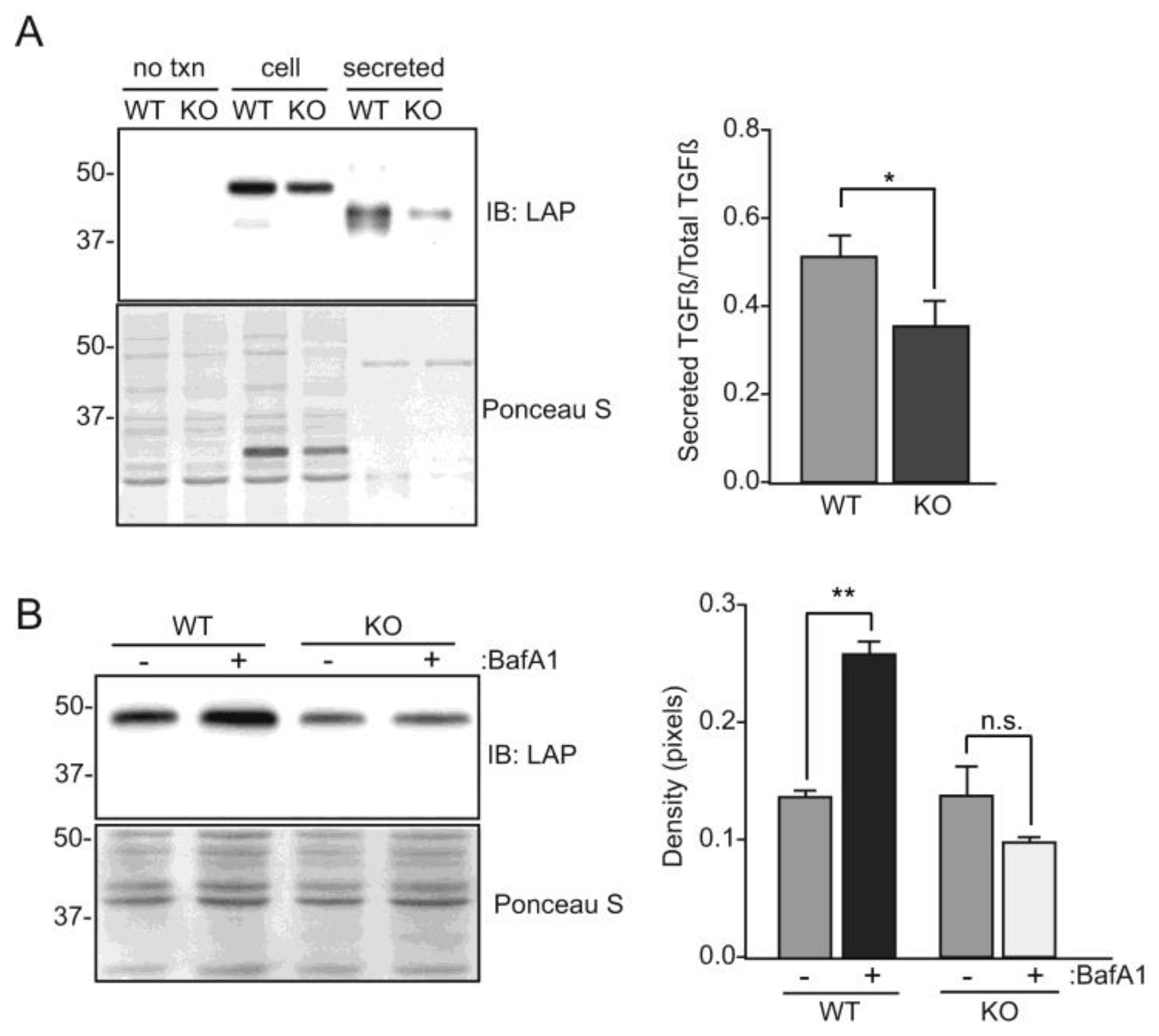
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Man-6-P | mannose 6-phosphate; |
| ML-II | mucolipidosis II; |
| TGFβ | transforming growth factor beta; |
| LAP | latency-associated peptide; |
| SLC | small latent complex; |
| LLC | large latent complex; |
| LTBP | latent TGFβ binding protein; |
| LAMP | lysosomal associated membrane protein; |
| DOC | deoxycholate; |
| LDL | low density lipoprotein; |
| APOB | apolipoprotein B; |
| BDNF | brain-derived neurotrophic factor; |
| RPL4 | ribosomal protein L4. |
References
- Reitman, M.L.; Varki, A.; Kornfeld, S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5’-diphosphate-N-acetylglucosamine: Glycoprotein N-acetylglucosaminylphosphotransferase activity. J. Clin. Investig. 1981, 67, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Tiede, S.; Storch, S.; Lubke, T.; Henrissat, B.; Bargal, R.; Raas-Rothschild, A.; Braulke, T. Mucolipidosis II is caused by mutations in GNPTA encoding the alpha/beta GlcNAc-1-phosphotransferase. Nat. Med. 2005, 11, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- David-Vizcarra, G.; Briody, J.; Ault, J.; Fietz, M.; Fletcher, J.; Savarirayan, R.; Wilson, M.; McGill, J.; Edwards, M.; Munns, C.; et al. The natural history and osteodystrophy of mucolipidosis types II and III. J. Paediatr. Child Health 2010, 46, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cathey, S.S.; Leroy, J.G.; Wood, T.; Eaves, K.; Simensen, R.J.; Kudo, M.; Stevenson, R.E.; Friez, M.J. Phenotype and genotype in mucolipidoses II and III alpha/beta: A study of 61 probands. J. Med. Genet. 2010, 47, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Saul, R.A.; Proud, V.; Taylor, H.A.; Leroy, J.G.; Spranger, J. Prenatal mucolipidosis type II (I-cell disease) can present as Pacman dysplasia. Am. J. Med. Genet. A 2005, 135, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Cathey, S.S.; Kudo, M.; Tiede, S.; Raas-Rothschild, A.; Braulke, T.; Beck, M.; Taylor, H.A.; Canfield, W.M.; Leroy, J.G.; Neufeld, E.F.; et al. Molecular order in mucolipidosis II and III nomenclature. Am. J. Med. Genet. A 2008, 146, 512–513. [Google Scholar] [CrossRef]
- Qian, Y.; van Meel, E.; Flanagan-Steet, H.; Yox, A.; Steet, R.; Kornfeld, S. Analysis of mucolipidosis II/III GNPTAB missense mutations identifies domains of UDP-GlcNAc:Lysosomal enzyme GlcNAc-1-phosphotransferase involved in catalytic function and lysosomal enzyme recognition. J. Biol. Chem. 2015, 290, 3045–3056. [Google Scholar] [CrossRef]
- Gelfman, C.M.; Vogel, P.; Issa, T.M.; Turner, C.A.; Lee, W.S.; Kornfeld, S.; Rice, D.S. Mice lacking alpha/beta subunits of GlcNAc-1-phosphotransferase exhibit growth retardation, retinal degeneration and secretory cell lesions. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5221–5228. [Google Scholar] [CrossRef]
- Boonen, M.; van Meel, E.; Oorschot, V.; Klumperman, J.; Kornfeld, S. Vacuolization of mucolipidosis type II mouse exocrine gland cells represents accumulation of autolysosomes. Mol. Biol. Cell 2011, 22, 1135–1147. [Google Scholar] [CrossRef]
- Vogel, P.; Payne, B.J.; Read, R.; Lee, W.S.; Gelfman, C.M.; Kornfeld, S. Comparative pathology of murine mucolipidosis types II and IIIC. Vet. Pathol. 2009, 46, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, K.; Damme, M.; Markmann, S.; Morelle, W.; Schweizer, M.; Hermans-Borgmeyer, I.; Rochert, A.K.; Pohl, S.; Lubke, T.; Michalski, J.C.; et al. Lysosomal dysfunction causes neurodegeneration in mucolipidosis II ‘knock-in’ mice. Brain 2012, 135, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Marschner, K.; Kollmann, K.; Schweizer, M.; Braulke, T.; Pohl, S. A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science 2011, 333, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Mazrier, H.; Van Hoeven, M.; Wang, P.; Knox, V.W.; Aguirre, G.D.; Holt, E.; Wiemelt, S.P.; Sleeper, M.M.; Hubler, M.; Haskins, M.E.; et al. Inheritance, biochemical abnormalities and clinical features of feline mucolipidosis II: The first animal model of human I-cell disease. J. Hered. 2003, 94, 363–373. [Google Scholar] [CrossRef]
- Flanagan-Steet, H.; Sias, C.; Steet, R. Altered chondrocyte differentiation and extracellular matrix homeostasis in a zebrafish model for mucolipidosis II. Am. J. Pathol. 2009, 175, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Petrey, A.C.; Flanagan-Steet, H.; Johnson, S.; Fan, X.; De la Rosa, M.; Haskins, M.E.; Nairn, A.V.; Moremen, K.W.; Steet, R. Excessive activity of cathepsin K is associated with cartilage defects in a zebrafish model of mucolipidosis II. Dis. Models Mech. 2012, 5, 177–190. [Google Scholar] [CrossRef]
- Flanagan-Steet, H.; Aarnio, M.; Kwan, B.; Guihard, P.; Petrey, A.; Haskins, M.; Blanchard, F.; Steet, R. Cathepsin-mediated alterations in TGFbeta-related signaling underlie disrupted cartilage and bone maturation associated with impaired lysosomal targeting. J. Bone Miner. Res. 2016, 31, 535–548. [Google Scholar] [CrossRef]
- Flanagan-Steet, H.; Christian, C.; Lu, P.N.; Aarnio-Peterson, M.; Sanman, L.; Archer-Hartmann, S.; Azadi, P.; Bogyo, M.; Steet, R.A. TGF-beta regulates cathepsin activation during normal and pathogenic development. Cell Rep. 2018, 22, 2964–2977. [Google Scholar] [CrossRef]
- Saito, T.; Kinoshita, A.; Yoshiura, K.; Makita, Y.; Wakui, K.; Honke, K.; NIIkawa, N.; Taniguchi, N. Domain-specific mutations of a transforming growth factor (TGF)-beta 1 latency-associated peptide cause Camurati-Engelmann disease because of the formation of a constitutively active form of TGF-beta 1. J. Biol. Chem. 2001, 276, 11469–11472. [Google Scholar] [CrossRef]
- Janssens, K.; Gershoni-Baruch, R.; Guanabens, N.; Migone, N.; Ralston, S.; Bonduelle, M.; Lissens, W.; Van Maldergem, L.; Vanhoenacker, F.; Verbruggen, L.; et al. Mutations in the gene encoding the latency-associated peptide of TGF-beta 1 cause Camurati-Engelmann disease. Nat. Genet. 2000, 26, 273–275. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef]
- Nistala, H.; Lee-Arteaga, S.; Siciliano, G.; Smaldone, S.; Ramirez, F. Extracellular regulation of transforming growth factor beta and bone morphogenetic protein signaling in bone. Ann. N. Y. Acad. Sci. 2010, 1192, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M., Jr. The new bone biology: Pathologic, molecular and clinical correlates. Am. J. Med. Genet. A 2006, 140, 2646–2706. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, C.; Cormier-Daire, V. From tall to short: The role of TGFbeta signaling in growth and its disorders. Am. J. Med. Genet. C Semin. Med. Genet. 2012, 160, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, C.; Morice-Picard, F.; Dagoneau, N.; Wang, L.W.; Perrot, C.; Crow, Y.J.; Bauer, F.; Flori, E.; Prost-Squarcioni, C.; Krakow, D.; et al. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat. Genet. 2008, 40, 1119–1123. [Google Scholar] [CrossRef]
- Bellesso, S.; Salvalaio, M.; Lualdi, S.; Tognon, E.; Costa, R.; Braghetta, P.; Giraudo, C.; Stramare, R.; Rigon, L.; Filocamo, M.; et al. FGF signaling deregulation is associated with early developmental skeletal defects in animal models for mucopolysaccharidosis type II (MPSII). Hum. Mol. Genet. 2018, 27, 2262–2275. [Google Scholar] [CrossRef]
- Costa, R.; Urbani, A.; Salvalaio, M.; Bellesso, S.; Cieri, D.; Zancan, I.; Filocamo, M.; Bonaldo, P.; Szabo, I.; Tomanin, R.; et al. Perturbations in cell signaling elicit early cardiac defects in mucopolysaccharidosis type II. Hum. Mol. Genet. 2017, 26, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Moro, E.; Tomanin, R.; Friso, A.; Modena, N.; Tiso, N.; Scarpa, M.; Argenton, F. A novel functional role of iduronate-2-sulfatase in zebrafish early development. Matrix Biol. 2010, 29, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Blain, S.W.; Lo, R.S. TGFbeta signaling in growth control, cancer and heritable disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef]
- Massague, J.; Chen, Y.G. Controlling TGF-beta signaling. Genes Dev. 2000, 14, 627–644. [Google Scholar] [PubMed]
- Taylor, A.W. Review of the activation of TGF-beta in immunity. J. Leukoc. Biol. 2009, 85, 29–33. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and bmp signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Kitisin, K.; Saha, T.; Blake, T.; Golestaneh, N.; Deng, M.; Kim, C.; Tang, Y.; Shetty, K.; Mishra, B.; Mishra, L. TGF-beta signaling in development. Sci. STKE 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Chang, C. TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res. C Embryo Today 2003, 69, 333–351. [Google Scholar] [CrossRef]
- Janssens, K.; ten Dijke, P.; Janssens, S.; Van Hul, W. Transforming growth factor-beta1 to the bone. Endocr. Rev. 2005, 26, 743–774. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Pardali, K.; Gaal, A.; Heldin, C.H. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol. Lett. 2002, 82, 85–91. [Google Scholar] [CrossRef]
- Gentry, L.E.; Webb, N.R.; Lim, G.J.; Brunner, A.M.; Ranchalis, J.E.; Twardzik, D.R.; Lioubin, M.N.; Marquardt, H.; Purchio, A.F. Type 1 transforming growth factor beta: Amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol. Cell Biol. 1987, 7, 3418–3427. [Google Scholar] [CrossRef]
- Gentry, L.E.; Lioubin, M.N.; Purchio, A.F.; Marquardt, H. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol. Cell Biol. 1988, 8, 4162–4168. [Google Scholar] [CrossRef]
- Rifkin, D.B.; Kojima, S.; Abe, M.; Harpel, J.G. TGF-beta: Structure, function and formation. Thromb. Haemost. 1993, 70, 177–179. [Google Scholar]
- Rifkin, D.B. Latent transforming growth factor-beta (TGF-beta) binding proteins: Orchestrators of TGF-beta availability. J. Biol. Chem. 2005, 280, 7409–7412. [Google Scholar] [CrossRef]
- Taipale, J.; Miyazono, K.; Heldin, C.H.; Keski-Oja, J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J. Cell Biol. 1994, 124, 171–181. [Google Scholar] [CrossRef]
- Miyazono, K.; Olofsson, A.; Colosetti, P.; Heldin, C.H. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991, 10, 1091–1101. [Google Scholar] [CrossRef]
- Chen, Q.; Sivakumar, P.; Barley, C.; Peters, D.M.; Gomes, R.R.; Farach-Carson, M.C.; Dallas, S.L. Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-beta (TGF-beta) by modulating assembly of latent TGF-beta-binding protein-1. J. Biol. Chem. 2007, 282, 26418–26430. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Sivakumar, P.; Jones, C.J.; Chen, Q.; Peters, D.M.; Mosher, D.F.; Humphries, M.J.; Kielty, C.M. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 2005, 280, 18871–18880. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.; Rifkin, D.B. Extracellular microfibrils: Contextual platforms for TGFbeta and BMP signaling. Curr. Opin. Cell Biol. 2009, 21, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Koli, K.; Hagood, J.S.; Miao, M.; Mavalli, M.; Rifkin, D.B.; Murphy-Ullrich, J.E. Latent transforming growth factor-beta-binding protein-4 regulates transforming growth factor-beta1 bioavailability for activation by fibrogenic lung fibroblasts in response to bleomycin. Am. J. Pathol. 2009, 174, 21–33. [Google Scholar] [CrossRef]
- Isogai, Z.; Ono, R.N.; Ushiro, S.; Keene, D.R.; Chen, Y.; Mazzieri, R.; Charbonneau, N.L.; Reinhardt, D.P.; Rifkin, D.B.; Sakai, L.Y. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003, 278, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Ota, M.; Rifkin, D.B. Matrix control of transforming growth factor-beta function. J. Biochem. 2012, 152, 321–329. [Google Scholar] [CrossRef]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. The integrin alpha V beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef]
- Crawford, S.E.; Stellmach, V.; Murphy-Ullrich, J.E.; Ribeiro, S.M.; Lawler, J.; Hynes, R.O.; Boivin, G.P.; Bouck, N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 1998, 93, 1159–1170. [Google Scholar] [CrossRef]
- Godar, S.; Horejsi, V.; Weidle, U.H.; Binder, B.R.; Hansmann, C.; Stockinger, H. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-beta1. Eur. J. Immunol. 1999, 29, 1004–1013. [Google Scholar] [CrossRef]
- Dennis, P.A.; Rifkin, D.B. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc. Natl. Acad. Sci. USA 1991, 88, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Warejcka, D.; Simpliciano, J.; Twining, S.; Steet, R. Latency-associated peptide of transforming growth factor-beta1 is not subject to physiological mannose phosphorylation. J. Biol. Chem. 2012, 287, 7526–7534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dabovic, B.; Annes, J.P.; Rifkin, D.B. Latent TGF-beta binding protein-3 (LTBP-3) requires binding to TGF-beta for secretion. FEBS Lett. 2002, 517, 277–280. [Google Scholar] [CrossRef]
- Barnes, J.; Lim, J.M.; Godard, A.; Blanchard, F.; Wells, L.; Steet, R. Extensive mannose phosphorylation on leukemia inhibitory factor (LIF) controls its extracellular levels by multiple mechanisms. J. Biol. Chem. 2011, 286, 24855–24864. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, J.; Schweizer, A.; Johnson, K.F.; Kornfeld, S. A determinant in the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor prevents trafficking to lysosomes. J. Cell Biol. 1995, 130, 1297–1306. [Google Scholar] [CrossRef]
- Steet, R.A.; Chung, S.; Wustman, B.; Powe, A.; Do, H.; Kornfeld, S.A. The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms. Proc. Natl. Acad. Sci. USA 2006, 103, 13813–13818. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Miyazono, K.; Thyberg, J.; Heldin, C.H. Retention of the transforming growth factor-beta 1 precursor in the golgi complex in a latent endoglycosidase H-sensitive form. J. Biol. Chem. 1992, 267, 5668–5675. [Google Scholar]
- Schaub, B.E.; Nair, P.; Rohrer, J. Analysis of protein transport to lysosomes. Curr. Protoc. Cell Biol. 2005, 27, 15–18. [Google Scholar] [CrossRef]
- Canuel, M.; Korkidakis, A.; Konnyu, K.; Morales, C.R. Sortilin mediates the lysosomal targeting of cathepsins d and h. Biochem. Biophys. Res. Commun. 2008, 373, 292–297. [Google Scholar] [CrossRef]
- Nykjaer, A.; Willnow, T.E. Sortilin: A receptor to regulate neuronal viability and function. Trends Neurosci. 2012, 35, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.; Ding, Q.; Edmondson, A.C.; Millar, J.S.; Sachs, K.V.; Li, X.; Kumaravel, A.; Wang, M.Y.; Ai, D.; Guo, L.; et al. Hepatic sortilin regulates both apolipoprotein B secretion and ldl catabolism. J. Clin. Investig. 2012, 122, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Steet, R.A.; Hullin, R.; Kudo, M.; Martinelli, M.; Bosshard, N.U.; Schaffner, T.; Kornfeld, S.; Steinmann, B. A splicing mutation in the alpha/beta GlcNAc-1-phosphotransferase gene results in an adult onset form of mucolipidosis III associated with sensory neuropathy and cardiomyopathy. Am. J. Med. Genet. A 2005, 132, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Steet, R.; Lee, W.S.; Kornfeld, S. Identification of the minimal lysosomal enzyme recognition domain in cathepsin D. J. Biol. Chem. 2005, 280, 33318–33323. [Google Scholar] [CrossRef]
- Evans, S.F.; Irmady, K.; Ostrow, K.; Kim, T.; Nykjaer, A.; Saftig, P.; Blobel, C.; Hempstead, B.L. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. J. Biol. Chem. 2011, 286, 29556–29567. [Google Scholar] [CrossRef]
- Kwon, S.; Christian, J.L. Sortilin associates with transforming growth factor-beta family proteins to enhance lysosome-mediated degradation. J. Biol. Chem. 2011, 286, 21876–21885. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Ieraci, A.; Teng, H.; Dall, H.; Meng, C.X.; Herrera, D.G.; Nykjaer, A.; Hempstead, B.L.; Lee, F.S. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J. Neurosci. 2005, 25, 6156–6166. [Google Scholar] [CrossRef]
- Gelling, C.L.; Dawes, I.W.; Perlmutter, D.H.; Fisher, E.A.; Brodsky, J.L. The endosomal protein-sorting receptor sortilin has a role in trafficking alpha-1 antitrypsin. Genetics 2012, 192, 889–903. [Google Scholar] [CrossRef]
- Strong, A.; Rader, D.J. Sortilin as a regulator of lipoprotein metabolism. Curr. Atheroscler. Rep. 2012, 14, 211–218. [Google Scholar] [CrossRef]
- Calkin, A.C.; Tontonoz, P. Genome-wide association studies identify new targets in cardiovascular disease. Sci. Transl. Med. 2010, 2, 48ps46. [Google Scholar] [CrossRef]
- Rogaeva, E.; Meng, Y.; Lee, J.H.; Gu, Y.; Kawarai, T.; Zou, F.; Katayama, T.; Baldwin, C.T.; Cheng, R.; Hasegawa, H.; et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007, 39, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Conlon, D.M. Role of sortilin in lipid metabolism. Curr. Opin. Lipidol. 2019, 30, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ciarlo, E.; Massone, S.; Penna, I.; Nizzari, M.; Gigoni, A.; Dieci, G.; Russo, C.; Florio, T.; Cancedda, R.; Pagano, A. An intronic ncRNA-dependent regulation of SORL1 expression affecting abeta formation is upregulated in post-mortem Alzheimer’s disease brain samples. Dis. Models Mech. 2012, 6, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.F.; Raines, S.M.; Steele, J.W.; Ehrlich, M.E.; Lah, J.A.; Small, S.A.; Tanzi, R.E.; Attie, A.D.; Gandy, S. Diabetes-associated SorCS1 regulates Alzheimer’s amyloid-beta metabolism: Evidence for involvement of SORL1 and the retromer complex. J. Neurosci. 2010, 30, 13110–13115. [Google Scholar] [CrossRef] [PubMed]
- Rohe, M.; Carlo, A.S.; Breyhan, H.; Sporbert, A.; Militz, D.; Schmidt, V.; Wozny, C.; Harmeier, A.; Erdmann, B.; Bales, K.R.; et al. Sortilin-related receptor with a-type repeats (SORLA) affects the amyloid precursor protein-dependent stimulation of ERK signaling and adult neurogenesis. J. Biol. Chem. 2008, 283, 14826–14834. [Google Scholar] [CrossRef]
- Musunuru, K.; Strong, A.; Frank-Kamenetsky, M.; Lee, N.E.; Ahfeldt, T.; Sachs, K.V.; Li, X.; Li, H.; Kuperwasser, N.; Ruda, V.M.; et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 2010, 466, 714–719. [Google Scholar] [CrossRef]
- Canuel, M.; Bhattacharyya, N.; Balbis, A.; Yuan, L.; Morales, C.R. Sortilin and prosaposin localize to detergent-resistant membrane microdomains. Exp. Cell Res. 2009, 315, 240–247. [Google Scholar] [CrossRef]
- Lyons, R.M.; Keski-Oja, J.; Moses, H.L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J. Cell Biol. 1988, 106, 1659–1665. [Google Scholar] [CrossRef]
- Palmieri, M.; Impey, S.; Kang, H.; di Ronza, A.; Pelz, C.; Sardiello, M.; Ballabio, A. Characterization of the clear network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 2011, 20, 3852–3866. [Google Scholar] [CrossRef]
- Sardiello, M.; Palmieri, M.; di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; Di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S.; et al. A gene network regulating lysosomal biogenesis and function. Science 2009, 325, 473–477. [Google Scholar] [CrossRef]
- Maeda, S.; Nobukuni, T.; Shimo-Onoda, K.; Hayashi, K.; Yone, K.; Komiya, S.; Inoue, I. Sortilin is upregulated during osteoblastic differentiation of mesenchymal stem cells and promotes extracellular matrix mineralization. J. Cell Physiol. 2002, 193, 73–79. [Google Scholar] [CrossRef] [PubMed]
- van Meel, E.; Lee, W.S.; Liu, L.; Qian, Y.; Doray, B.; Kornfeld, S. Multiple Domains of GlcNAc-1-phosphotransferase Mediate Recognition of Lysosomal Enzymes. J. Biol. Chem. 2016, 291, 8295–8307. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnes, J.W.; Aarnio-Peterson, M.; Norris, J.; Haskins, M.; Flanagan-Steet, H.; Steet, R. Upregulation of Sortilin, a Lysosomal Sorting Receptor, Corresponds with Reduced Bioavailability of Latent TGFβ in Mucolipidosis II Cells. Biomolecules 2020, 10, 670. https://doi.org/10.3390/biom10050670
Barnes JW, Aarnio-Peterson M, Norris J, Haskins M, Flanagan-Steet H, Steet R. Upregulation of Sortilin, a Lysosomal Sorting Receptor, Corresponds with Reduced Bioavailability of Latent TGFβ in Mucolipidosis II Cells. Biomolecules. 2020; 10(5):670. https://doi.org/10.3390/biom10050670
Chicago/Turabian StyleBarnes, Jarrod W., Megan Aarnio-Peterson, Joy Norris, Mark Haskins, Heather Flanagan-Steet, and Richard Steet. 2020. "Upregulation of Sortilin, a Lysosomal Sorting Receptor, Corresponds with Reduced Bioavailability of Latent TGFβ in Mucolipidosis II Cells" Biomolecules 10, no. 5: 670. https://doi.org/10.3390/biom10050670
APA StyleBarnes, J. W., Aarnio-Peterson, M., Norris, J., Haskins, M., Flanagan-Steet, H., & Steet, R. (2020). Upregulation of Sortilin, a Lysosomal Sorting Receptor, Corresponds with Reduced Bioavailability of Latent TGFβ in Mucolipidosis II Cells. Biomolecules, 10(5), 670. https://doi.org/10.3390/biom10050670




