Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Cell Lines and Cell Culture Conditions
2.3. In Vitro Cell Proliferation Assay
2.4. Cell Cycle Analysis
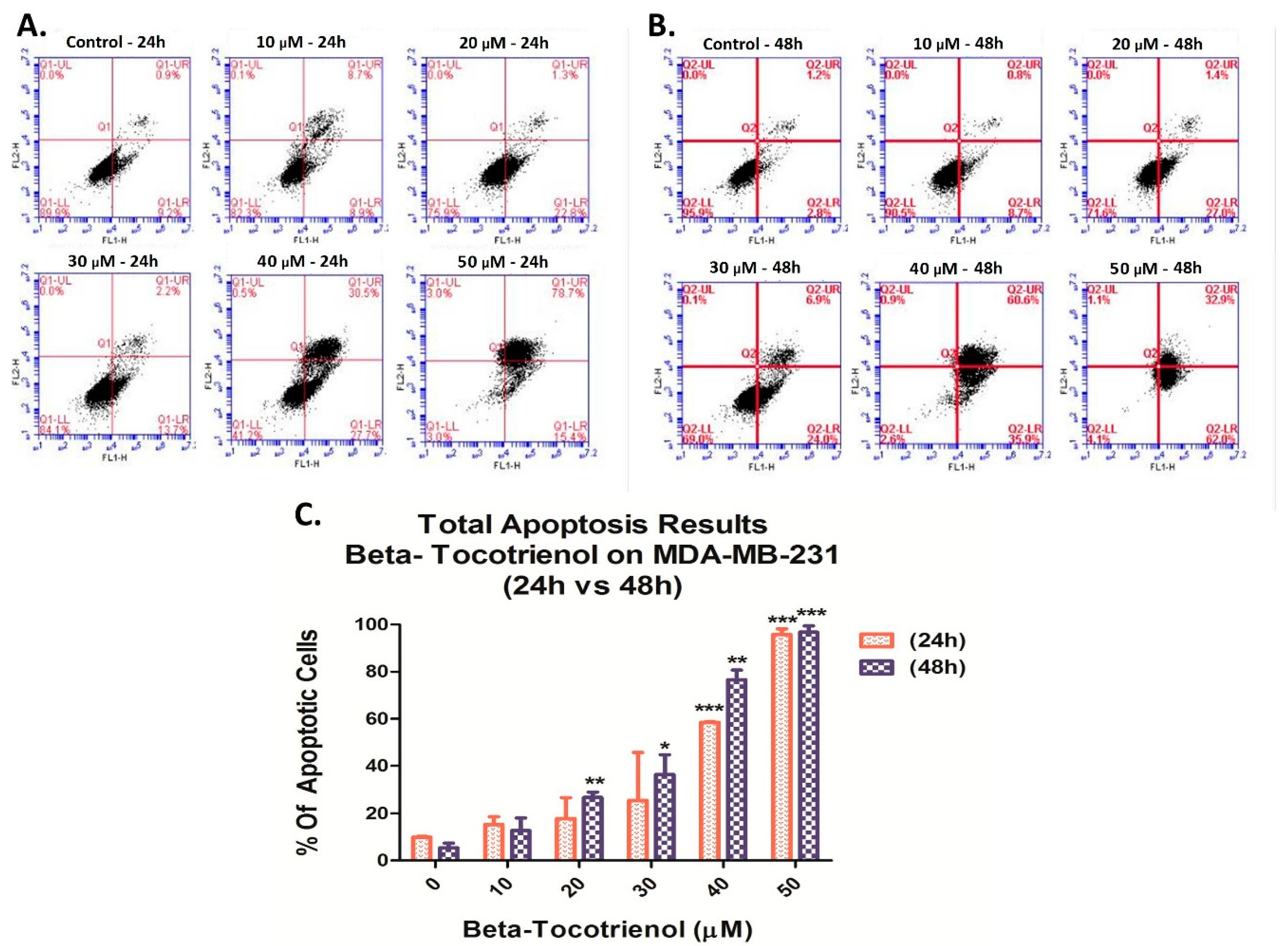
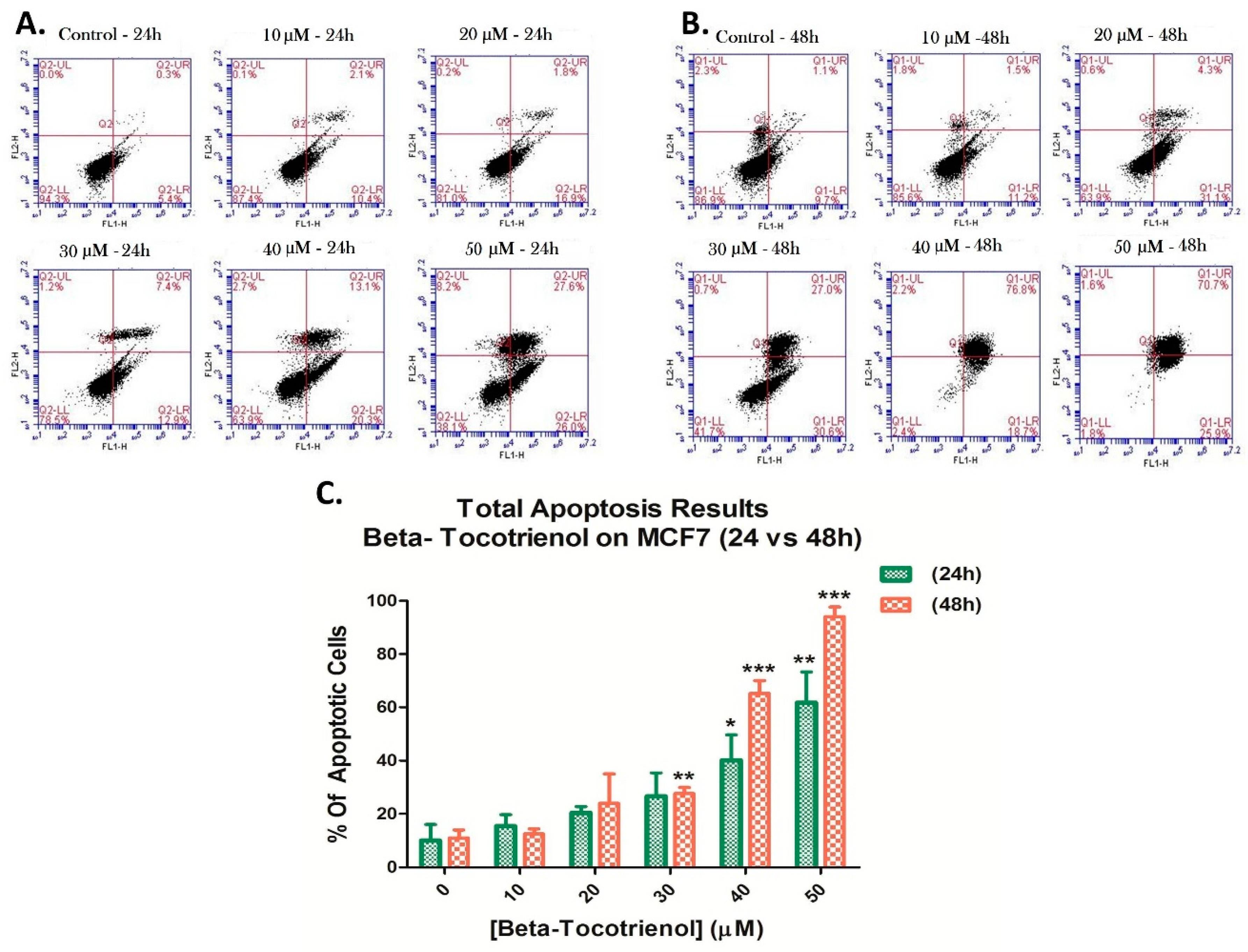
2.5. Cell Apoptosis Detection by Annexin V/PI Staining
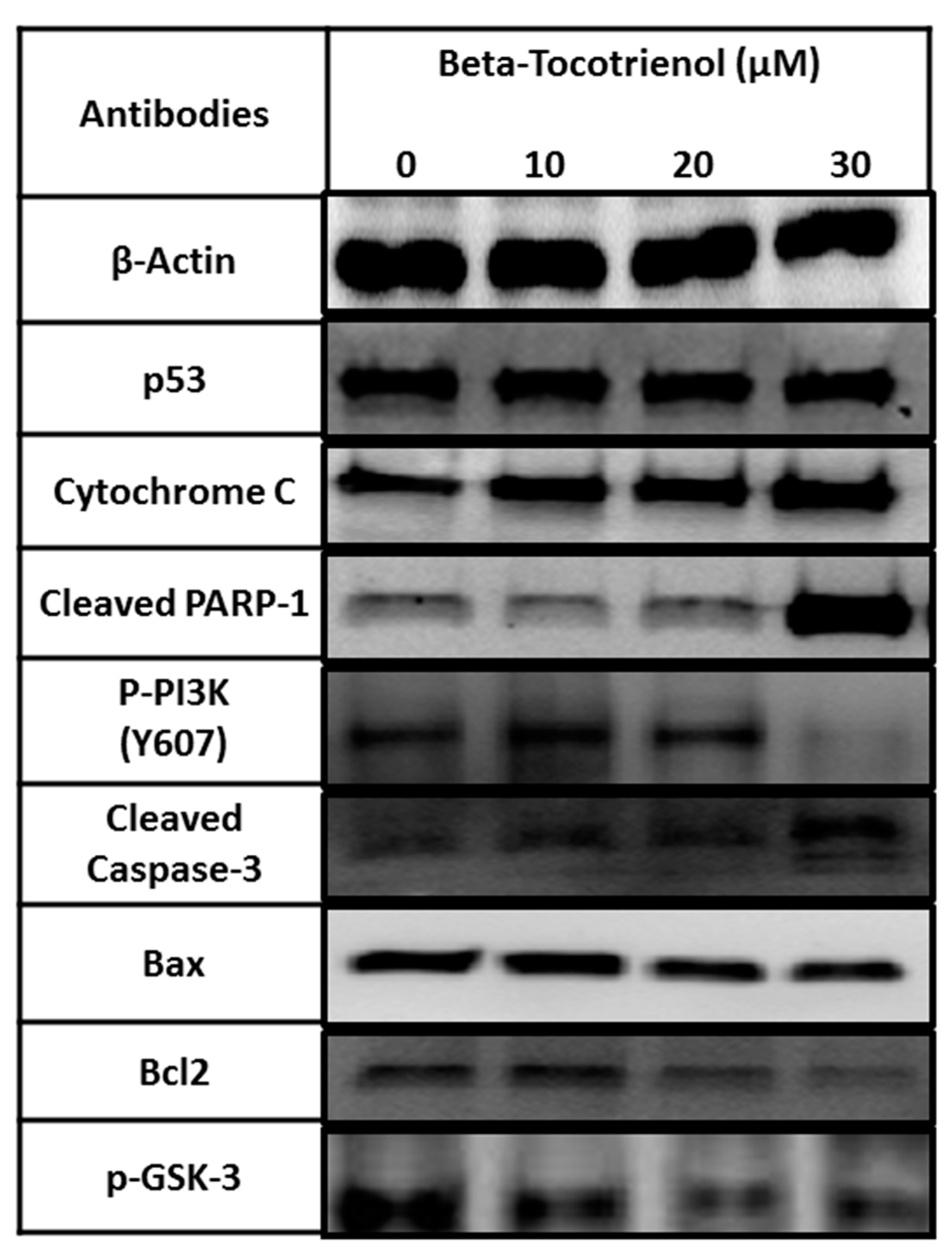
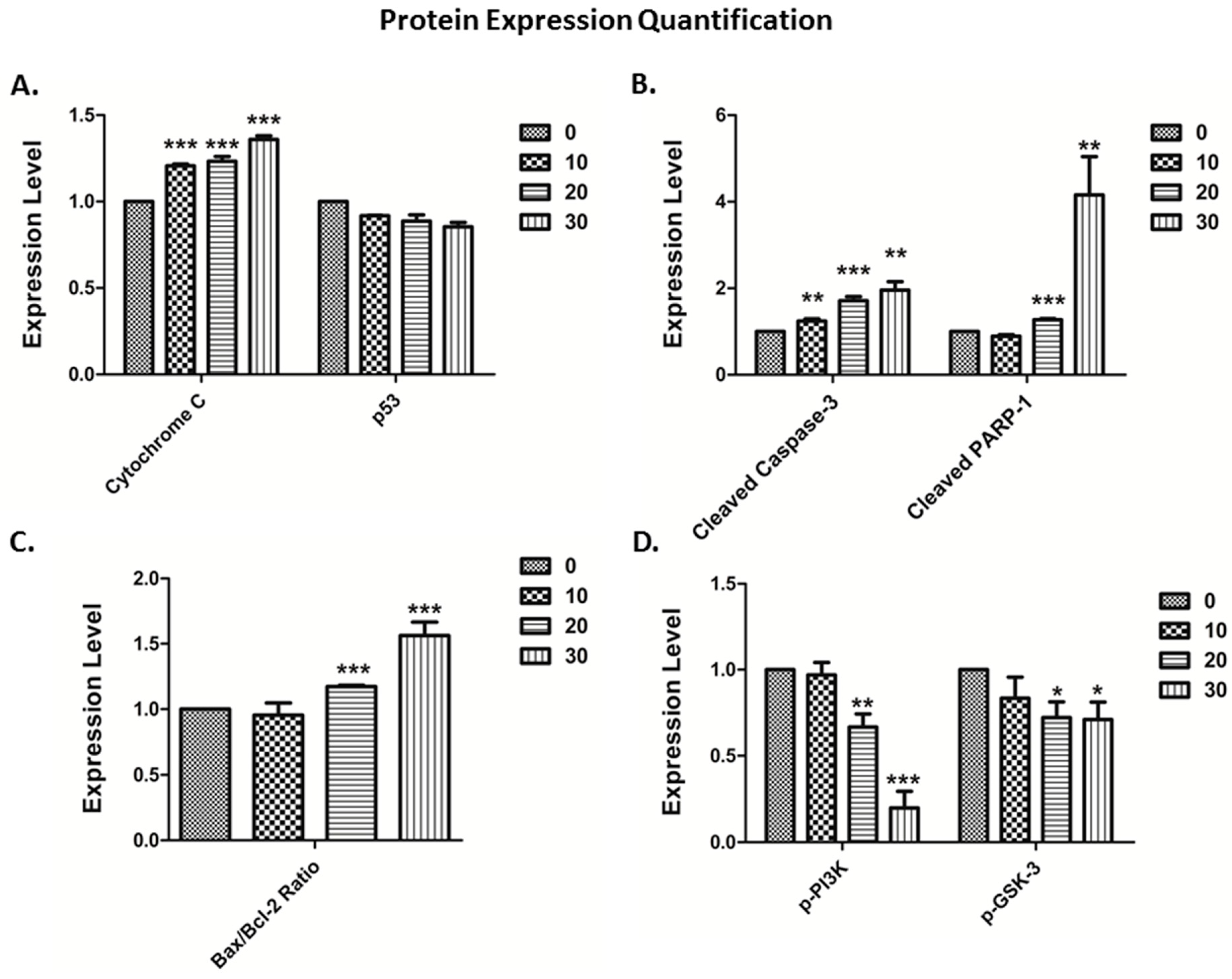
2.6. Protein Extraction and Western Blots
2.7. Statistical Analysis
3. Results
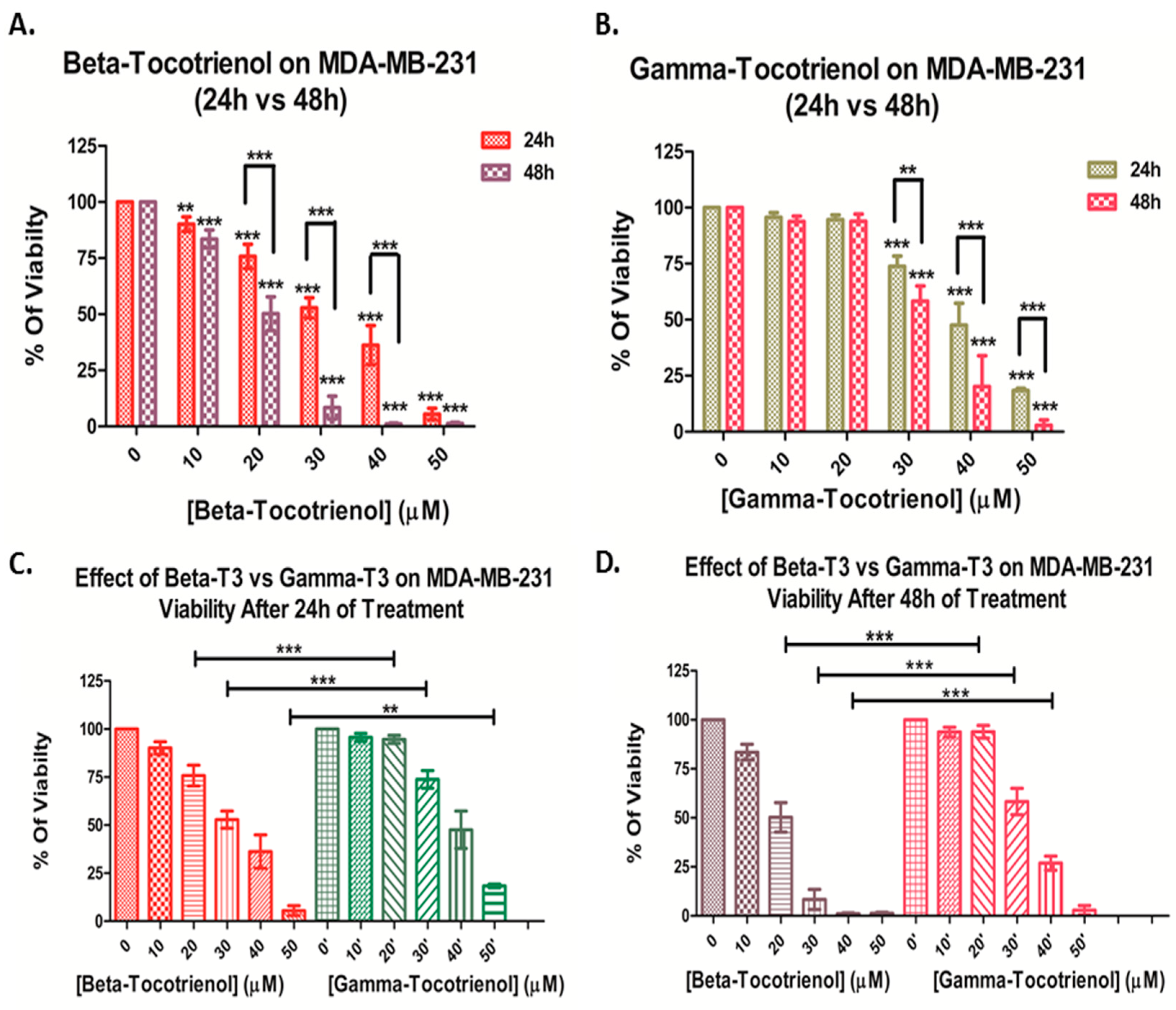
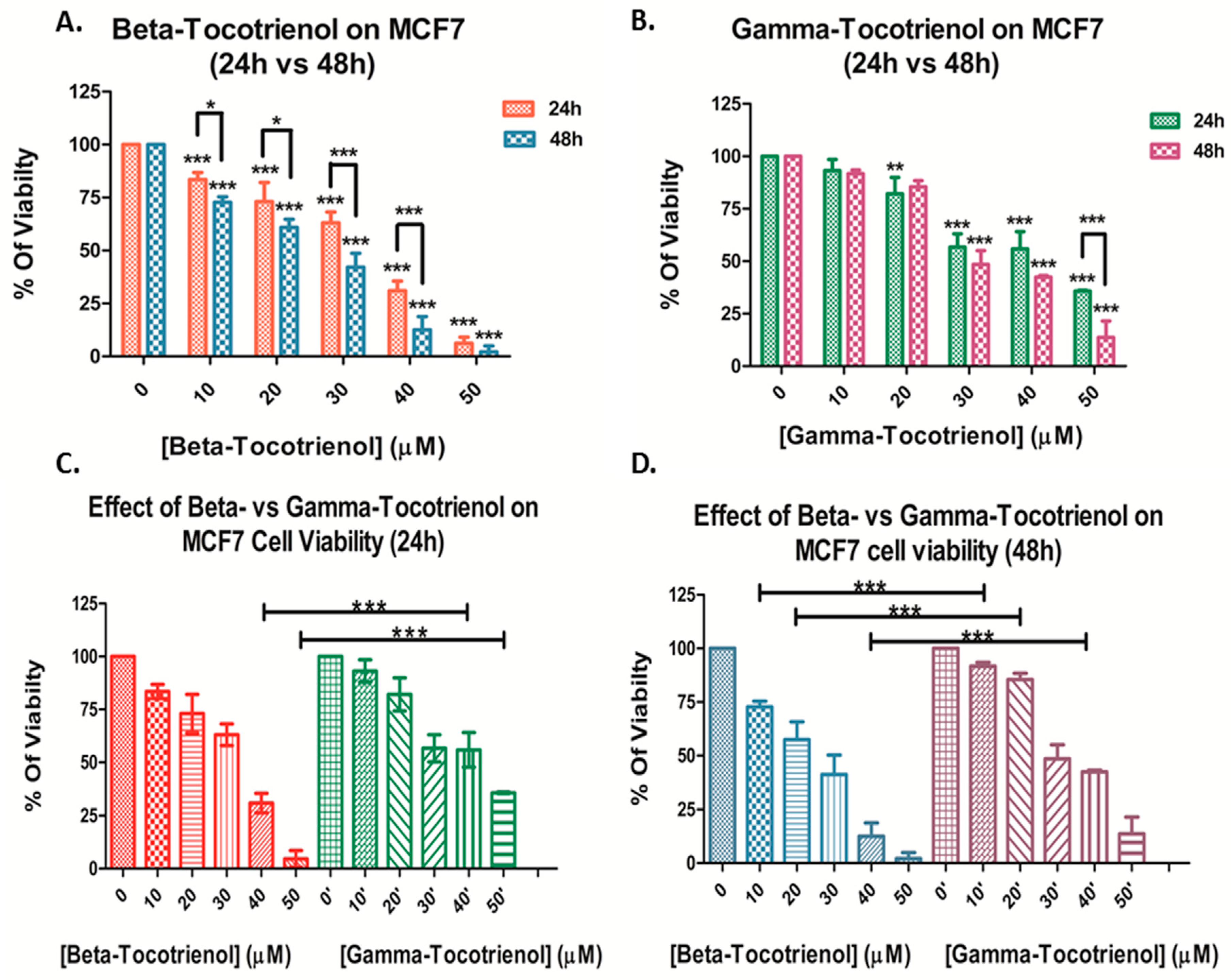
3.1. Effect of Beta- and Gamma- Tocotrienols on the Cell Proliferation of MDA-MB-231 and MCF7 cells
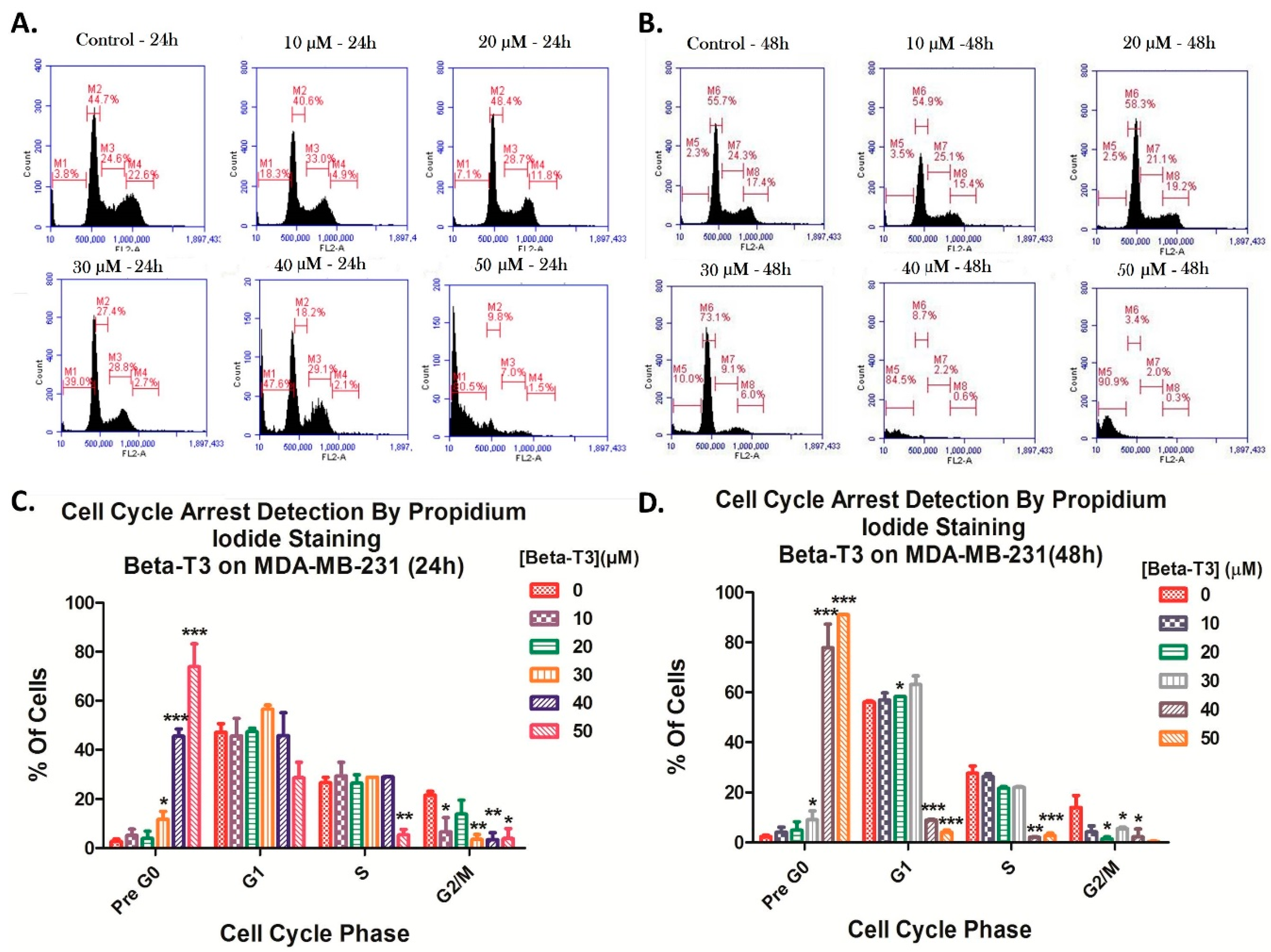
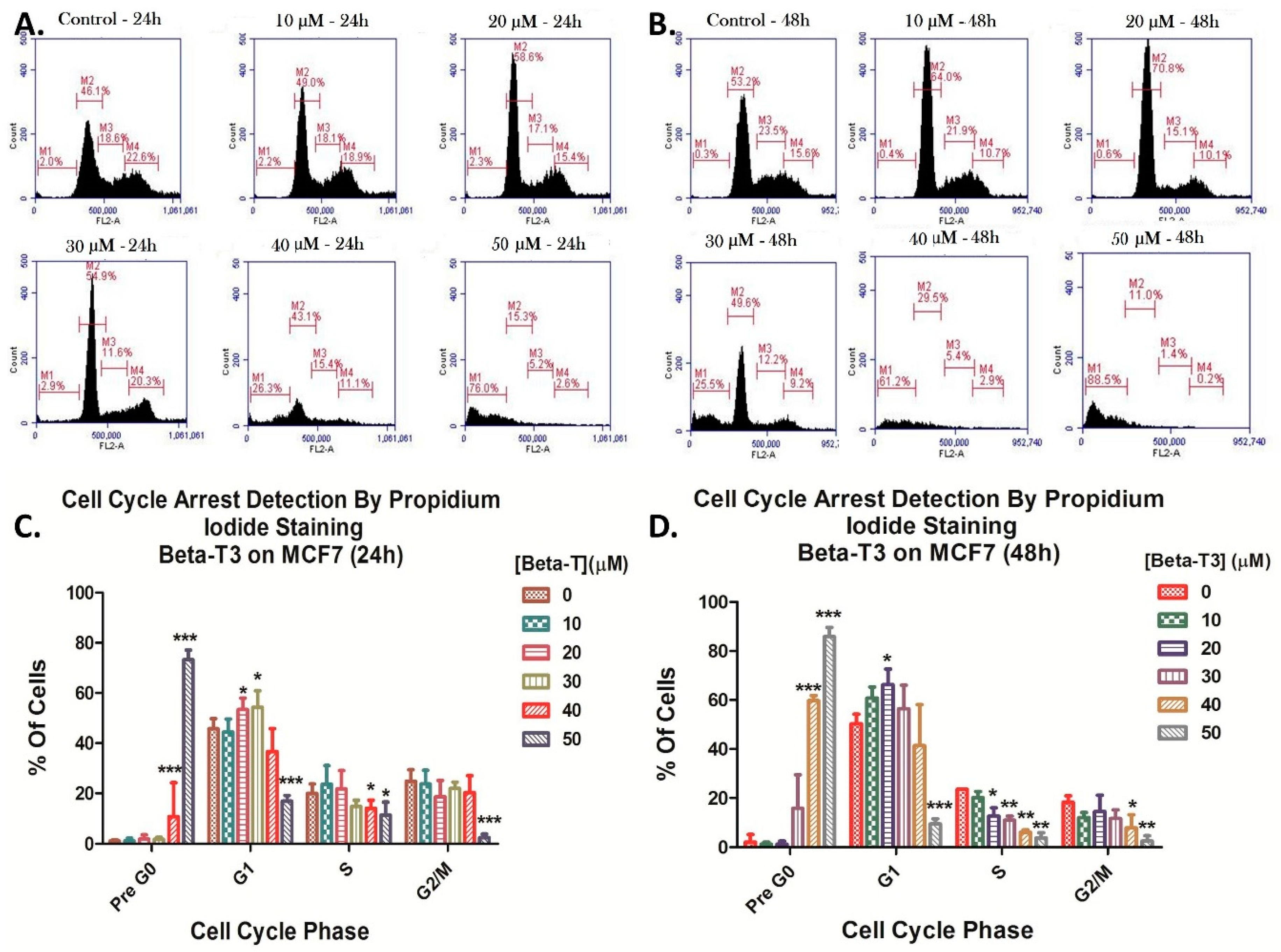
3.2. Effect of Beta-Tocotrienol on the Cell Cycle Progression of BC Cell Lines
3.3. Beta-Tocotrienol Induces Apoptosis in BC Cell Lines
3.4. The Vitamin E Beta-T3 Triggers Pro-Apoptotic Proteins Up-Regulation by a p53- Independent Mechanism in the MDA-MB-231 Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nesaretnam, K.; Meganathan, P.; Veerasenan, S.D.; Selvaduray, K.R. Tocotrienols and breast cancer: The evidence to date. Genes Nutr. 2012, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; Van Leeuwen, F.E. Subtyping of Breast Cancer by Immunohistochemistry to Investigate a Relationship between Subtype and Short and Long Term Survival: A Collaborative Analysis of Data for 10,159 Cases from 12 Studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef] [PubMed]
- Cadoo, K.A.; Fornier, M.N.; Morris, P.G. Biological subtypes of breast cancer: Current concepts and implications for recurrence patterns. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 312–321. [Google Scholar] [PubMed]
- Wu, N.; Fu, F.; Chen, L.; Lin, Y.; Yang, P.; Wang, C. Single hormone receptor-positive breast cancer patients experienced poor survival outcomes: A systematic review and meta-analysis. Clin. Transl. Oncol. 2019, 22, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Theile, K.; Bohm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, P.W. Targeting met mediated epithelial-mesenchymal transition in the treatment of breast cancer. Clin. Transl. Med. 2014, 3, 30. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Karmowski, J.; Hintze, V.; Kschonsek, J.; Killenberg, M.; Böhm, V. Antioxidant activities of tocopherols/tocotrienols and lipophilic antioxidant capacity of wheat, vegetable oils, milk and milk cream by using photochemiluminescence. Food Chem. 2015, 175, 593–600. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- Ng, M.H.; Choo, Y.M.; Ma, A.N.; Chuah, C.H.; Hashim, M.A. Separation of vitamin E (tocopherol, tocotrienol, and tocomonoenol) in palm oil. Lipids 2004, 39, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols. A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, B.; Atanasiu, V.; Cercasov, C.; Stoian, I.; Oprea, E.; Buşu, C. So many options but one choice: The human body prefers α-tocopherol. A matter of stereochemistry. J. Med. Life. 2008, 1, 376–382. [Google Scholar] [PubMed]
- Kiyose, C.; Muramatsu, R.; Kameyama, Y.; Ueda, T.; Igarashi, O. Biodiscrimination of alpha-tocopherol stereoisomers in humans after oral administration. Am. J. Clin. Nutr. 1997, 65, 785–789. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.; Tangen, C.M.; Lucia, M.S.; Goodman, P.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; Karp, D.; et al. Vitamin E and the risk of prostate cancer: Updated results of the Selenium and Vitamin E Cancer Prevention Trial (SELECT). 2012. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Vlajinac, H.D.; Marinković, J.M.; Ilić, M.D.; Kocev, N.I. Diet and prostate cancer: A case-control study. Eur. J. Cancer 1997, 33, 101–107. [Google Scholar] [CrossRef]
- Yin, Y.; Ni, J.; Chen, M.; DiMaggio, M.A.; Guo, Y.; Yeh, S. The therapeutic and preventive effect of RRR-alpha-vitamin E succinate on prostate cancer via induction of insulin-like growth factor binding protein-3. Clin. Cancer Res. 2007, 13, 2271–2280. [Google Scholar] [CrossRef]
- Shun, M.C.; Yu, W.; Gapor, A.; Parsons, R.; Atkinson, J.; Sanders, B.G.; Kline, K. Pro-apoptotic mechanisms of action of a novel vitamin E analog (alpha-TEA) and a naturally occurring form of vitamin E (delta-tocotrienol) in MDA-MB-435 human breast cancer cells. Nutr. Cancer 2004, 48, 95–105. [Google Scholar] [CrossRef]
- Wada, S.; Satomi, Y.; Murakoshi, M.; Noguchi, N.; Yoshikawa, T.; Nishino, H. Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett. 2005, 229, 181–191. [Google Scholar] [CrossRef]
- Yu, W.; Jia, L.; Park, S.K.; Li, J.; Gopalan, A.; Simmons-Menchaca, M.; Sanders, B.G.; Kline, K. Anti-cancer actions of natural and synthetic vitamin E forms: RRR-alpha- tocopherol blocks the anti-cancer actions of gamma-tocopherol. Mol. Nutr. Food Res. 2009, 53, 1573–1581. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A. Present trends in vitamin E research. BioFactors 2010, 36, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.N.; Yap, W.N.; Lee, D.T.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. Evidence of gamma-tocotrienol as an apoptosis inducing, invasion-suppressing, and chemotherapy drug-sensitizing agent in human melanoma cells. Nutr. Cancer 2009, 61, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Nakagawa, K.; Sookwong, P.; Tsuduki, T.; Tomita, S.; Shirakawa, H.; Komai, M.; Miyazawa, T. Tocotrienol inhibits secretion of angiogenic factors from human colorectal adenocarcinoma cells by suppressing hypoxia-inducible factor-1alpha. J. Nutr. 2008, 138, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yin, X.; Lill, M.A.; Danielson, M.L.; Freiser, H.; Huang, J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA 2008, 105, 20464–20469. [Google Scholar] [CrossRef]
- Samant, G.V.; Wali, V.B.; Sylvester, P.W. Anti-proliferative effects of gamma-tocotrienol on mammary tumour cells are associated with suppression of cell cycle progression. Cell Prolif. 2010, 43, 77–83. [Google Scholar] [CrossRef]
- Renuka Devi, R.; Arumughan, C. Antiradical efficacy of phytochemical extracts from defatted rice bran. Food Chem. Toxicol. 2007, 45, 2014–2021. [Google Scholar] [CrossRef]
- Minhajuddin, M.; Beg, Z.H.; Iqbal, J. Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem. Toxicol. 2005, 43, 747–753. [Google Scholar] [CrossRef]
- Montagnani Marelli, M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Moretti, R.M.; Limonta, P. Anti-cancer properties of tocotrienols: A review of cellular mechanisms and molecular targets. J. Cell. Physiol. 2019, 234, 1147–1164. [Google Scholar] [CrossRef]
- Ghanem, P.; Zouein, A.; Mohamad, M.; Hodroj, M.H.; Haykal, T.; Abou Najem, S.; Naim, H.Y.; Rizk, S. The Vitamin E Derivative Gamma Tocotrienol Promotes Anti-Tumor Effects in Acute Myeloid Leukemia Cell Lines. Nutrients 2019, 11, 2808. [Google Scholar] [CrossRef]
- Mosselman, S.; Polman, J.; Dijkema, R. ER beta: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef]
- Agarwal, M.K.; Agarwal, M.L.; Athar, M.; Gupta, S. Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle 2004, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.A.; Makpol, S.; Jamal, R.; Harun, R.; Mokhtar, N.; Ngah, W.Z.W. Tocotrienol-rich fraction, [6]-gingerol and epigallocatechin gallate inhibit proliferation and induce apoptosis of glioma cancer cells. Molecules 2014, 19, 14528–14541. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Gupta, S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem. Biophys. Res. Commun. 2006, 346, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.N.; Chang, P.N.; Han, H.Y.; Lee, D.T.W.; Ling, M.T.; Wong, Y.; Yap, Y.L. Gamma- Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br. J. Cancer 2008, 99, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-L.; Liu, J.-R.; Liu, H.-K.; Qi, G.-Y.; Sun, X.-R.; Sun, W.-G.; Chen, B.-Q. Inhibition of proliferation and induction of apoptosis by gamma-tocotrienol in human colon carcinoma HT-29 cells. Nutrition 2009, 25, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Okabe, M.; Tachibana, H.; Yamada, K. Apoptosis induction by gamma-tocotrienol in human hepatoma Hep3B cells. J. Nutr. Biochem. 2006, 17, 672–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Loo, G. Disruption of mitochondria during tocotrienol-induced apoptosis in MDA-MB-231 human breast cancer cells. Biochem. Pharmacol. 2004, 67, 315–324. [Google Scholar] [CrossRef]
- Nikolic, K.; Agababa, D. Design and QSAR study of analogs of g-tocotrienol with enhanced antiproliferative activity against human breast cancer cells. J. Mol. Graph. Model. 2009, 27, 777–783. [Google Scholar] [CrossRef]
- Tan, B.; Brzuskiewicz, L. Separation of tocopherol and tocotrienol isomers using normal- and reverse-phase liquid chromatography. Anal. Biochem. 1989, 180, 368–373. [Google Scholar] [CrossRef]
- Kooyenga, D.K.; Geller, M.; Watkins, T.R.; Gapor, A.; Diakoumakis, E.; Bierenbaum, M.L. Palm oil antioxidant effects in patients with hyperlipidaemia and carotid stenosis-2 year experience. Asia. Pac. J. Clin. Nutr. 1997, 6, 72–75. [Google Scholar]
- Shin-Kang, S.; Ramsauer, V.P.; Lightner, J.; Chakraborty, K.; Stone, W.; Campbell, S.; Reddy, S.A.; Krishnan, K. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic. Biol. Med. 2011, 51, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Samant, G.V.; Sylvester, P.W. Gamma-tocotrienol inhibits ErbB3-dependent PI3K/Akt mitogenic signaling in neoplastic mammary epithelial cells. Cell Prolif. 2006, 39, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Shirode, A.B.; Sylvester, P.W. Synergistic anti-cancer effects of combined gamma-tocotrienol and celecoxib treatment are associated with suppression in Akt and NF-kappa B signaling. Biomed. Pharmacother. 2010, 64, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Loh, H.S.; Ting, K.N.; Bradshaw, T.D.; Zeenathul, N.A. Antiproliferation and induction of caspase-8-dependent mitochondria-mediated apoptosis by β-tocotrienol in human lung and brain cancer cell lines. Biomed. Pharmacother. 2014, 68, 1105–1115. [Google Scholar] [CrossRef]
- Haykal, T.; Nasr, P.; Hodroj, M.H.; Taleb, R.I.; Sarkis, R.; Moujabber, M.N.E.; Rizk, S. Annona cherimola Seed Extract Activates Extrinsic and Intrinsic Apoptotic Pathways in Leukemic Cells. Toxins 2019, 11, 506. [Google Scholar] [CrossRef]
- Ammoury, C.; Younes, M.; El Khoury, M.; Hodroj, M.H.; Haykal, T.; Nasr, P.; Sily, M.; Taleb, R.I.; Sarkis, R.; Khalife, R.; et al. The pro-apoptotic effect of a Terpene-rich Annona cherimola leaf extract on leukemic cell lines. BMC Complement. Altern. Med. 2019, 19, 365. [Google Scholar] [CrossRef]
- Elias, A.; Shebaby, W.N.; Nehme, B.; Faour, W.; Bassil, B.S.; El Hakim, J.; Iskandar, R.; Dib-Jalbout, N.; Mroueh, M.; Daher, C.; et al. In Vitro and In Vivo Evaluation of the Anti-cancer and Anti-inflammatory Activities of 2-Himachelen-7-ol isolated from Cedrus Libani. Sci. Rep. 2019, 9, 12855. [Google Scholar] [CrossRef]
- Najem, S.A.; Khawaja, G.; Hodroj, M.H.; Rizk, S. Synergistic effect of epigenetic inhibitors Decitabine and Suberoylanilide Hydroxamic acid on colorectal Cancer in vitro. Curr. Mol. Pharmacol. 2019, 12, 281–300. [Google Scholar] [CrossRef]
- Najem, S.A.; Khawaja, G.; Hodroj, M.H.; Babikian, P.; Rizk, S. Adjuvant Epigenetic Therapy of Decitabine and Suberoylanilide Hydroxamic Acid Exerts Anti-Neoplastic Effects in Acute Myeloid Leukemia Cells. Cells 2019, 8, 1480. [Google Scholar] [CrossRef]
- Shebaby, W.; Elias, A.; Mroueh, M.; Nehme, B.; El Jalbout, N.D.; Iskandar, R.; Daher, J.C.; Zgheib, M.; Ibrahim, P.; Dwairi, V.; et al. Himachalol induces apoptosis in B16-F10 murine melanoma cells and protects against skin carcinogenesis. J. Ethnopharmacol. 2020, 243, 112545. [Google Scholar] [CrossRef]
- Hodroj, M.H.; Jardaly, A.; Raad, S.A.; Zouein, A.; Rizk, S. Andrographolide potentiates the antitumor effect of topotecan in acute myeloid leukemia cells through an intrinsic apoptotic pathway. Cancer Manag. Res. 2018, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, B.S.; Briski, K.P.; Tirmenstein, M.A.; Fariss, M.W.; Gapor, A.; Sylvester, P.W. Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids 2000, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Okabe, M.; Yamasaki, M.; Tachibana, H.; Yamada, K. Induction of apoptosis by tocotrienol in rat hepatoma dRLh-84 cells. Anti-cancer Res. 2004, 24, 1683–1688. [Google Scholar]
- Mo, H.; Elson, C.E. Apoptosis and cell-cycle arrest in human and murine tumor cells are initiated by isoprenoids. J. Nutr. 1999, 129, 804–813. [Google Scholar] [CrossRef] [PubMed]
- McAnally, J.A.; Gupta, J.; Sodhani, S.; Bravo, L.; Mo, H. Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo. Exp. Biol. Med. 2007, 232, 523–531. [Google Scholar] [CrossRef]
- Nesaretnam, K.; Stephen, R.; Dils, R.; Darbre, P. Tocotrienols inhibits the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids 1998, 33, 461–469. [Google Scholar] [CrossRef]
- McIntyre, B.S.; Briski, K.P.; Gapor, A.; Sylvester, P.W. Antiproliferative and Apoptotic Effects of Tocopherols and Tocotrienols on Preneoplastic and Neoplastic Mouse Mammary Epithelial Cells. PSEBM 2000, 224, 292–301. [Google Scholar] [CrossRef]
- Sailo, B.L.; Banik, K.; Padmavathi, G.; Javadi, M.; Bordoloi, D.; Kunnumakkara, A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018, 130, 259–272. [Google Scholar] [CrossRef]
- Ibrahim, F.; Attia, H.; Maklad, Y.; Kawkab, A.; Ramadan, M. Biochemical characterization, anti-inflammatory properties and ulcerogenic traits of some cold-pressed oils in experimental animals. Pharm. Biol. 2017, 55, 740–748. [Google Scholar] [CrossRef]
- Loganathan, R.; Selvaduray, K.R.; Nesaretnam, K.; Radhakrishnan, A.K. Tocotrienols promote apoptosis in human breast cancer cells by inducing poly(ADP-ribose) polymerase cleavage and inhibiting nuclear factor kappa-B activity. Cell Prolif. 2013, 46, 203–213. [Google Scholar] [CrossRef]
- Lerner, L.J.; Jordan, V.C. Development of anti-estrogens and their use in breast cancer: Eighth Cain memorial award lecture. Cancer Res. 1990, 50, 4177–4189. [Google Scholar] [PubMed]
- Jaiyesimi, I.A.; Buzdar, A.U.; Decker, D.A.; Hortobagyi, G.N. Use of tamoxifen for breast cancer: Twenty-eight years later. J. Clin. Oncol. 1995, 13, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Patacsil, D.; Tran, A.T.; Cho, Y.S.; Suy, S.; Saenz, F.; Malyukova, I.; Ressom, H.; Collins, S.P.; Clarke, R.; Kumar, D. Gamma-tocotrienol induced apoptosis is associated with unfolded protein response in human breast cancer cells. J. Nutr. Biochem. 2012, 23, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Nesaretnam, K.; Dorasamy, S.; Darbre, P.D. Tocotrienols inhibit growth of ZR-75-1 breast cancer cells. Int. J. Food Sci. Nutr. 2000, 51, 95–103. [Google Scholar] [CrossRef]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular Mechanisms of Action of Tocotrienols in Cancer: Recent Trends and Advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef]
- Jia, L.T.; Chen, S.Y.; Yang, A.G. Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat. Rev. 2012, 38, 868–876. [Google Scholar] [CrossRef]
- Sylvester, P.W.; Akl, M.R.; Malaviya, A.; Parajuli, P.; Roshan, S.A.; Tiwari, V.; Ayoub, N.M. Potential role of tocotrienols in the treatment and prevention of breast cancer. Biofactors 2014, 40, 49–58. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.; Rosenthal, D.; Iyer, S.; Boulares, H.; Smulson, M. Transient Poly(ADP-ribosyl)ation of Nuclear Proteins and Role of Poly(ADP-ribose) Polymerase in the Early Stages of Apoptosis. J. Biol. Chem. 1998, 273, 13703–13712. [Google Scholar] [CrossRef]
- Pierpaoli, E.; Viola, V.; Pilolli, F.; Piroddi, M.; Galli, F.; Provinciali, M. γ- and δ-tocotrienols exert a more potent anti-cancer effect than α-tocopheryl succinate on breast cancer cell lines irrespective of HER-2/neu expression. Life Sci. 2010, 86, 668–675. [Google Scholar] [CrossRef]
- Tiwari, R.V.; Parajuli, P.; Sylvester, P.W. γ-Tocotrienol-induced endoplasmic reticulum stress and autophagy act concurrently to promote breast cancer cell death. Biochem. Cell Biol. 2015, 93, 306–320. [Google Scholar] [CrossRef]
- Park, S.K.; Sanders, B.G.; Kline, K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signalling. Breast Cancer Res. Treat. 2010, 124, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Wali, V.B.; Bachawal, S.V.; Sylvester, P.W. Endoplasmic reticulum stress mediates gamma-tocotrienol-induced apoptosis in mammary tumor cells. Apoptosis 2009, 14, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Rink, C.; Roy, S. Tocotrienols: The emerging face of natural vitamin E. Vitam. Horm. 2007, 76, 203–261. [Google Scholar] [CrossRef] [PubMed]
- Nesaretnam, K.; Khor, H.T.; Ganeson, J.; Chong, Y.H.; Sundram, K.; Gapor, A. The effect of vitamin E tocotrienols from palm oil on chemically induced mammary carcinogenesis in female rats. Nutr. Res. 1992, 12, 879–892. [Google Scholar] [CrossRef]
- Sylvester, P.W.; Ayoub, N.M. Tocotrienols target PI3K/Akt signaling in anti-breast cancer therapy. Anti-cancer Agents Med. Chem. 2013, 13, 1039–1047. [Google Scholar] [CrossRef]
- Nagini S: Breast cancer: Current molecular therapeutic targets and new players. Anti-cancer Agents Med. Chem. 2017, 17, 152–163. [CrossRef]
- Woodgett, J. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990, 9, 2431–2438. [Google Scholar] [CrossRef]
- Welsh, G.; Proud, C. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem. J. 1993, 294, 625–629. [Google Scholar] [CrossRef]
- Quintayo, M.A.; Munro, A.F.; Thomas, J.; Kunkler, I.H.; Jack, W.; Kerr, G.R.; Dixon, J.M.; Chetty, U.; Barlett, J.M. GSK3β and cyclin D1 expression predicts outcome in early breast cancer patients. Breast Cancer Res. Treat. 2012, 136, 161–168. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Zhang, X.; Farrell, A.S.; Daniel, C.J.; Arnold, H.; Scanlan, C.; Laraway, B.J.; Janghorban, M.; Lum, L.; Chen, D.; Troxell, M.; et al. Mechanistic insight into Myc stabilization in breast cancer involving aberrant Axin1 expression. Proc. Natl. Acad. Sci. USA 2012, 109, 2790–2795. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.A.; Qi, Y.; Hann, S.R. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J. Biol. Chem. 2003, 278, 51606–51612. [Google Scholar] [CrossRef] [PubMed]
- Welcker, M.; Orian, A.; Jin, J.; Grim, J.E.; Harper, J.W.; Eisenman, R.N.; Clurman, B.E. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl Acad. Sci. USA 2004, 101, 9085–9090. [Google Scholar] [CrossRef]
- Hughes, K.; Nikolakaki, E.; Plyte, S.E.; Totty, N.F.; Woodgett, J.R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993, 12, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Fiol, C.J.; DePaoli-Roach, A.A.; Roach, P.J. Glycogen synthase kinase-3β is a dual specificity kinase differentially regulated by tyrosine and serine/ threonine phosphorylation. J. Biol. Chem. 1994, 269, 14566–14574. [Google Scholar] [PubMed]
- Maniam, G.; Mai, C.W.; Jusoh, M.; Zulkefeli, M.; Dufes, C.; Tan, D.M.Y.; FU, J.Y. Challenges and opportunities of nanotechnology as delivery platform for tocotrienols in cancer therapy. Front. Pharmacol. 2018, 9, 1358. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Rink, C.; Gordillo, G.M.; Khanna, S.; Gnyawali, U.; Roy, S.; Shneker, B.; Ganesh, K.; Phillips, G.; More, J.L.; et al. Oral tocotrienols are transported to human tissues and delay the progression of the model for end-stage liver disease score in patients. J. Nutr. 2012, 142, 513–519. [Google Scholar] [CrossRef]
- Yap, S.P.; Yuen, K.H.; Lim, A.B. Influence of route of administration on the absorption and disposition of alpha-, gamma- and delta-tocotrienols in rats. J. Pharm. Pharmacol. 2003, 55, 53–58. [Google Scholar] [CrossRef]
| Beta-Tocotrienol IC50 (μM) | Gamma-Tocotrienol IC50 (μM) | |||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| MDA-MB-231 | 30.0 ± 1.8 | 21.1 ± 2.8 | 39.0 ± 5.5 * | 31.0 ± 3.9 ** |
| MCF7 | 30.5 ± 2.3 | 24.3 ± 1.3 | 41.1 ± 3.9 ** | 32.9 ± 3.6 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idriss, M.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway. Biomolecules 2020, 10, 577. https://doi.org/10.3390/biom10040577
Idriss M, Hodroj MH, Fakhoury R, Rizk S. Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway. Biomolecules. 2020; 10(4):577. https://doi.org/10.3390/biom10040577
Chicago/Turabian StyleIdriss, Maya, Mohammad Hassan Hodroj, Rajaa Fakhoury, and Sandra Rizk. 2020. "Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway" Biomolecules 10, no. 4: 577. https://doi.org/10.3390/biom10040577
APA StyleIdriss, M., Hodroj, M. H., Fakhoury, R., & Rizk, S. (2020). Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway. Biomolecules, 10(4), 577. https://doi.org/10.3390/biom10040577






