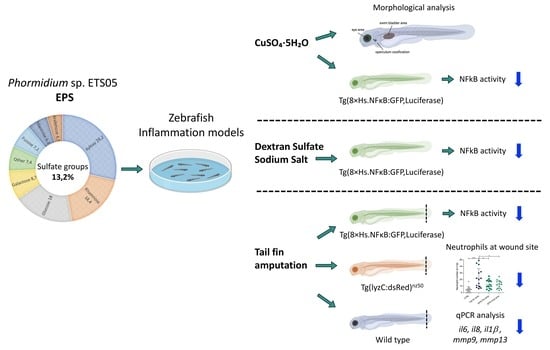
Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Phormidium sp. ETS05 and Extraction of Exopolysaccharides
2.2. EPS Characterization
2.3. Zebrafish Lines
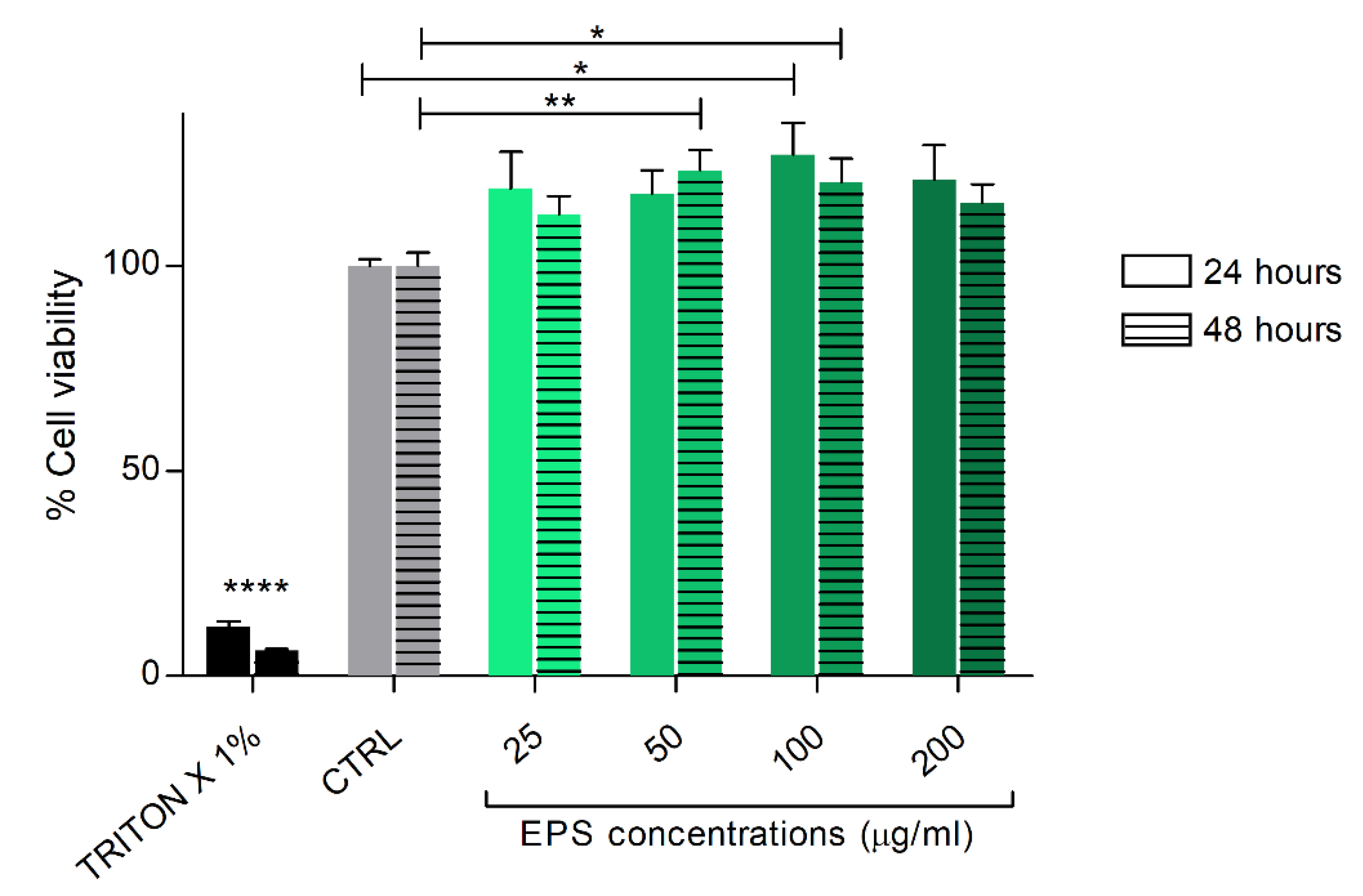
2.4. In Vitro Cell Viability Assay
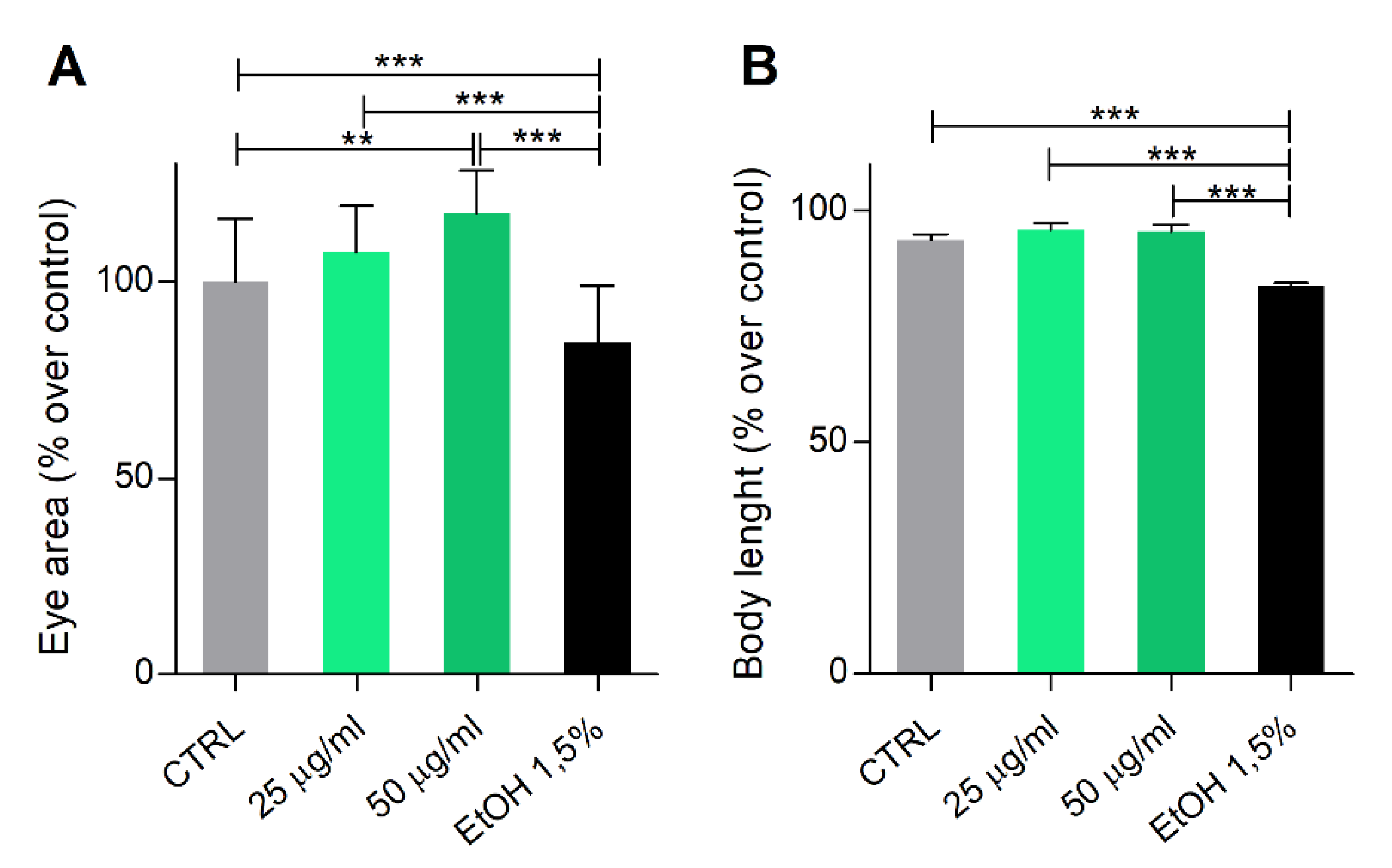
2.5. Zebrafish Embryo/Larvae Developmental Toxicity Assay
2.6. Co-Cultivation of Phormidium sp. ETS05 and Zebrafish to Evaluate Cyanobacterium Toxicity
2.7. Chemicals Treatments and Caudal Fin Amputation of Zebrafish Larvae
2.8. Analysis of Luciferase in NFκB:GFP,Luc Larvae
2.9. Analysis of lysC:DsRed Transgenic Larvae
2.10. Zebrafish Operculum Area Analysis
2.11. Morphological Analysis and Image Processing
2.12. RNA Isolation, cDNA Synthesis, and Expression Analyses
2.13. Statistical Analyses
3. Results
3.1. Chemical Characterization of the EPS Produced by Phormidium sp. ETS05 Pure Culture
3.2. Phormidium EPS Treatment Does Not Interfere with In Vitro Cell Viability of Human Skin Fibroblast Cells
3.3. Phormidium EPS Treatment Has No Toxicological or Teratogenic Effects on Zebrafish Development
3.4. Co-Cultivation of Phormidium sp. ETS05 and Zebrafish to Evaluate Cyanobacterium Toxicity
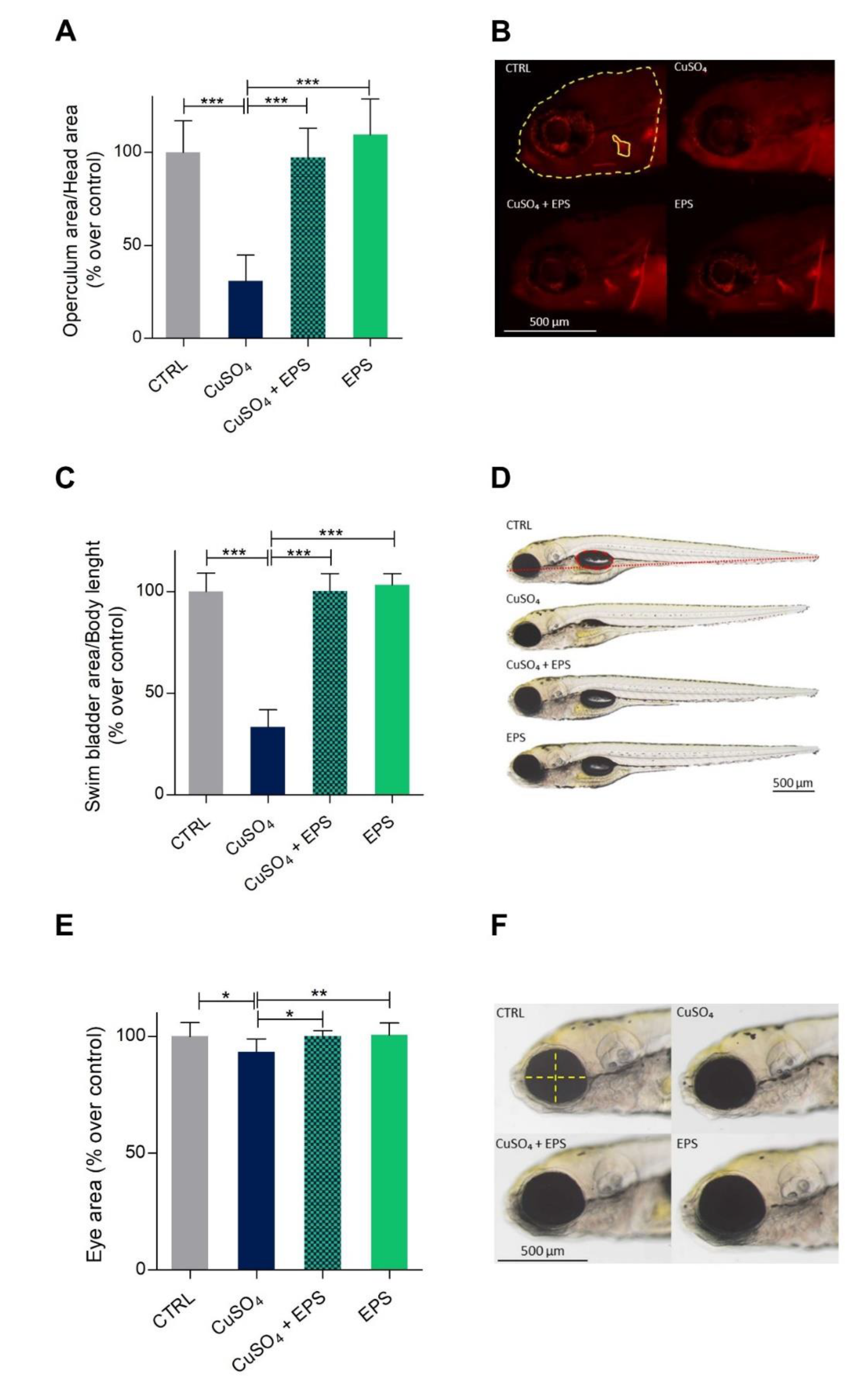
3.5. Zebrafish Developmental Delay due to CuSO4·5H2O Treatment is Rescued by Phormidium EPS Treatment
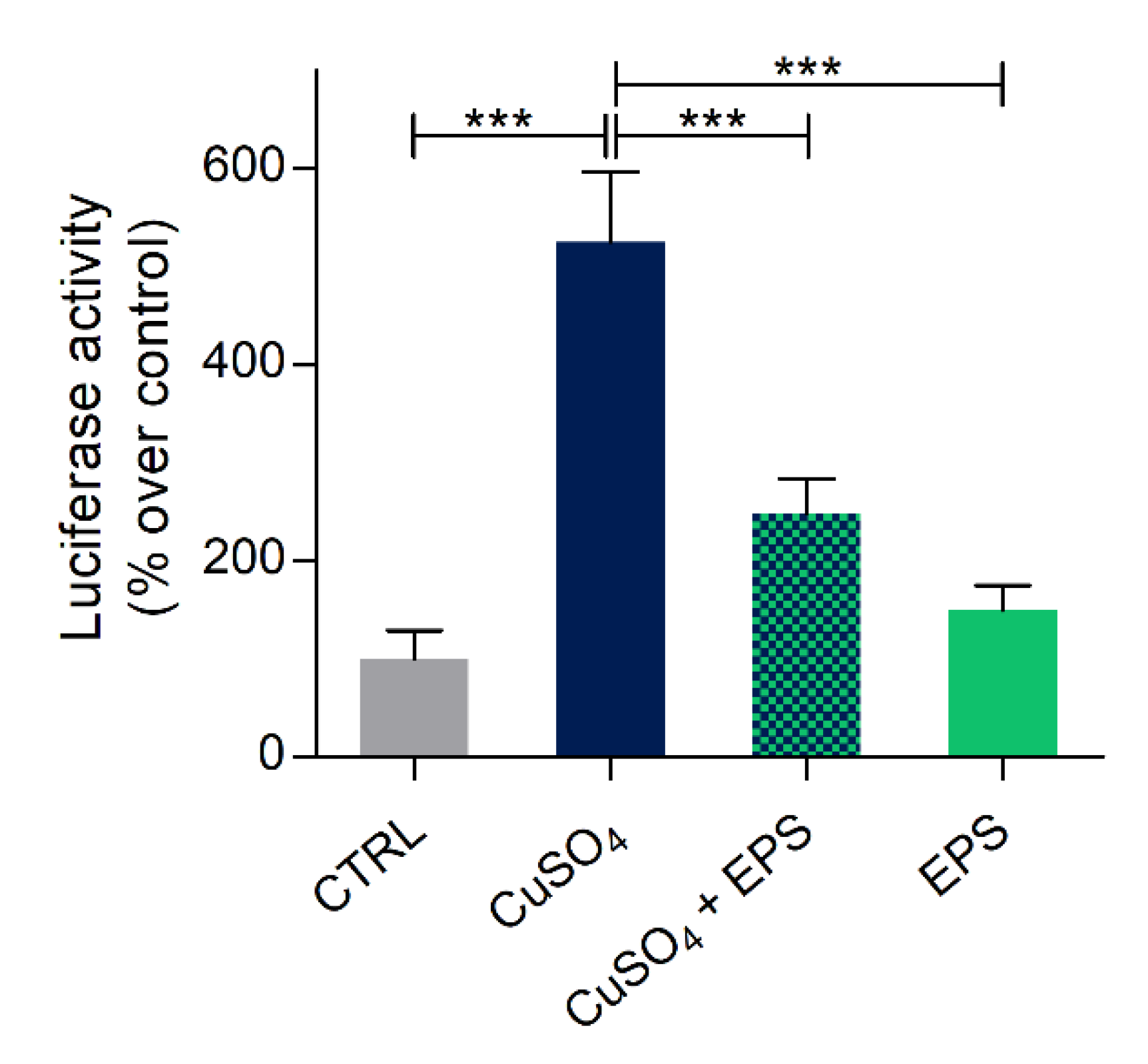
3.6. Copper Induced Inflammatory Status Can Be Recovered after Phormidium EPS Treatment
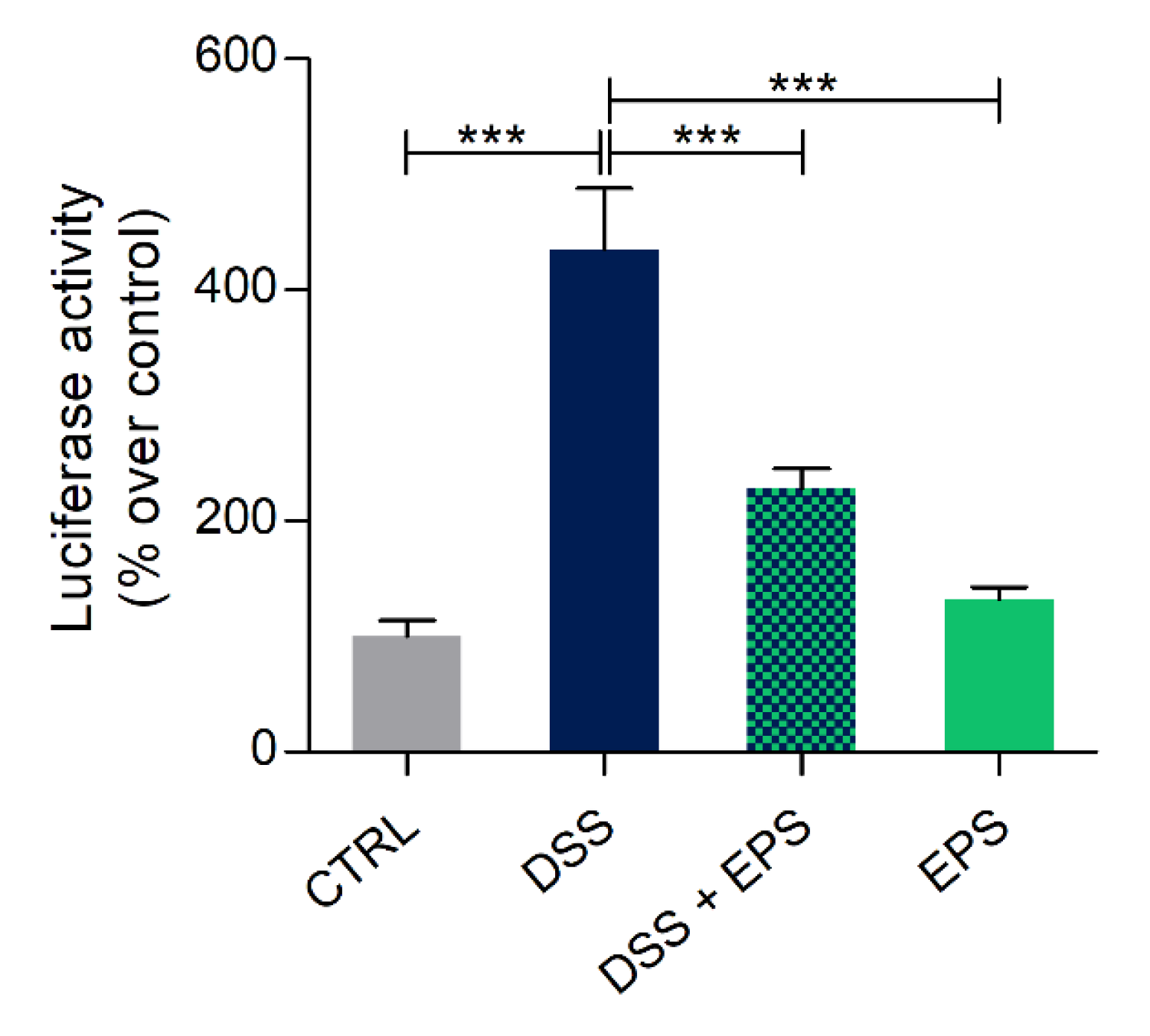
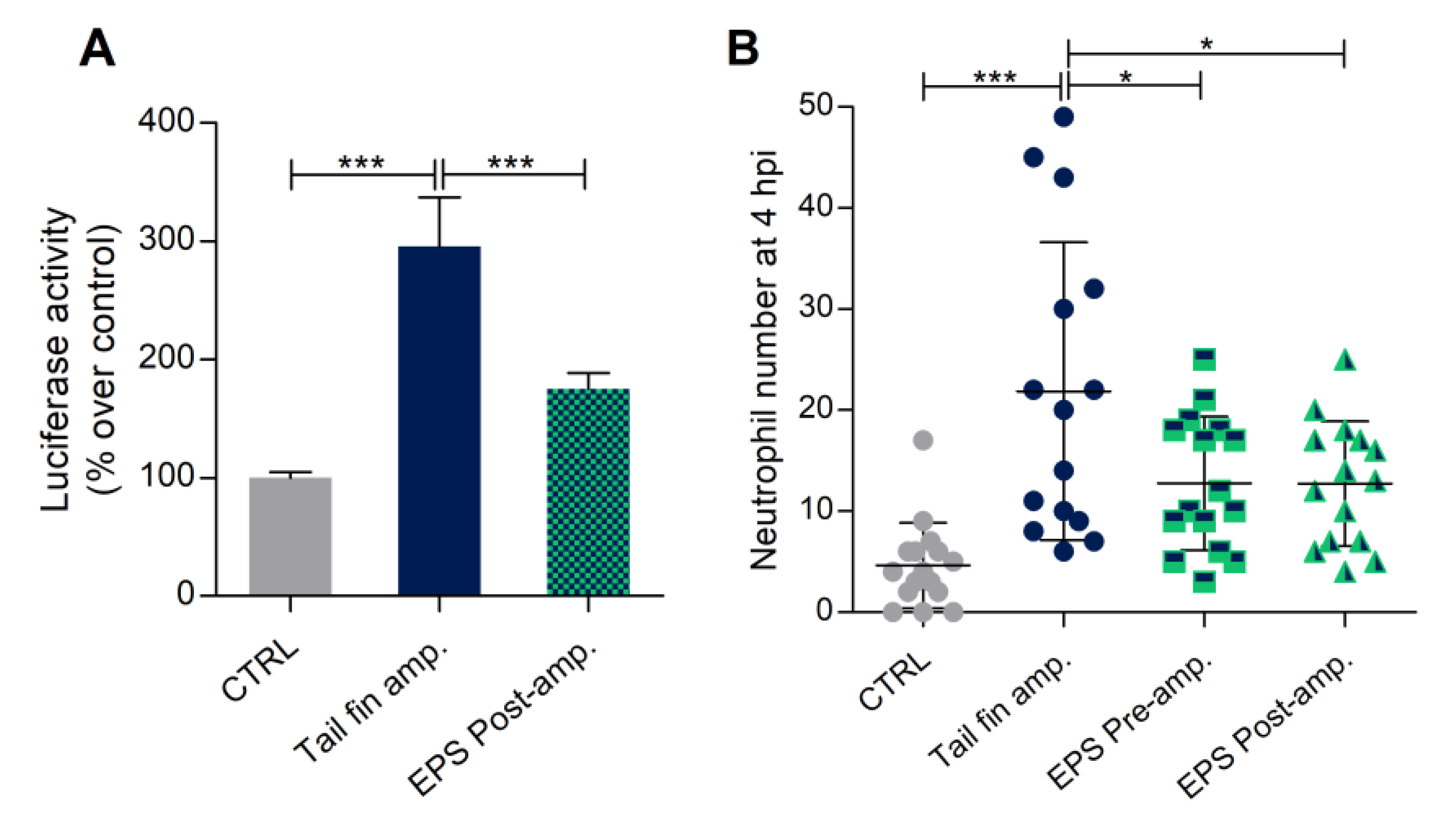
3.7. Phormidium EPS Can Reduce the Inflammatory Response Induced by DSS and Caudal Fin Amputation
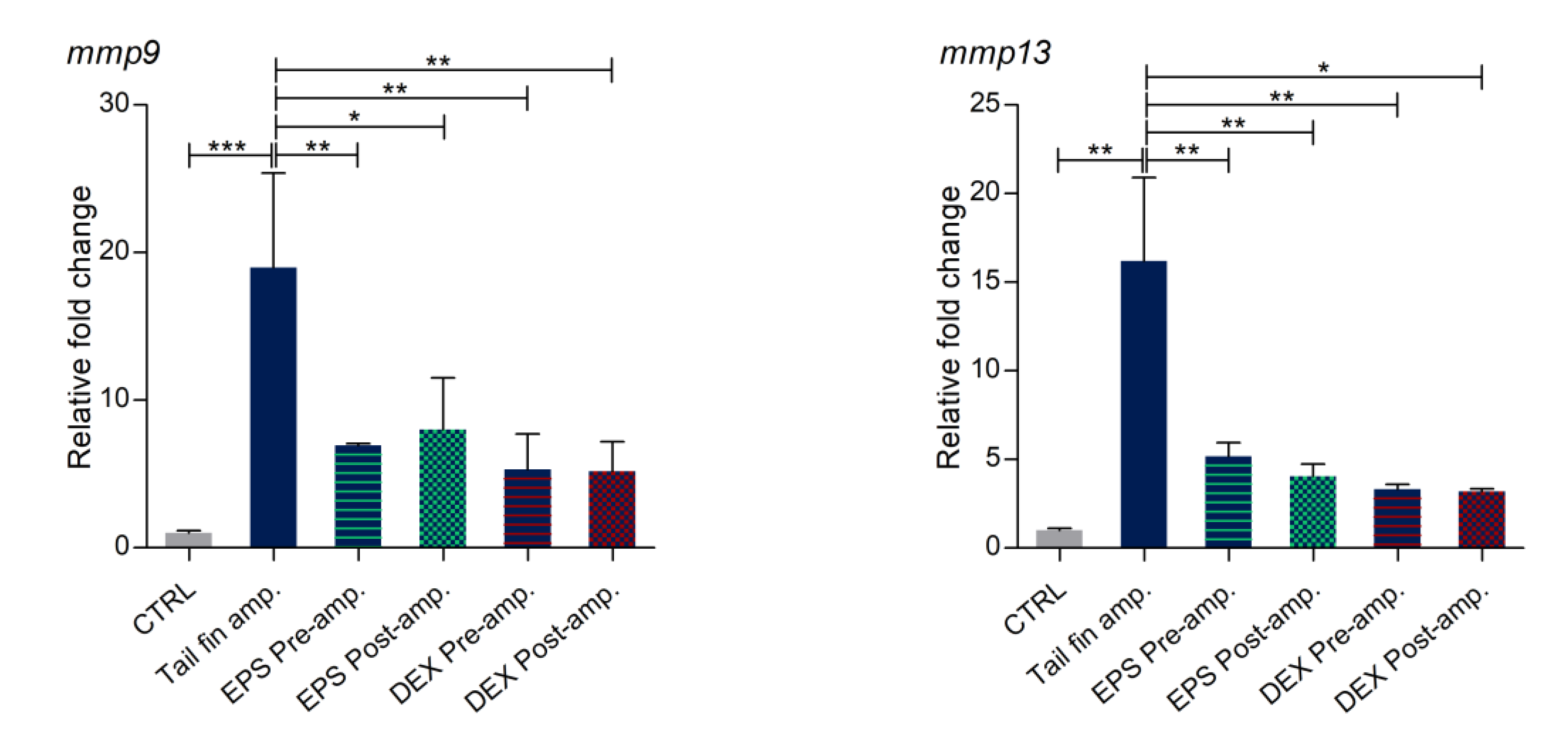
3.8. Effects of Phormidium EPS on the Expression of Inflammatory Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- BUR. Regolamento d’Uso del Marchio Collettivo d’Origine Fango D.O.C.—Thermae Abano Montegrotto—Regione Veneto; venerdì 27 marzo 2015. Anno XLVI–N. 30; Bollettino Ufficiale Regione del Veneto: Venezia, Italy, 2015; pp. 33–81. [Google Scholar]
- Andreoli, C.; Rascio, N. The Algal Flora in the Thermal Baths of Montegrotto Terme (Padua). Its Distribution Over One-Year Period. Int. Rev. Hydrobiol. 1975, 60, 857–871. [Google Scholar] [CrossRef]
- Bruno, A.; Rossi, C.; Marcolongo, G.; Di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective In Vivo Anti-Inflammatory Action of the Glactolipid Monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, V.; Lenti, M.; Gentili, C.; Marcolongo, G.; Cancedda, R.; Descalzi Cancedda, F. Anti-Inflammatory Activity of Monogalactosyldiacylglycerol in Human Articular Cartilage In Vitro: Activation of an Anti-Inflammatory Cyclooxygenase-2 (COX-2) Pathway. Arthritis Res. Ther. 2011, 13. [Google Scholar] [CrossRef]
- Gris, B.; Sforza, E.; Morosinotto, T.; Bertucco, A.; La Rocca, N. Influence of Light and Temperature on Growth and High-Value Molecules Productivity from Cyanobacterium aponinum. J. Appl. Phycol. 2017, 29, 1781–1790. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.J.; Prasad, S.M. Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef]
- Ceschi Berrini, C.; De Appolonia, F.; Dalla Valle, L.; Komárek, J.; Andreoli, C. Morphological and Molecular Characterization of a Thermophilic Cyanobacterium (Oscillatoriales) from the Euganean Thermal Springs (Padua, Italy). Algol. Stud. 2004, 113, 73–85. [Google Scholar] [CrossRef]
- Marcolongo, G.; De Appolonia, F.; Venzo, A.; Berrie, C.P.; Carofiglio, T.; Ceschi Berrini, C. Diacylglycerolipids Isolated from a Thermophile Cyanobacterium from the Euganean Hot Springs. Nat. Prod. Res. 2006, 20, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.; Gentili, C.; Pianezzi, A.; Marcolongo, G.; Lalli, A.; Cancedda, R.; Descalzi Cancedda, F. Monogalactosyldiacylglycerol Anti-inflammatory Activity on Adult Articular Cartilage. Nat. Prod. Res. 2009, 23, 754–762. [Google Scholar] [CrossRef]
- Lalli, A.; Andreoli, C.; Ceshi Berrini, C.; De Appolonia, F.; Marcolongo, G. Anti-inflammatory Active Principles in Euganean Thermal Mud. European Patent 1571203 (B1), 24 July 2013. [Google Scholar]
- De Jesus Raposo, M.F.; Bernardo De Morais, M.A.; Costa de Morais, R.M.S. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Gugliandolo, C.; Spanò, A.; Maugeri, T.L.; Poli, A.; Arena, A.; Nicolaus, B. Role of Bacterial Exopolysaccharides as Agents in Counteracting Immune Disorders Induced by Herpes Virus. Microorganisms 2015, 3, 464–483. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, M. Present and Future Medical Applications of Microbial Exopolysaccharides. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; García-mauriño, S.; Ávila-román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Hamidi, M.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Pierre, G.; Michaud, P.; Delattre, C. Marine Bacteria versus Microalgae: Who Is the Best for Biotechnological Production of Bioactive Compounds with Antioxidant Properties and Other Biological Applications? Mar. Drugs 2018, 18, 28. [Google Scholar] [CrossRef]
- Silambarasan, G.; Ramanathan, T.; Nabeell, M.A.; Kalaichelvan, V.; Kathiresan, K.; Balasubramanian, T. Anti-Inflammatory Activity of the Marine Cyanobacterium Trichodesmium Erythraeum Against Carrageenan-Induced Paw Oedema in Wistar Albino RATS. Eur. J. Inflamm. 2011, 9, 53–56. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.B.; Omarsdottir, S.; Brynjolfsdottir, A.; Paulsen, B.S.; Olafsdottir, E.S.; Brynjolfsdottir, A.; Paulsen, B.S.; Olafsdottir, E.S.; Freysdottir, J. Exopolysaccharides from Cyanobacterium aponinum from the Blue Lagoon in Iceland Increase IL-10 Secretion by Human Dendritic Cells and Their Ability to Reduce The IL-17+Rorγt+/IL-10+Foxp3+ Ratio in CD4+ T Cells. Immunol. Lett. 2015, 163, 157–162. [Google Scholar] [CrossRef]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Abu Hashim, I.I.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; et al. Anti-inflammatory Effects of Novel Polysaccharide Sacran Extracted from Cyanobacterium Aphanothece sacrum in Various Inflammatory Animal Models. Biol. Pharm. Bull. 2016, 39, 1172–1178. [Google Scholar] [CrossRef]
- Belhaj, D.; Frikha, D.; Athmouni, K.; Jerbi, B.; Boshir, M.; Bouallagui, Z.; Kallel, M.; Maalej, S.; Zhou, J.; Ayadi, H. Box-Behnken Design for Extraction Optimization of Crude Polysaccharides from Tunisian Phormidium versicolor Cyanobacteria (NCC 466): Partial Characterization, In Vitro Antioxidant and Antimicrobial Activities. Int. J. Biol. Macromol. 2017, 105, 1501–1510. [Google Scholar] [CrossRef]
- Belhaj, D.; Athmouni, K.; Boshir, M.; Aoiadni, N.; El Feki, A.; Zhou, J.L.; Ayadi, H. Polysaccharides from Phormidium versicolor (NCC466) Protecting Hepg2 Human Hepatocellular Carcinoma Cells and Rat Liver Tissues from Cadmium Toxicity: Evidence from In Vitro and In Vivo Tests. Int. J. Biol. Macromol. 2018, 113, 813–820. [Google Scholar] [CrossRef]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.J.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of Inflammation: State of the Art, Definitions and Terms. FASEB J. 2007, 21, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.M.; Loynes, C.A.; Whyte, M.K.B.; Renshaw, S.A. Zebrafish as a Model for the Study of Neutrophil Biology. J. Leukoc. Biol. 2013, 94, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.O.; Van Dyke, T.E. Natural Resolution of Inflammation. Periodontol. 2000 2013, 63, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Phillips, J.B.; Westerfield, M. Zebrafish Models in Translational Research: Tipping the Scales Toward Advancements in Human Health. Dis. Models Mech. 2014, 7, 739–743. [Google Scholar] [CrossRef]
- Mandrekar, N.; Thakur, N.L. Significance of the Zebrafish Model in the Discovery of Bioactive Molecules from Nature. Biotechnol. Lett. 2009, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y. Development and Maturation of the Immune System in Zebrafish, Danio Rerio: A Gene Expression Profiling, In Situ Hybridization and Immunological Study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Herbomel, P.; Thisse, B.; Thisse, C. Ontogeny and Behaviour of Early Macrophages in the Zebrafish Embryo. Development 1999, 126, 3735–3745. [Google Scholar]
- Morales Fénero, C.I.; Colombo Flores, A.A.; Saraiva Câmara, N.O. Inflammatory Diseases Modelling in Zebrafish. World J. Exp. Med. 2016, 6, 9–20. [Google Scholar] [CrossRef]
- Forn-Cuní, G.; Varela, M.; Pereiro, P.; Novoa, B.; Figueras, A. Conserved Gene Regulation During Acute Inflammation Between Zebrafish and Mammals. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Mugnai, G.; Simiani, A.; De Philippis, R. Soil Type and Cyanobacteria Species Influence the Macromolecular and Chemical Characteristics of the Polysaccharidic Matrix in Induced Biocrusts. Microb. Ecol. 2019, 78, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Lima, R.T.; Adessi, A.; Sousa, A.; Pereira, S.B.; Granja, P.L.; De Philippis, R.; Soares, P.; Tamagnini, P. Characterization and Antitumor Activity of the Extracellular Carbohydrate Polymer from the Cyanobacterium Synechocystis ΔsigF mutant. Int. J. Biol. Macromol. 2019, 136, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Kuri, P.; Ellwanger, K.; Kufer, T.A.; Leptin, M.; Bajoghli, B. A High-Sensitivity Bi-Directional Reporter to Monitor NF-kb Activity in Cell Culture and Zebrafish in Real Time. J. Cell Sci. 2017, 130, 648–657. [Google Scholar] [CrossRef]
- Hall, C.; Flores, M.V.; Storm, T.; Crosier, K.; Crosier, P. The Zebrafish Lysozyme C Promoter Drives Myeloid-Specific Expression in Transgenic Fish. BMC Dev. Biol. 2007, 7. [Google Scholar] [CrossRef]
- Franzolin, E.; Coletta, S.; Ferraro, P.; Pontarin, G.; D’Aronco, G.; Stevanoni, M.; Palumbo, E.; Cagnin, S.; Bertoldi, L.; Feltrin, E.; et al. SAMHD1-Deficient Fibroblasts from Aicardi-Goutières Syndrome Patients Can Escape Senescence and Accumulate Mutations. FASEB J. 2020, 34, 631–647. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a Novel Exopolysaccharide with Antitumor Activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhang, J.; Wang, X.; Liu, M.; Zhang, C.; Jia, L. Hepatoprotective and In Vitro Antioxidant Effects of Native Depolymerised-Exopolysaccharides Derived from Termitomyces albuminosus. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides Produced by Lactobacillus Strains Suppress HT-29 Cell Growth Via Induction of G0/G1 Cell Cycle Arrest and Apoptosis. Oncol. Lett. 2018, 16, 3577–3586. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar] [CrossRef]
- Qian, H.; Liu, G.; Lu, T.; Sun, L. Developmental neurotoxicity of Microcystis aeruginosa in the early life stages of zebrafish. Ecotoxicol. Environ. Saf. 2018, 151, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Tarasco, M.; Laizé, V.; Cardeira, J.; Cancela, M.L.; Gavaia, P.J. The Zebrafish Operculum: A Powerful System to Assess Osteogenic Bioactivities of Molecules with Pharmacological and Toxicological Relevance. Comp. Biochem. Physiol. Part C 2017, 197, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, S.; Kim, E.; Lee, J.; Kim, Y.; Yu, S.; Chae, J.B.; Choe, I.; Cho, J.; Jeon, Y. Antioxidant Activity of Polysaccharide Purified from Acanthopanax koreanum Nakai Stems In Vitro and In Vivo Zebrafish Model. Carbohydr. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- Jin, J.; Nguyen, T.T.H.; Kim, C.; Kim, D. Antimelanogenesis Effects of Fungal Exopolysaccharides Prepared from Submerged Culture of Fomitopsis castanea Mycelia. J. Microbiol. Biotechnol. 2019, 29, 1204–1211. [Google Scholar] [CrossRef]
- D’Alençon, C.A.; Peña, O.A.; Wittmann, C.; Gallardo, V.E.; Jones, R.A.; Loosli, F.; Liebel, U.; Grabher, C.; Allende, M.L. A High-Throughput Chemically Induced Inflammation Assay in Zebrafish. BMC Biol. 2010, 8. [Google Scholar] [CrossRef]
- Wittmann, C.; Reischl, M.; Shah, A.H.; Mikut, R.; Liebel, U.; Grabher, C. Facilitating Drug Discovery: An Automated High-content Inflammation Assay in Zebrafish. J. Vis. Exp. 2012, 65, 1–7. [Google Scholar] [CrossRef]
- Pereira, S.; Micheletti, E.; Zille, A.; Santos, A.; Moradas-ferreira, P.; Tamagnini, P.; De Philippis, R. Using Extracellular Polymeric Substances (EPS)-Producing Cyanobacteria for the Bioremediation of Heavy Metals: Do Cations Compete for the EPS Functional Groups and also Accumulate Inside the Cell? Microbiology 2011, 157, 451–458. [Google Scholar] [CrossRef]
- Witeska, M.; Sarnowski, P.; Ługowska, K.; Kowal, E. The Effects of Cadmium and Copper on Embryonic and Larval Development of Ide Leuciscus idus L. Fish Physiol. Biochem. 2014, 40, 151–163. [Google Scholar] [CrossRef]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran Sodium Sulfate (DSS) Induces Colitis in Mice by Forming Nano-Lipocomplexes with Medium-Chain-Length Fatty Acids in the Colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, D.; Huang, Z.; Liu, Y. The Vertical Microdistribution of Cyanobacteria and Green Algae within Desert Crusts and the Development of the Algal Crusts. Plant Soil 2003, 257, 97–111. [Google Scholar] [CrossRef]
- Xue, Y.; Rossi, F.; Colica, G.; Deng, S.; De Philippis, R.; Chen, L. Use of Cyanobacterial Polysaccharides to Promote Shrub Performances in Desert Soils: A Potential Approach for the Restoration of Desertified Areas. Biol. Fertil. Soils 2012, 49. [Google Scholar] [CrossRef]
- Gloaguen, V.; Garbacki, N.; Petit, D.; Morvan, H.; Hoffmann, L. Bioactive Capsular Polysaccharide from the Thermophilic Mastigocladus laminosus (Cyanophyceae/Cyanobacteria): Demonstration of Anti-Inflammatory Properties. Algol. Stud. Arch. Für Hydrobiol. 2003, 108, 63–73. [Google Scholar]
- Gloaguen, V.; Morvan, H.; Hoffmann, L.; Sainte Catherine, O.; Kraemer, M.; Krausz, P. Bioactive Capsular Polysaccharide from the Thermophilic Cyanophyte/Cyanobacterium Mastigocladus laminosus—Cytotoxic Properties. Planta Med. 2007, 73, 1402–1406. [Google Scholar] [CrossRef]
- Okajima, M.K.; Kaneko, D.; Mitsumata, T.; Kaneko, T.; Watanabe, J. Cyanobacteria that Produce Megamolecules with Efficient Self-Orientations. Macromolecules 2009, 42, 3057–3062. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of Cyanobacterial Exopolysaccharides: Composition, Structures, Inducing Factors and Putative Genes Involved in Their Biosynthesis and Assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Richert, L.; Golubic, S.; Le Guédès, R.; Ratiskol, J.; Payri, C.; Guezennec, J. Characterization of Exopolysaccharides Produced by Cyanobacteria Isolated from Polynesian Microbial Mats. Curr. Microbiol. 2005, 51, 379–384. [Google Scholar] [CrossRef]
- Hussein, M.H.; Abou-ElWafa, G.S.; Shaaban-Dessuuki, S.A.; Hassan, N.I. Characterization and Antioxidant Activity of Exopolysaccharide Secreted by Nostoc carneum. Int. J. Pharmacol. 2015, 11, 432–439. [Google Scholar] [CrossRef]
- Mota, R.; Vidal, R.; Pandeirada, C.; Flores, C.; Adessi, A.; De Philippis, R.; Nunes, C.; Coimbra, M.A.; Tamagnini, P. Cyanoflan: A Cyanobacterial Sulfated Carbohydrate Polymer with Emulsifying Properties. Carbohydr. Polym. 2020, 229. [Google Scholar] [CrossRef]
- Garbacki, N.; Gloaguen, V.; Damas, J.; Hoffmann, L.; Tits, M.; Angenot, L. Inhibition of Croton Oil-Induced Oedema in Mice Ear Skin by Capsular Polysaccharides from Cyanobacteria. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 361, 460–464. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhao, F.; Shi, M.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Chen, X.-L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-L.; Chen, J.-F.; Tan, L.; Jin, M.-Y.; Ju, F.; Cao, Z.-X.; Deng, F.; Wang, L.-N.; Gu, Y.-C.; Deng, Y. Terpene Glycosides from Sanguisorba officinalis and Their Anti-Inflammatory Effect. Molecules 2019, 24, 2906. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Le, H.D.; Kim, T.N.T.; The, H.P.; Nguyen, T.M.; Cornet, V.; Lambert, J.; Kestemont, P. Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, L.; Wu, J.; Wang, W.; Mei, J.; Ma, X.; Liu, J. Transcriptional Responses and Mechanisms of Copper-Induced Dysfunctional Locomotor Behavior in Zebrafish Embryos. Toxicol. Sci. 2015, 148, 299–310. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef]
- Jimi, E.; Huang, F.; Nakatomi, C. NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Mol. Sci. 2019, 20, 6275. [Google Scholar] [CrossRef]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.I.; Elworthy, S.; Ingham, P.W.; Whyte, M.K.B. A Transgenic Zebrafish Model of Neutrophilic Inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The Anti-Inflammatory and Immunosuppressive Effects of Glucocorticoids, Recent Developments and Mechanistic Insights. Mol. Cell. Endocrinol. 2011, 2–13. [Google Scholar] [CrossRef]
- Alan, I.S.; Alan, B. Side Effects of Glucocorticoids. In Pharmacokinetics and Adverse Effects of Drugs—Mechanisms and Risks Factors; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, M.; Yang, F.; Zhu, J.; Xiao, Q.; Zhang, L. Matrix Metalloproteinases: Inflammatory Regulators of Cell Behaviors in Vascular Formation and Remodeling. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Sakao, K.; Takahashi, K.A.; Arai, Y.; Saito, M.; Honjo, K.; Hiraoka, N.; Asada, H.; Shin-Ya, M.; Imanishi, J.; Mazda, O.; et al. Osteoblasts Derived from Osteophytes Produce Interleukin-6, Interleukin-8, and Matrix Metalloproteinase-13 in Osteoarthritis. J. Bone Miner. Metab. 2009, 27, 412–423. [Google Scholar] [CrossRef]
- Hashizume, M.; Mihara, M. The Roles of Interleukin-6 in the Pathogenesis of Rheumatoid Arthritis. Arthritis 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, A.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Akeson, G.; Malemud, C.J. A Role for Soluble IL-6 Receptor in Osteoarthritis. Funct. Morphol. Kinesiol. 2017, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Mimura, T. Matrix Metalloproteinase Gene Activation Resulting from Disordered Epigenetic Mechanisms in Rheumatoid Arthritis. Mol. Sci. 2017, 18, 905. [Google Scholar] [CrossRef] [PubMed]

| Molar % | Mean | st. dev. |
|---|---|---|
| Xylose | 28.2 | 1.5 |
| Rhamnose | 18.4 | 3.2 |
| Glucose | 18.0 | 0.7 |
| Galactose | 08.7 | 0.6 |
| Arabinose | 04.7 | 0.1 |
| Fucose | 07.2 | 0.4 |
| Glucosamine | 03.6 | 0.4 |
| Mannose | 06.1 | 0.7 |
| Glucuronic acid | 02.4 | 0.6 |
| Galacturonic acid | 01.4 | 1.5 |
| Galactosamine | Traces | - |
| Fructose | Traces | - |
| Ribose | Traces | - |
| % (w/w) | mean | st. dev |
| Sulfates | 13.2 | 2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules 2020, 10, 582. https://doi.org/10.3390/biom10040582
Zampieri RM, Adessi A, Caldara F, Codato A, Furlan M, Rampazzo C, De Philippis R, La Rocca N, Dalla Valle L. Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules. 2020; 10(4):582. https://doi.org/10.3390/biom10040582
Chicago/Turabian StyleZampieri, Raffaella Margherita, Alessandra Adessi, Fabrizio Caldara, Alessia Codato, Mattia Furlan, Chiara Rampazzo, Roberto De Philippis, Nicoletta La Rocca, and Luisa Dalla Valle. 2020. "Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model" Biomolecules 10, no. 4: 582. https://doi.org/10.3390/biom10040582
APA StyleZampieri, R. M., Adessi, A., Caldara, F., Codato, A., Furlan, M., Rampazzo, C., De Philippis, R., La Rocca, N., & Dalla Valle, L. (2020). Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules, 10(4), 582. https://doi.org/10.3390/biom10040582