Construction of Alizarin Conjugated Graphene Oxide Composites for Inhibition of Candida albicans Biofilms
Abstract
1. Introduction
2. Materials and Method
2.1. Strains and Culture Conditions
2.2. Synthesis of Dye Conjugated Graphene Oxide
2.3. Characterizations of Nanocomposites
2.4. Antifungal Evaluation
2.5. Antibiofilm Assay
2.6. Visualization of Biofilm Inhibition
2.7. Hyphae Inhibition Assay
2.8. Anti-Virulence Assay Using C. elegans as a Host
2.9. Statistical Analysis
3. Results and Discussion
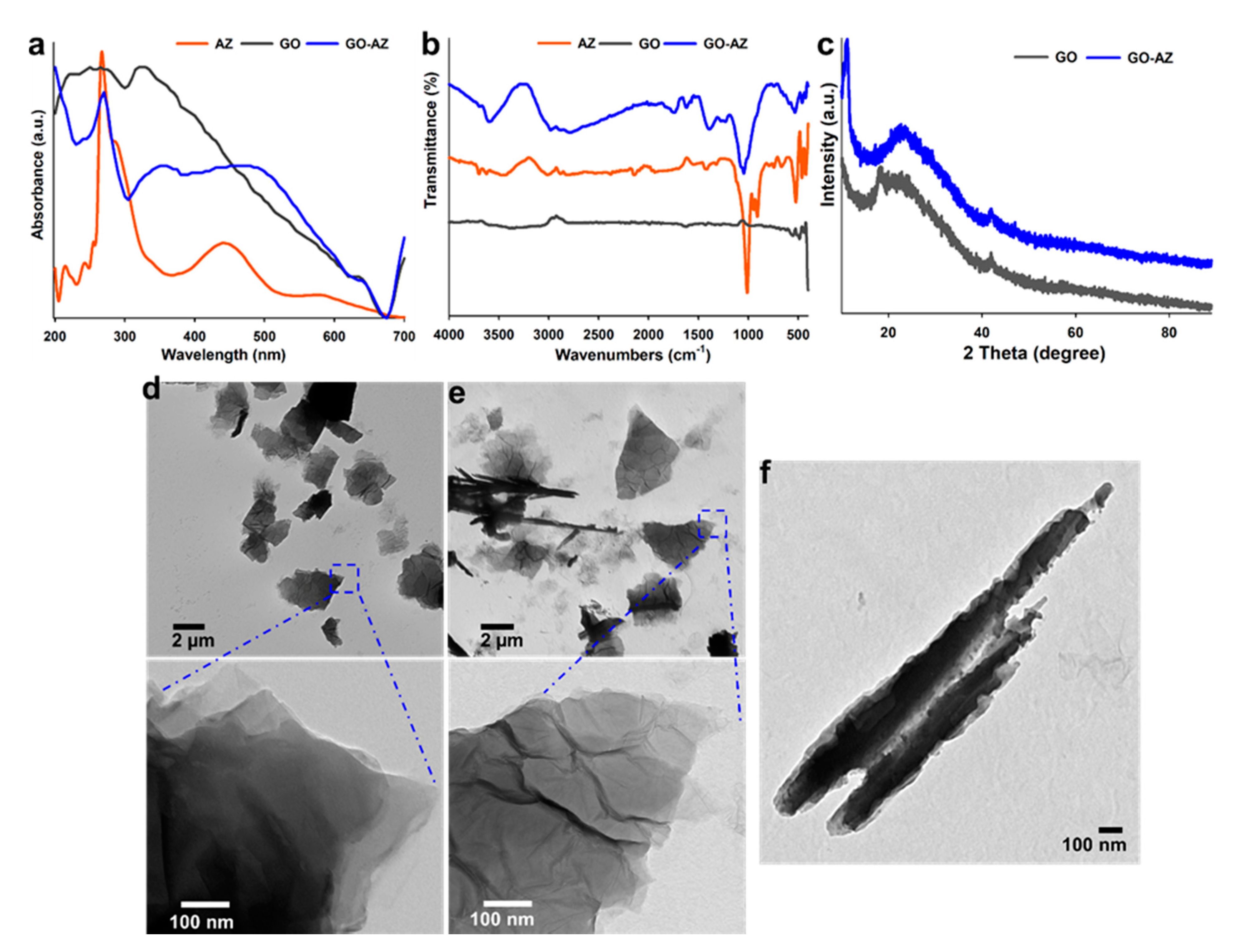
3.1. Characterizations of GO-AZ Conjugate
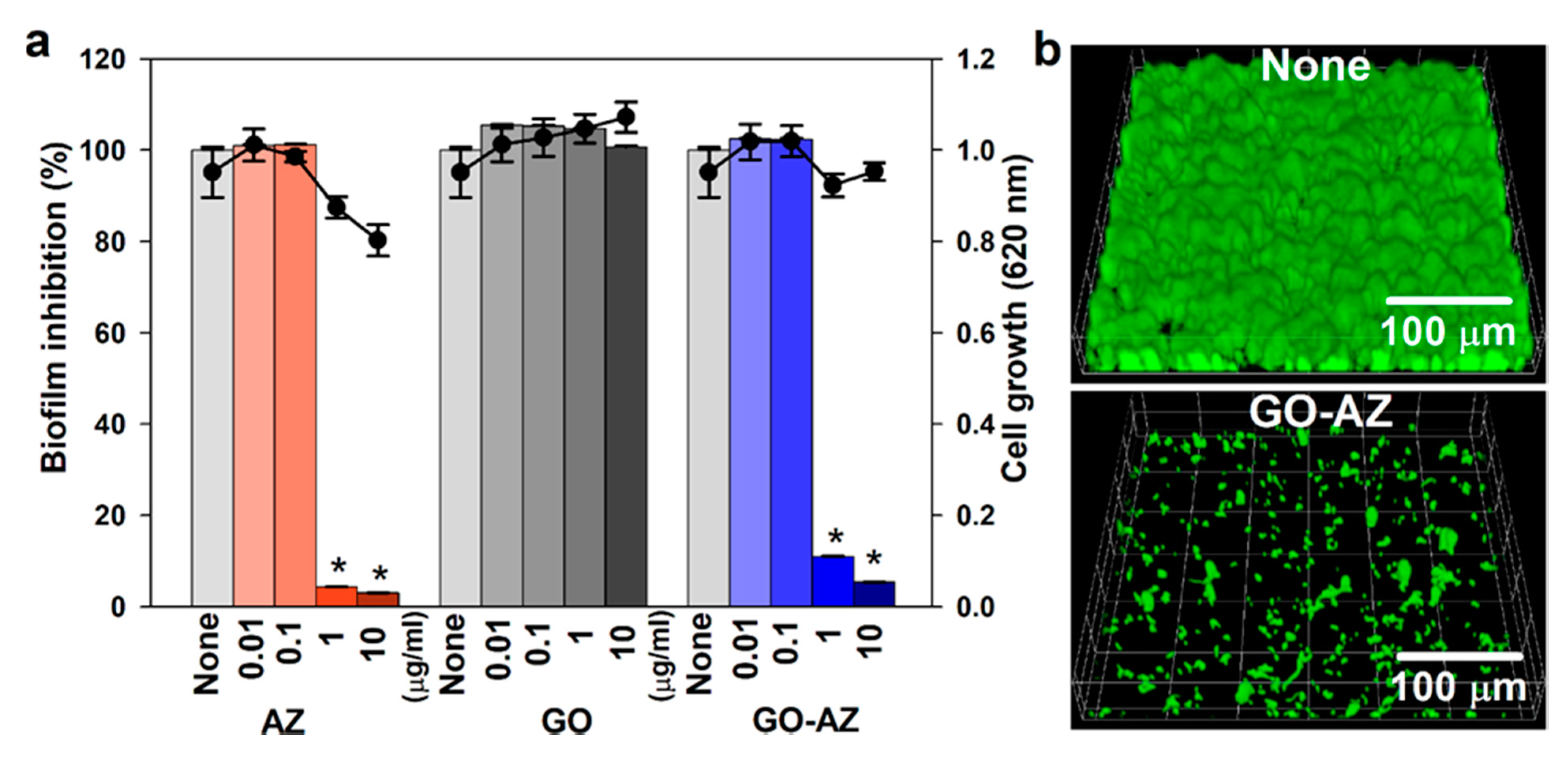
3.2. Antifungal and Antibiofilm Activities of GO-AZ Against C. albicans
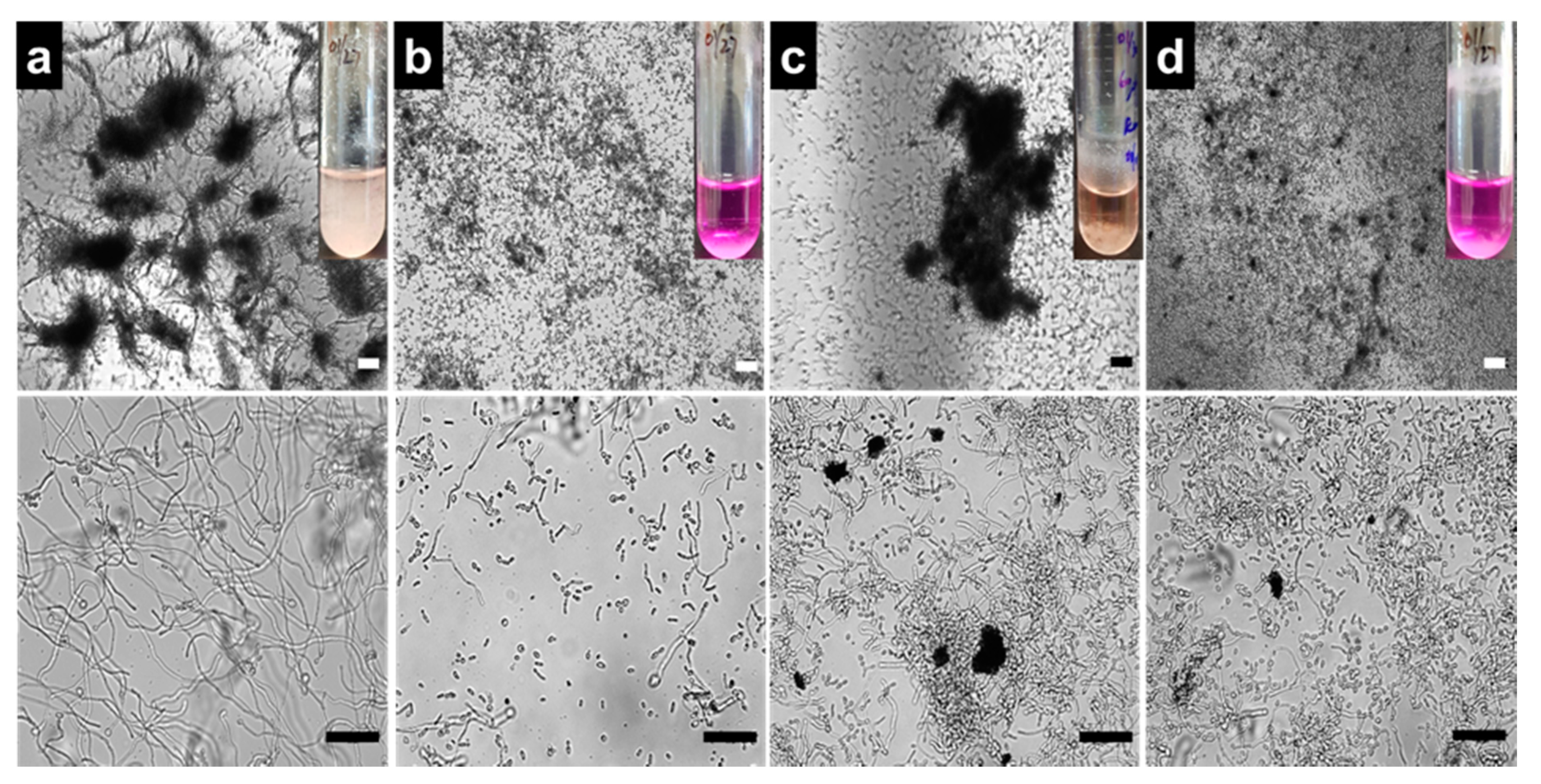
3.3. Antibiofilm Mechanism of GO-AZ via Hyphae Inhibition
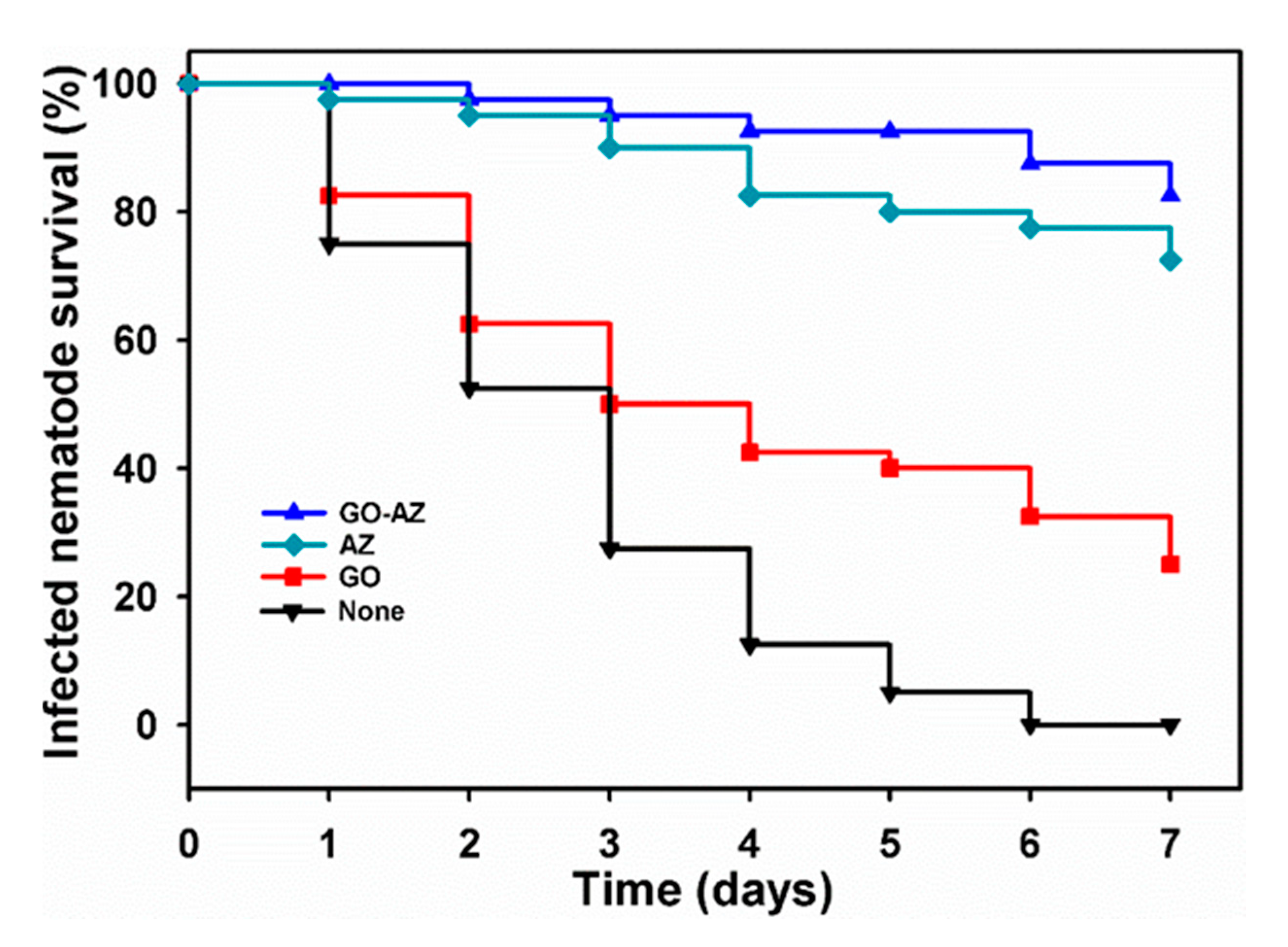
3.4. Inhibition of Candida Virulence by GO-AZ in the Nematode Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weber, K.; Sohr, R.; Schulz, B.; Fleischhacker, M.; Ruhnke, M. Secretion of E, E-farnesol and biofilm formation in eight different Candida species. Antimicrob. Agents Chemother. 2008, 52, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Krasner, R.I. The Microbial Challenge: Human-Microbe Interactions; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Ramage, G.; Martínez, J.P.; López-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Mitchell, A.P. Genetics and genomics of Candida albicans biofilm formation. Cell. Microbiol. 2006, 8, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. H 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Shunmugaperumal, T. Biofilm Eradication and Prevention. A Pharmaceutical Approach to Medical Device Infections; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Ramasamy, M.; Lee, J. Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. Biomed Res. Int. 2016, 2016, 1851242. [Google Scholar] [CrossRef]
- Mihu, M.R.; Cabral, V.; Pattabhi, R.; Tar, M.T.; Davies, K.P.; Friedman, A.J.; Martinez, L.R.; Nosanchuk, J.D. Sustained nitric oxide-releasing nanoparticles interfere with methicillin-resistant staphylococcus aureus adhesion and biofilm formation in a rat central venous catheter model. Antimicrob. Agents Chemother. 2017, 61, e02020-16. [Google Scholar] [CrossRef]
- Palmieri, V.; Bugli, F.; Cacaci, M.; Perini, G.; Maio, F.D.; Delogu, G.; Torelli, R.; Conti, C.; Sanguinetti, M.; Spirito, M.D. Graphene oxide coatings prevent Candida albicans biofilm formation with a controlled release of curcumin-loaded nanocomposites. Nanomedicine (Lond.) 2018, 13, 2867–2879. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int. J. Nanomed. 2017, 12, 2813. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Perkas, N.; Nitzan, Y.; Gedanken, A.; Banin, E. ZnO nanoparticle-coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility. RSC Adv. 2012, 2, 2314–2321. [Google Scholar] [CrossRef]
- Andrea, A.; Molchanova, N.; Jenssen, H. Antibiofilm peptides and peptidomimetics with focus on surface immobilization. Biomolecules 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Pickard, R.; Lam, T.; MacLennan, G.; Starr, K.; Kilonzo, M.; McPherson, G.; Gillies, K.; McDonald, A.; Walton, K.; Buckley, B. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: A multicentre randomised controlled trial. Lancet 2012, 380, 1927–1935. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Han, L.; Xu, J.; Wang, Z.; Jiang, L.; Wang, K.; Zhang, H.; Ren, F.; Tang, Y. Biomimetic mineralized hierarchical graphene oxide/chitosan scaffolds with adsorbability for immobilization of nanoparticles for biomedical applications. ACS Appl. Mater. Interfaces 2016, 8, 1707–1717. [Google Scholar] [CrossRef]
- Henriques, P.C.; Borges, I.; Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Fabrication and antimicrobial performance of surfaces integrating graphene-based materials. Carbon 2018, 132, 709–732. [Google Scholar] [CrossRef]
- Da Silva, G.N.S.; Primon-Barros, M.; Macedo, A.J.; Gnoatto, S.C.B. Triterpene derivatives as relevant scaffold for new antibiofilm drugs. Biomolecules 2019, 9, 58. [Google Scholar] [CrossRef]
- Bai A, J.; Vittal, R.R. Quorum sensing inhibitory and anti-biofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotechnol. 2014, 28, 269–292. [Google Scholar] [CrossRef]
- Zhu, H.; He, C.C.; Chu, Q.H. Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett. Appl. Microbiol. 2011, 52, 269–274. [Google Scholar] [CrossRef]
- Cherian, T.; Ali, K.; Saquib, Q.; Faisal, M.; Wahab, R.; Musarrat, J. Cymbopogon citratus functionalized green synthesis of CuO-nanoparticles: Novel prospects as antibacterial and antibiofilm agents. Biomolecules 2020, 10, 169. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Ryu, S.Y.; Lee, J. Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci. Rep. 2016, 6, 19267. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Alizarin and chrysazin inhibit biofilm and hyphal formation by Candida albicans. Front Cell Infect. Microbiol. 2017, 7, 447. [Google Scholar] [CrossRef]
- Nanda, S.S.; An, S.S.A.; Yi, D.K. Oxidative stress and antibacterial properties of a graphene oxide-cystamine nanohybrid. Int. J. Nanomed. 2015, 10, 549. [Google Scholar]
- Alastruey-Izquierdo, A.; Melhem, M.S.; Bonfietti, L.X.; Rodriguez-Tudela, J.L. Susceptibility test for fungi: Clinical and laboratorial correlations in medical mycology. Revista do Instituto de Medicina Tropical de São Paulo 2015, 57, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Dubey, A.; Shukla, S.; Saini, C.P.; Gupta, G.; Priyadarshini, R.; Lochab, B. Graphene oxide-coated surface: Inhibition of bacterial biofilm formation due to specific surface–interface interactions. ACS Omega 2017, 2, 3070–3082. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, M.H.; Lee, J. 3-Indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ. Microbiol. 2011, 13, 62–73. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibiofilm properties. Colloids. Surf. B Biointerfaces 2017, 160, 639–648. [Google Scholar] [CrossRef]
- An, N.; An, Y.; Hu, Z.; Guo, B.; Yang, Y.; Lei, Z. Graphene hydrogels non-covalently functionalized with alizarin: An ideal electrode material for symmetric supercapacitors. J. Mater. Chem. A 2015, 3, 22239–22246. [Google Scholar] [CrossRef]
- Chen, X.; Lai, X.; Hu, J. An easy and novel approach to prepare Fe 3 O 4–reduced graphene oxide composite and its application for high-performance lithium-ion batteries. RSC Adv. 2015, 5, 62913–62920. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Yi, H.; Wang, X.; Yan, X.; Guo, Z. Anthraquinone on porous carbon nanotubes with improved supercapacitor performance. J. Phys. Chem. C 2014, 118, 8262–8270. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, F.; Guo, Y.; Wang, S.; Liu, Y. One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities. ACS Appl. Mater. Interfaces 2013, 5, 2227–2233. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.-P.; Huang, G.; Huang, P.; Shen, G.; Guo, S.; Mei, Y.; Cui, D. Rolling up graphene oxide sheets into micro/nanoscrolls by nanoparticle aggregation. J. Mater. Chem. 2012, 22, 17441–17444. [Google Scholar] [CrossRef]
- Braga, S.F.; Coluci, V.R.; Legoas, S.B.; Giro, R.; Galvão, D.S.; Baughman, R.H. Structure and dynamics of carbon nanoscrolls. Nano Lett. 2004, 4, 881–884. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Choi, P.; Ham, J.; Park, J.G.; Lee, J. Antibiofilm and antivirulence activities of 6-gingerol and 6-shogaol against Candida albicans due to hyphal inhibition. Front Cell Infect. Microbiol. 2018, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Candida biofilms associated with CVC and medical devices. Mycoses 2012, 55, 46–57. [Google Scholar] [CrossRef]
- White, T.C.; Marr, K.A.; Bowden, R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M. Antifungal drug resistance mechanisms in pathogenic fungi: From bench to bedside. Clin. Microbiol. Infect. 2014, 20 (Suppl. S6), 54–59. [Google Scholar] [CrossRef] [PubMed]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Bar-Yosef, H.; Gonzalez, N.V.; Ben-Aroya, S.; Kron, S.J.; Kornitzer, D. Chemical inhibitors of Candida albicans hyphal morphogenesis target endocytosis. Sci. Rep. 2017, 7, 5692. [Google Scholar] [CrossRef]
- Tampakakis, E.; Okoli, I.; Mylonakis, E. A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat. Protoc. 2008, 3, 1925. [Google Scholar] [CrossRef]
- Wu, Q.; Yin, L.; Li, X.; Tang, M.; Zhang, T.; Wang, D. Contributions of altered permeability of intestinal barrier and defecation behavior to toxicity formation from graphene oxide in nematode Caenorhabditis elegans. Nanoscale 2013, 5, 9934–9943. [Google Scholar] [CrossRef] [PubMed]
- Zanni, E.; De Bellis, G.; Bracciale, M.P.; Broggi, A.; Santarelli, M.L.; Sarto, M.S.; Palleschi, C.; Uccelletti, D. Graphite nanoplatelets and Caenorhabditis elegans: Insights from an in vivo model. Nano Lett. 2012, 12, 2740–2744. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasamy, M.; Nanda, S.S.; Lee, J.-H.; Lee, J. Construction of Alizarin Conjugated Graphene Oxide Composites for Inhibition of Candida albicans Biofilms. Biomolecules 2020, 10, 565. https://doi.org/10.3390/biom10040565
Ramasamy M, Nanda SS, Lee J-H, Lee J. Construction of Alizarin Conjugated Graphene Oxide Composites for Inhibition of Candida albicans Biofilms. Biomolecules. 2020; 10(4):565. https://doi.org/10.3390/biom10040565
Chicago/Turabian StyleRamasamy, Mohankandhasamy, Sitansu Sekhar Nanda, Jin-Hyung Lee, and Jintae Lee. 2020. "Construction of Alizarin Conjugated Graphene Oxide Composites for Inhibition of Candida albicans Biofilms" Biomolecules 10, no. 4: 565. https://doi.org/10.3390/biom10040565
APA StyleRamasamy, M., Nanda, S. S., Lee, J.-H., & Lee, J. (2020). Construction of Alizarin Conjugated Graphene Oxide Composites for Inhibition of Candida albicans Biofilms. Biomolecules, 10(4), 565. https://doi.org/10.3390/biom10040565






