Spirochete Flagella and Motility
Abstract
1. Introduction
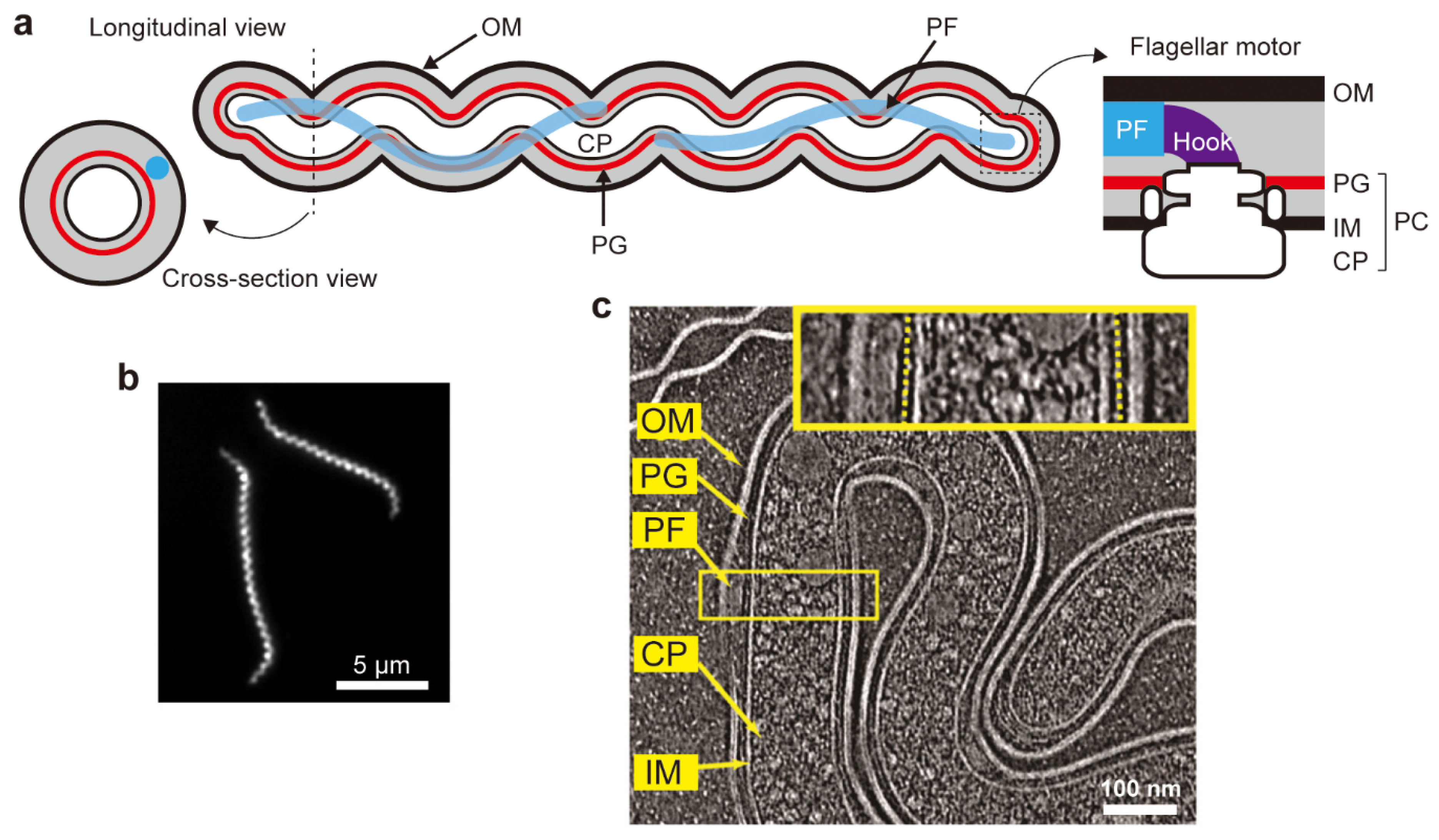
2. Cell Structure
3. Periplasmic Flagella
3.1. Physical Properties of the PF Filament
3.2. Structure of the PF Filament
3.3. Flagellar Motor
4. Swimming Motility
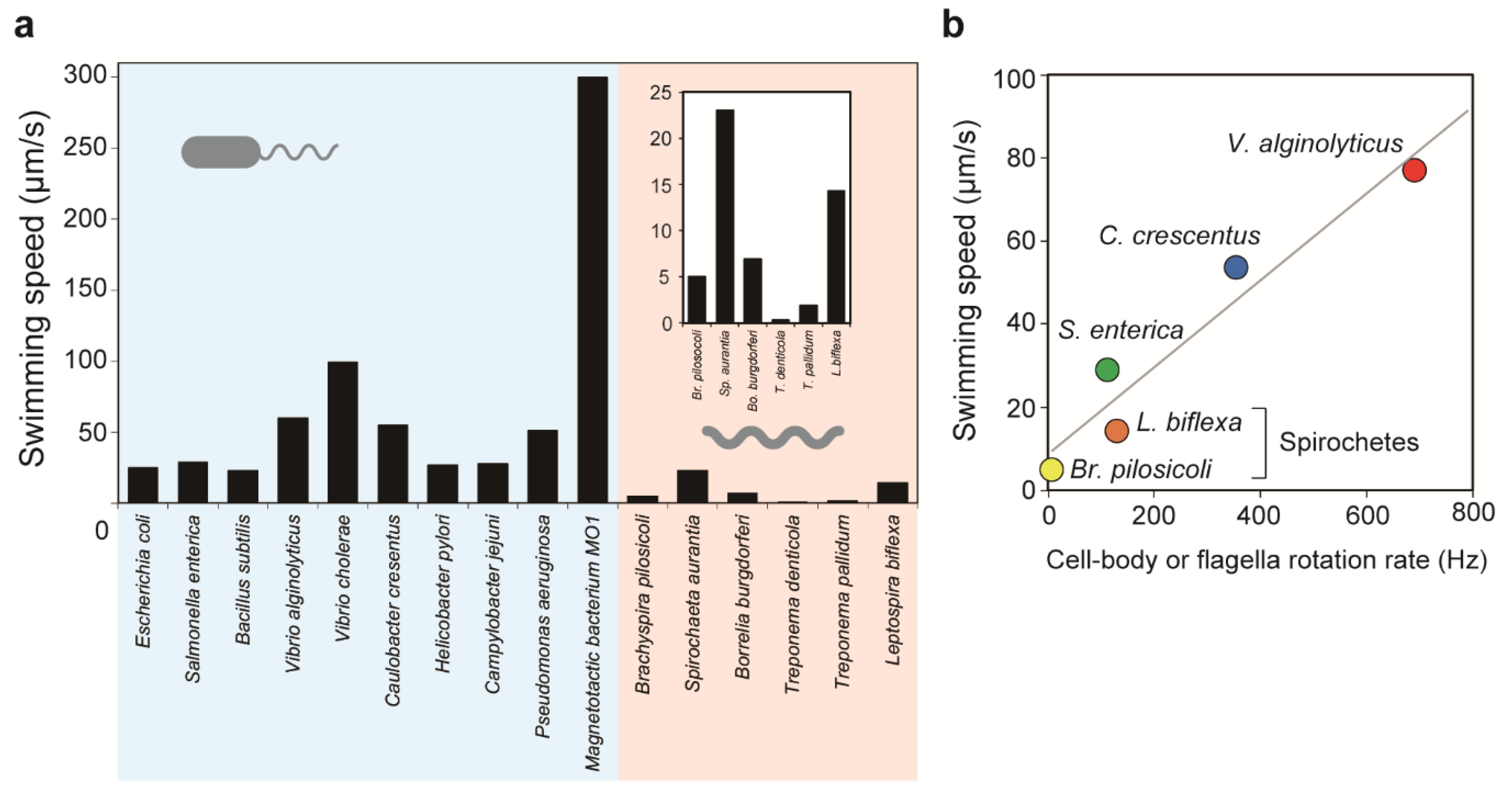
4.1. PF-Dependent Swimming
4.2. Energy Input for Spirochete Motility
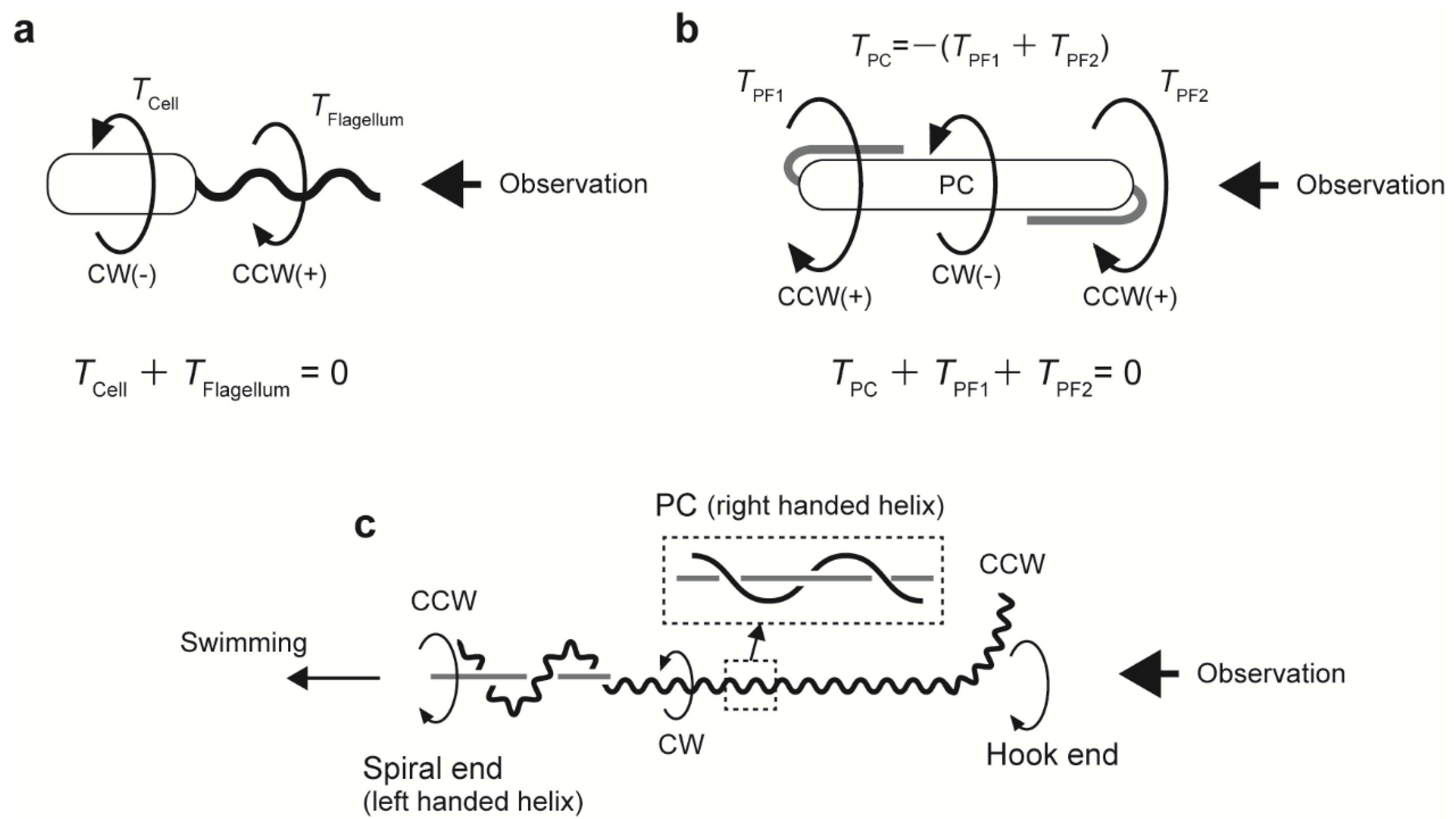
4.3. Coordinated Rotation of PFs
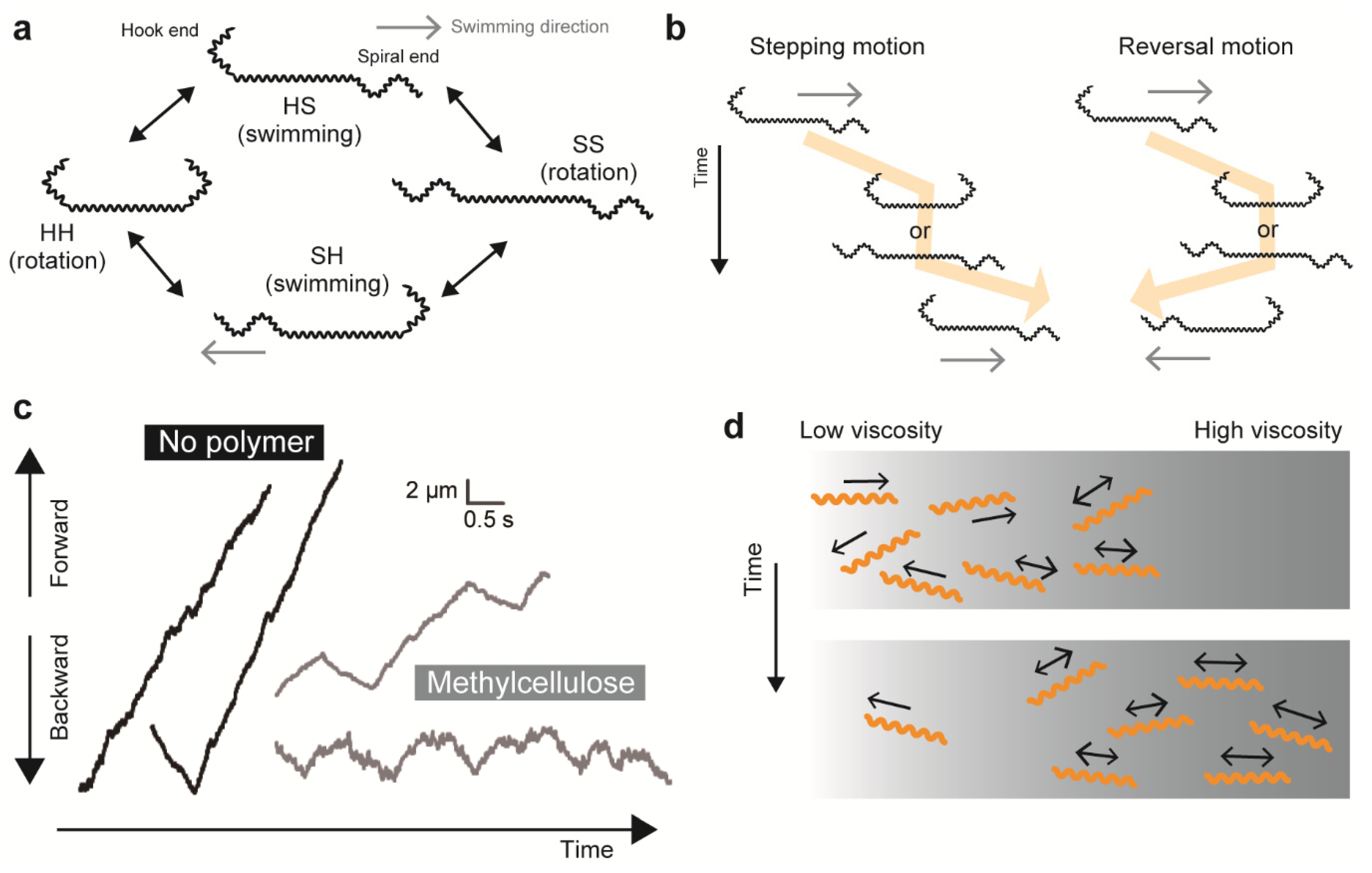
4.4. Translation Versus Rotation
4.5. Effect of Viscosity on Swimming Motility
5. Chemotaxis
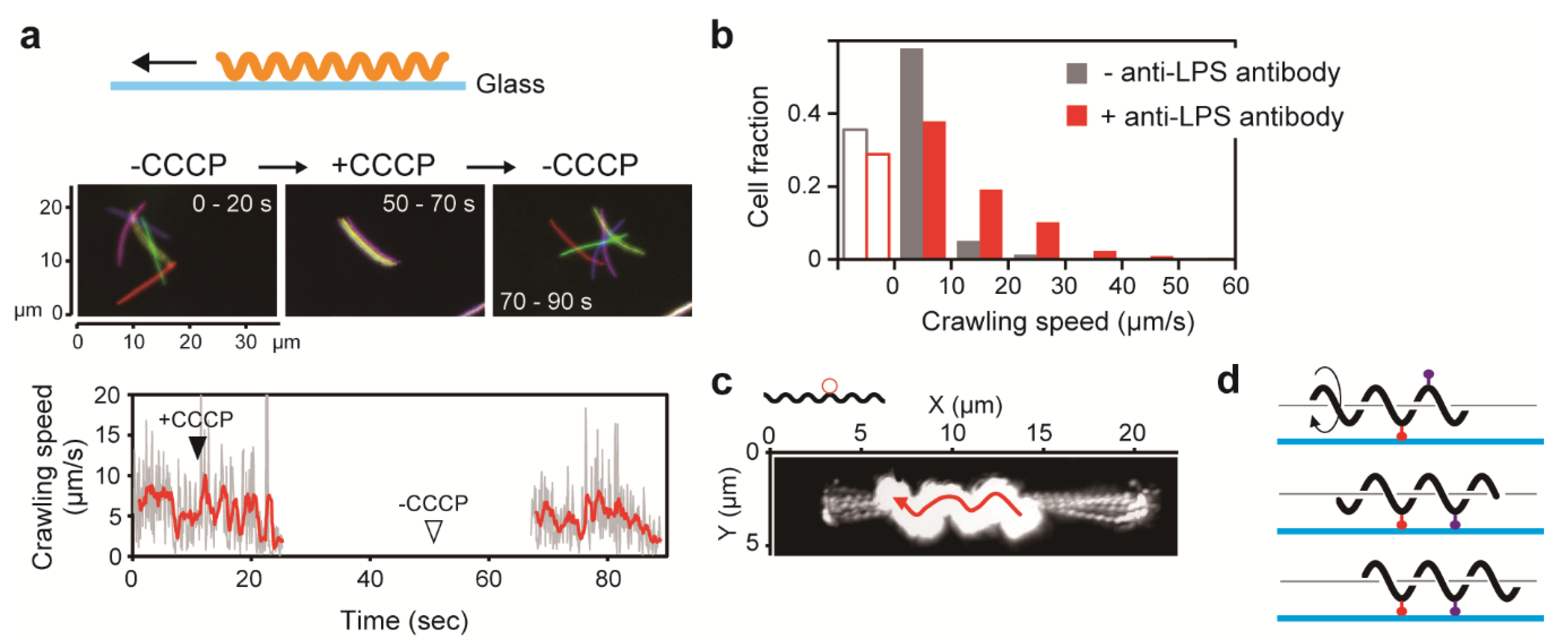
6. Movement on Solid Surfaces
7. Motility as A Virulence Factor
8. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Miyata, M.; Robinson, R.C.; Uyeda, T.Q.P.; Fukumori, Y.; Fukushima, S.; Haruta, S.; Homma, M.; Inaba, K.; Ito, M.; Kaito, C.; et al. Tree of motility – A proposed history of motility systems in the tree of life. Genes Cells 2020, 25, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, K.F.; McBride, M.J. The surprisingly diverse ways that prokaryotes move. Nat. Rev. Micro. 2008, 6, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, K.; Boquoi, T.; Hu, B.; Motaleb, M.A.; Miller, K.A.; James, M.E.; Charon, N.W.; Manson, M.D.; Norris, S.J.; et al. Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia Burgdorferi. PNAS 2013, 110, 14390–14395. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.F.; Buttle, K.F.; Charon, N.W. Structural analysis of the Leptospiraceae and Borrelia burgdorferi by high-voltage electron microscopy. J. Bacteriol. 1996, 178, 6539–6545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Charon, N.W.; Goldstein, S.F.; Marko, M.; Hsieh, C.; Gebhardt, L.L.; Motaleb, M.A.; Wolgemuth, C.W.; Limberger, R.J.; Rowe, N. The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J. Bacteriol. 2009, 191, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Charon, N.W.; Cockburn, A.; Li, C.; Liu, J.; Miller, K.A.; Miller, M.R.; Motaleb, M.A.; Wolgemuth, C.W. The unique paradigm of spirochete motility and chemotaxis. Annu. Rev. Microbiol. 2012, 66, 349–370. [Google Scholar] [CrossRef]
- Goldstein, S.F.; Charon, N.W.; Kreiling, J.A. Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proc. Natl. Acad. Sci. USA 1994, 91, 3433–3437. [Google Scholar] [CrossRef]
- Nakamura, S.; Adachi, Y.; Goto, T.; Magariyama, Y. Improvement in motion efficiency of the spirochete Brachyspira pilosicoli in viscous environments. Biophys. J. 2006, 90, 3019–3026. [Google Scholar] [CrossRef]
- Tasu, C.; Nakamura, S.; Tazawa, H.; Hara, H.; Adachi, Y. Morphological properties of a human intestinal spirochete first isolated from a patient with diarrhea in Japan. Microbiol. Immunol. 2003, 47, 989–996. [Google Scholar] [CrossRef]
- Nakamura, S.; Leshansky, A.; Magariyama, Y.; Namba, K.; Kudo, S. Direct measurement of helical cell motion of the spirochete leptospira. Biophys. J. 2014, 106, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.C.; Bromley, D.B.; Charon, N.W. Leptospiral motility. Symp. Soc. Gen. Microbiol. 1978, 28, 285–294. [Google Scholar]
- Goldstein, S.F.; Charon, N.W. Multiple-exposure photographic analysis of a motile spirochete. Proc. Natl. Acad. Sci. USA 1990, 87, 4895–4899. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Tahara, H.; Islam, M.S.; Affroze, S.; Kudo, S.; Nakamura, S. Viscosity-dependent variations in the cell shape and swimming manner of Leptospira. Microbiology 2017, 163, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Kawamoto, A.; Tahara, H.; Kudo, S.; Nakamura, S. Implications of coordinated cell-body rotations for Leptospira motility. Biochem. Biophys. Res. Commun. 2017, 491, 1040–1046. [Google Scholar] [CrossRef]
- Bromley, D.B.; Charon, N.W. Axial filament involvement in the motility of Leptospira interrogans. J. Bacteriol. 1979, 137, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Motaleb, A.; Sal, M.; Goldstein, S.F.; Charon, N.W. Spirochete periplasmic flagella and motility. J. Mol. Microbiol. Biotechnol. 2000, 2, 345–354. [Google Scholar]
- Li, C.; Corum, L.; Morgan, D.; Rosey, E.L.; Stanton, T.B.; Charon, N.W. The spirochete FlaA periplasmic flagellar sheath protein impacts flagellar helicity. J. Bacteriol. 2000, 182, 6698–6706. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wolgemuth, C.W.; Marko, M.; Morgan, D.G.; Charon, N.W. Genetic analysis of spirochete flagellin proteins and their involvement in motility, filament assembly, and flagellar morphology. J. Bacteriol. 2008, 190, 5607–5615. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kawamoto, A.; Tahara, H.; Kasuga, K.; Sato, R.; Ohnishi, M.; Nakamura, S.; Koizumi, N. Leptospiral flagellar sheath protein FcpA interacts with FlaA2 and FlaB1 in Leptospira biflexa. PLoS ONE 2018, 13, e0194923. [Google Scholar] [CrossRef]
- Lambert, A.; Picardeau, M.; Haake, D.A.; Sermswan, R.W.; Srikram, A.; Adler, B.; Murray, G.A. FlaA proteins in Leptospira interrogans are essential for motility and virulence but are not required for formation of the flagellum sheath. Infect. Immun. 2012, 80, 2019–2025. [Google Scholar] [CrossRef]
- Wunder, E.A.; Figueira, C.P.; Benaroudj, N.; Hu, B.; Tong, B.A.; Trajtenberg, F.; Liu, J.; Reis, M.G.; Charon, N.W.; Buschiazzo, A.; et al. A novel flagellar sheath protein, FcpA, determines filament coiling, translational motility and virulence for the Leptospira spirochete. Mol. Microbiol. 2016, 101, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Wunder, E.A.J.; Slamti, L.; Suwondo, D.N.; Gibson, K.H.; Shang, Z.; Sindelar, C.V.; Trajtenberg, F.; Buschiazzo, A.; Ko, A.I.; Picardeau, M. FcpB Is a surface filament protein of the endoflagellum required for the motility of the spirochete Leptospira. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.H.; Trajtenberg, F.; Wunder, E.A.; Brady, M.R.; San Martin, F.; Mechaly, A.; Shang, Z.; Liu, J.; Picardeau, M.; Ko, A.; et al. An asymmetric sheath controls flagellar supercoiling and motility in the leptospira spirochete. eLife 2020, 9, e53672. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Minamino, T. Flagella-driven motility of bacteria. Biomolecules 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.D.; Li, H.; Kuramitsu, H.; Norris, S.J.; Goldstein, S.F.; Buttle, K.F.; Charon, N.W. Relationship of Treponema denticola periplasmic flagella to irregular cell morphology. J. Bacteriol. 1997, 179, 1628–1635. [Google Scholar] [CrossRef][Green Version]
- Motaleb, M.A.; Corum, L.; Bono, J.L.; Elias, A.F.; Rosa, P.; Samuels, D.S.; Charon, N.W. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. PNAS 2000, 97, 10899–10904. [Google Scholar] [CrossRef]
- Sal, M.S.; Li, C.; Motalab, M.A.; Shibata, S.; Aizawa, S.; Charon, N.W. Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook-basal body structure. J. Bacteriol. 2008, 190, 1912–1921. [Google Scholar] [CrossRef]
- Slamti, L.; de Pedro, M.A.; Guichet, E.; Picardeau, M. Deciphering morphological determinants of the helix-shaped Leptospira. J. Bacteriol. 2011, 193, 6266–6275. [Google Scholar] [CrossRef][Green Version]
- Kan, W.; Wolgemuth, C.W. The shape and dynamics of the Leptospiraceae. Biophys. J. 2007, 93, 54–61. [Google Scholar] [CrossRef]
- Dombrowski, C.; Kan, W.; Motaleb, M.A.; Charon, N.W.; Goldstein, R.E.; Wolgemuth, C.W. The elastic basis for the shape of Borrelia burgdorferi. Biophys. J. 2009, 96, 4409–4417. [Google Scholar] [CrossRef][Green Version]
- Vig, D.K.; Wolgemuth, C.W. Swimming dynamics of the lyme disease spirochete. Phys. Rev. Lett. 2012, 109, 218104. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Miller, K.A.; Bian, J.; James, M.E.; Zhang, S.; Lynch, M.J.; Callery, P.S.; Hettick, J.M.; Cockburn, A.; Liu, J.; et al. Spirochaete flagella hook proteins self-catalyse a lysinoalanine covalent crosslink for motility. Nat. Microbiol. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Berg, H.C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef] [PubMed]
- Beeby, M.; Ribardo, D.A.; Brennan, C.A.; Ruby, E.G.; Jensen, G.J.; Hendrixson, D.R. Diverse high-torque bacterial flagellar motors assemble wider stator rings using a conserved protein scaffold. PNAS 2016, 113, E1917–E1926. [Google Scholar] [CrossRef] [PubMed]
- Lele, P.P.; Hosu, B.G.; Berg, H.C. Dynamics of mechanosensing in the bacterial flagellar motor. PNAS 2013, 110, 11839–11844. [Google Scholar] [CrossRef] [PubMed]
- Tipping, M.J.; Delalez, N.J.; Lim, R.; Berry, R.M.; Armitage, J.P. Load-dependent assembly of the bacterial flagellar motor. MBio 2013, 4, e00551-13. [Google Scholar] [CrossRef] [PubMed]
- Nord, A.L.; Gachon, E.; Perez-Carrasco, R.; Nirody, J.A.; Barducci, A.; Berry, R.M.; Pedaci, F. Catch bond drives stator mechanosensitivity in the bacterial flagellar motor. Proc. Natl. Acad. Sci. USA 2017, 114, 12952–12957. [Google Scholar] [CrossRef]
- Castillo, D.J.; Nakamura, S.; Morimoto, Y.V.; Che, Y.-S.; Kami-ike, N.; Kudo, S.; Minamino, T.; Namba, K. The C-terminal periplasmic domain of MotB is responsible for load-dependent control of the number of stators of the bacterial flagellar motor. Biophysics 2013, 9, 173–181. [Google Scholar] [CrossRef]
- Murphy, G.E.; Leadbetter, J.R.; Jensen, G.J. In situ structure of the complete Treponema primitia flagellar motor. Nature 2006, 442, 1062–1064. [Google Scholar] [CrossRef]
- Raddi, G.; Morado, D.R.; Yan, J.; Haake, D.A.; Yang, X.F.; Liu, J. Three-dimensional structures of pathogenic and saprophytic Leptospira species revealed by cryo-electron tomography. J. Bacteriol. 2012, 194, 1299–1306. [Google Scholar] [CrossRef]
- Liu, J.; Howell, J.K.; Bradley, S.D.; Zheng, Y.; Zhou, Z.H.; Norris, S.J. Cellular architecture of Treponema pallidum: Novel flagellum, periplasmic cone, and cell envelope as revealed by cryo electron tomography. J. Mol. Biol. 2010, 403, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Sowa, Y.; Berry, R.M. Bacterial flagellar motor. Q. Rev. Biophys. 2008, 41, 103–132. [Google Scholar] [CrossRef] [PubMed]
- DiLuzio, W.R.; Turner, L.; Mayer, M.; Garstecki, P.; Weibel, D.B.; Berg, H.C.; Whitesides, G.M. Escherichia coli swim on the right-hand side. Nature 2005, 435, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bakker, R.G.; Motaleb, M.A.; Sartakova, M.L.; Cabello, F.C.; Charon, N.W. Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc. Natl. Acad. Sci. USA 2002, 99, 6169–6174. [Google Scholar] [CrossRef] [PubMed]
- Thormann, K.M.; Paulick, A. Tuning the flagellar motor. Microbiology 2010, 156, 1275–1283. [Google Scholar] [CrossRef]
- Kawagishi, I.; Maekawa, Y.; Atsumi, T.; Homma, M.; Imae, Y. Isolation of the polar and lateral flagellum-defective mutants in Vibrio alginolyticus and identification of their flagellar driving energy sources. J. Bacteriol. 1995, 177, 5158–5160. [Google Scholar] [CrossRef]
- Kawagishi, I.; Imagawa, M.; Imae, Y.; McCarter, L.; Homma, M. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol. Microbiol. 1996, 20, 693–699. [Google Scholar] [CrossRef]
- Ito, M.; Hicks, D.B.; Henkin, T.M.; Guffanti, A.A.; Powers, B.D.; Zvi, L.; Uematsu, K.; Krulwich, T.A. MotPS is the stator-force generator for motility of alkaliphilic Bacillus, and its homologue is a second functional Mot in Bacillus subtilis: Alkaliphile MotPS and its B. subtilis homologue. Mol. Microbiol. 2004, 53, 1035–1049. [Google Scholar] [CrossRef]
- Ito, M.; Terahara, N.; Fujinami, S.; Krulwich, T.A. Properties of motility in Bacillus subtilis powered by the H+-coupled MotAB flagellar stator, Na+-coupled MotPS or hybrid stators MotAS or MotPB. J. Mol. Biol. 2005, 352, 396–408. [Google Scholar] [CrossRef]
- Minamino, T.; Terahara, N.; Kojima, S.; Namba, K. Autonomous control mechanism of stator assembly in the bacterial flagellar motor in response to changes in the environment. Mol. Microbiol. 2018, 109, 723–734. [Google Scholar] [CrossRef]
- Shi, W.; Yang, Z.; Geng, Y.; Wolinsky, L.E.; Lovett, M.A. Chemotaxis in Borrelia burgdorferi. J. Bacteriol. 1998, 180, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Goulbourne, E.A.; Greenberg, E.P. Relationship between proton motive force and motility in Spirochaeta aurantia. J. Bacteriol. 1980, 143, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Morimoto, Y.V.; Kudo, S.; Nakamura, S. H+ and Na+ are involved in flagellar rotation of the spirochete Leptospira. Biochem. Biophys. Res. Commun. 2015, 466, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Guasto, J.S.; Stocker, R. Bacteria can exploit a flagellar buckling instability to change direction. Nat. Phys. 2013, 9, 494–498. [Google Scholar] [CrossRef]
- Bray, D. Cell Movements: From Molecules to Motility; Garland Science: New York, NY, USA, 2000; ISBN 978-0-203-83358-2. [Google Scholar]
- Terasawa, S.; Fukuoka, H.; Inoue, Y.; Sagawa, T.; Takahashi, H.; Ishijima, A. Coordinated reversal of flagellar motors on a single Escherichia coli cell. Biophys. J. 2011, 100, 2193–2200. [Google Scholar] [CrossRef]
- Li, Z.-H.; Dong, K.; Yuan, J.-P.; Hu, B.-Y.; Liu, J.-X.; Zhao, G.-P.; Guo, X.-K. Characterization of the cheY genes from Leptospira interrogans and their effects on the behavior of Escherichia coli. Biochem. Biophys. Res. Commun. 2006, 345, 858–866. [Google Scholar] [CrossRef]
- Novak, E.A.; Sekar, P.; Xu, H.; Moon, K.H.; Manne, A.; Wooten, R.M.; Motaleb, M.A. The Borrelia burgdorferi CheY3 response regulator is essential for chemotaxis and completion of its natural infection cycle. Cell. Microbiol. 2016, 18, 1782–1799. [Google Scholar] [CrossRef]
- Motaleb, M.A.; Sultan, S.Z.; Miller, M.R.; Li, C.; Charon, N.W. CheY3 of Borrelia burgdorferi is the key response regulator essential for chemotaxis and forms a long-lived phosphorylated intermediate. J. Bacteriol. 2011, 193, 3332–3341. [Google Scholar] [CrossRef]
- Bellgard, M.I.; Wanchanthuek, P.; La, T.; Ryan, K.; Moolhuijzen, P.; Albertyn, Z.; Shaban, B.; Motro, Y.; Dunn, D.S.; Schibeci, D.; et al. Genome sequence of the pathogenic intestinal spirochete Brachyspira hyodysenteriae reveals adaptations to its lifestyle in the porcine large intestine. PLoS ONE 2009, 4, e4641. [Google Scholar] [CrossRef]
- Charon, N.W.; Daughtry, G.R.; McCuskey, R.S.; Franz, G.N. Microcinematographic analysis of tethered Leptospira illini. J. Bacteriol. 1984, 160, 1067–1073. [Google Scholar] [CrossRef]
- Segall, J.E.; Ishihara, A.; Berg, H.C. Chemotactic signaling in filamentous cells of Escherichia coli. J. Bacteriol. 1985, 161, 51–59. [Google Scholar] [CrossRef]
- Li, C.; Xu, H.; Zhang, K.; Liang, F.T. Inactivation of a putative flagellar motor switch protein FliG1 prevents Borrelia burgdorferi from swimming in highly viscous media and blocks its infectivity. Mol. Microbiol. 2010, 75, 1563–1576. [Google Scholar] [CrossRef]
- Magariyama, Y.; Sugiyama, S.; Kudo, S. Bacterial swimming speed and rotation rate of bundled flagella. FEMS Microbiol. Lett. 2001, 199, 125–129. [Google Scholar] [CrossRef]
- Minamino, T.; Imae, Y.; Oosawa, F.; Kobayashi, Y.; Oosawa, K. Effect of intracellular pH on rotational speed of bacterial flagellar motors. J. Bacteriol. 2003, 185, 1190–1194. [Google Scholar] [CrossRef]
- Faulds-Pain, A.; Birchall, C.; Aldridge, C.; Smith, W.D.; Grimaldi, G.; Nakamura, S.; Miyata, T.; Gray, J.; Li, G.; Tang, J.X.; et al. Flagellin redundancy in Caulobacter crescentus and its implications for flagellar filament assembly. J. Bacteriol. 2011, 193, 2695–2707. [Google Scholar] [CrossRef]
- Kojima, S.; Yamamoto, K.; Kawagishi, I.; Homma, M. The polar flagellar motor of Vibrio cholerae is driven by an Na+ motive force. J. Bacteriol. 1999, 181, 1927–1930. [Google Scholar] [CrossRef]
- Ruan, J.; Kato, T.; Santini, C.-L.; Miyata, T.; Kawamoto, A.; Zhang, W.-J.; Bernadac, A.; Wu, L.-F.; Namba, K. Architecture of a flagellar apparatus in the fast-swimming magnetotactic bacterium MO-1. Proc. Natl. Acad. Sci. USA 2012, 109, 20643–20648. [Google Scholar] [CrossRef]
- Takabe, K.; Nakamura, S.; Ashihara, M.; Kudo, S. Effect of osmolarity and viscosity on the motility of pathogenic and saprophytic Leptospira. Microbiol. Immunol. 2013, 57, 236–239. [Google Scholar] [CrossRef]
- Harman, M.W.; Dunham-Ems, S.M.; Caimano, M.J.; Belperron, A.A.; Bockenstedt, L.K.; Fu, H.C.; Radolf, J.D.; Wolgemuth, C.W. The heterogeneous motility of the Lyme disease spirochete in gelatin mimics dissemination through tissue. PNAS 2012, 109, 3059–3064. [Google Scholar] [CrossRef]
- Harman, M.; Vig, D.K.; Radolf, J.D.; Wolgemuth, C.W. Viscous dynamics of Lyme disease and syphilis spirochetes reveal flagellar torque and drag. Biophys. J. 2013, 105, 2273–2280. [Google Scholar] [CrossRef]
- Magariyama, Y.; Sugiyama, S.; Muramoto, K.; Kawagishi, I.; Imae, Y.; Kudo, S. Simultaneous measurement of bacterial flagellar rotation rate and swimming speed. Biophys. J. 1995, 69, 2154–2162. [Google Scholar] [CrossRef]
- Tahara, H.; Takabe, K.; Sasaki, Y.; Kasuga, K.; Kawamoto, A.; Koizumi, N.; Nakamura, S. The mechanism of two-phase motility in the spirochete Leptospira: Swimming and crawling. Sci. Adv. 2018, 4, eaar7975. [Google Scholar] [CrossRef]
- Nakamura, S.; Morimoto, Y.V.; Kami-ike, N.; Minamino, T.; Namba, K. Role of a conserved prolyl residue (Pro173) of MotA in the mechanochemical reaction cycle of the proton-driven flagellar motor of Salmonella. J. Mol. Biol. 2009, 393, 300–307. [Google Scholar] [CrossRef]
- Atsumi, T.; Maekawa, Y.; Yamada, T.; Kawagishi, I.; Imae, Y.; Homma, M. Effect of viscosity on swimming by the lateral and polar flagella of Vibrio alginolyticus. J. Bacteriol. 1996, 178, 5024–5026. [Google Scholar] [CrossRef]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Keates, S.; Ghiran, I.; Kelly, C.P.; Ewoldt, R.H.; McKinley, G.H.; So, P.; Erramilli, S.; et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14321–14326. [Google Scholar] [CrossRef]
- Apel, D.; Ellermeier, J.; Pryjma, M.; DiRita, V.J.; Gaynor, E.C. Characterization of Campylobacter jejuni RacRS reveals roles in the heat shock response, motility, and maintenance of cell length homogeneity. J. Bacteriol. 2012, 194, 2342–2354. [Google Scholar] [CrossRef]
- Shigematsu, M.; Meno, Y.; Misumi, H.; Amako, K. The measurement of swimming velocity of Vibrio cholerae and Pseudomonas aeruginosa using the video tracking methods. Microbiol. Immunol. 1995, 39, 741–744. [Google Scholar] [CrossRef]
- Greenberg, E.P.; Canale-Parola, E. Chemotaxis in Spirochaeta aurantia. J. Bacteriol. 1977, 130, 485–494. [Google Scholar] [CrossRef]
- Ruby, J.D.; Charon, N.W. Effect of temperature and viscosity on the motility of the spirochete Treponema denticola. FEMS Microbiol. Lett. 1998, 169, 251–254. [Google Scholar] [CrossRef][Green Version]
- Li, G.; Tang, J.X. Low flagellar motor torque and high swimming efficiency of Caulobacter crescentus swarmer cells. Biophys. J. 2006, 91, 2726–2734. [Google Scholar] [CrossRef]
- Kaiser, G.E.; Doetsch, R.N. Enhanced translational motion of Leptospira in viscous environments. Nature 1975, 255, 656–657. [Google Scholar] [CrossRef]
- Kimsey, R.B.; Spielman, A. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J. Infect. Dis. 1990, 162, 1205–1208. [Google Scholar] [CrossRef]
- Magariyama, Y.; Kudo, S. A Mathematical explanation of an increase in bacterial swimming speed with viscosity in linear-polymer solutions. Biophys. J. 2002, 83, 733–739. [Google Scholar] [CrossRef]
- Berg, H.C.; Turner, L. Movement of microorganisms in viscous environments. Nature 1979, 278, 349–351. [Google Scholar] [CrossRef]
- Petrino, M.G.; Doetsch, R.N. “Viscotaxis”, a new behavioural response of Leptospira interrogans (biflexa) strain B16. J. Gen. Microbiol. 1978, 109, 113–117. [Google Scholar] [CrossRef]
- Melton, T.; Hartman, P.E.; Stratis, J.P.; Lee, T.L.; Davis, A.T. Chemotaxis of Salmonella typhimurium to Amino Acids and Some Sugars. J. Bacteriol. 1978, 133, 708–716. [Google Scholar] [CrossRef]
- Tso, W.-W.; Adler, J. Negative chemotaxis in Escherichia coli. J. Bacteriol. 1974, 118, 560–576. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Yancey, R.J. Motility and chemotaxis in Serpulina hyodysenteriae. Vet. Microbiol. 1996, 49, 21–30. [Google Scholar] [CrossRef]
- Lambert, A.; Takahashi, N.; Charon, N.W.; Picardeau, M. Chemotactic behavior of pathogenic and nonpathogenic Leptospira species. Appl. Environ. Microbiol. 2012, 78, 8467–8469. [Google Scholar] [CrossRef]
- Islam, M.S.; Takabe, K.; Kudo, S.; Nakamura, S. Analysis of the chemotactic behaviour of Leptospira using microscopic agar-drop assay. FEMS Microbiol. Lett. 2014, 356, 39–44. [Google Scholar] [CrossRef]
- Affroze, S.; Islam, M.S.; Takabe, K.; Kudo, S.; Nakamura, S. Characterization of leptospiral chemoreceptors using a microscopic agar drop assay. Curr. Microbiol. 2016, 73, 202–205. [Google Scholar] [CrossRef]
- Yuri, K.; Takamoto, Y.; Okada, M.; Hiramune, T.; Kikuchi, N.; Yanagawa, R. Chemotaxis of leptospires to hemoglobin in relation to virulence. Infect. Immun. 1993, 61, 2270–2272. [Google Scholar] [CrossRef]
- Butenko, A.V.; Mogilko, E.; Amitai, L.; Pokroy, B.; Sloutskin, E. Coiled to diffuse: Brownian motion of a helical bacterium. Langmuir 2012, 28, 12941–12947. [Google Scholar] [CrossRef]
- Wall, D.; Kaiser, D. Type IV pili and cell motility. Mol. Microbiol. 1999, 32, 1–10. [Google Scholar] [CrossRef]
- Cox, P.J.; Twigg, G.I. Leptospiral motility. Nature 1974, 250, 260–261. [Google Scholar] [CrossRef]
- Miyata, M. Unique centipede mechanism of Mycoplasma gliding. Annu. Rev. Microbiol. 2010, 64, 519–537. [Google Scholar] [CrossRef]
- Faure, L.M.; Fiche, J.-B.; Espinosa, L.; Ducret, A.; Anantharaman, V.; Luciano, J.; Lhospice, S.; Islam, S.T.; Tréguier, J.; Sotes, M.; et al. The mechanism of force transmission at bacterial focal adhesion complexes. Nature 2016, 539, 530–535. [Google Scholar] [CrossRef]
- Charon, N.W.; Lawrence, C.W.; O’Brien, S. Movement of antibody-coated latex beads attached to the spirochete Leptospira interrogans. Proc. Natl. Acad. Sci. USA 1981, 78, 7166–7170. [Google Scholar] [CrossRef]
- Miyahara, S.; Saito, M.; Kanemaru, T.; Villanueva, S.Y.A.M.; Gloriani, N.G.; Yoshida, S. Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil’s disease. Int. J. Exp. Path. 2014, 95, 271–281. [Google Scholar] [CrossRef]
- Haiko, J.; Westerlund-Wikström, B. The role of the bacterial flagellum in adhesion and virulence. Biology 2013, 2, 1242. [Google Scholar] [CrossRef]
- Josenhans, C.; Suerbaum, S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Rosey, E.L.; Kennedy, M.J.; Yancey, R.J. Dual flaA1 flaB1 mutant of Serpulina hyodysenteriae expressing periplasmic flagella is severely attenuated in a murine model of swine dysentery. Infect. Immun. 1996, 64, 4154–4162. [Google Scholar] [CrossRef] [PubMed]
- Kraaz, W.; Pettersson, B.; Thunberg, U.; Engstrand, L.; Fellström, C. Brachyspira aalborgi infection diagnosed by culture and 16S ribosomal DNA sequencing using human colonic biopsy specimens. J. Clin. Microbiol. 2000, 38, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Picardeau, M. Virulence of the zoonotic agent of leptospirosis: Still terra incognita? Nat. Rev. Micro. 2017, 15, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Koizumi, N.; Nakamura, S. Adhesivity and motility of a zoonotic spirochete: Implications in host-dependent pathogenicity. bioRxiv 2020. [Google Scholar] [CrossRef]
| Species (Disease) | Cell Morphology | Cell Body Parameters | PF | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length | Width | Wavelength | Number | Shape | Overlap | Proteins | ||||
| Borrelia burgdorferi (Lyme disease) |  | Flat wave | ~20 μm | ~0.3 μm | ~2.8 μm | 14~22 | Left-handed helix | Yes | FlaA FlaB | [4,5,6,7] |
| Brachyspira hyodysenteriae (Swine dysentery) |  | Flat wave? | ~10 μm | ~0.3 μm | ~4 μm | 16~18 | Left-handed helix | Yes | FlaA FlaB1,2,3 | [8,16,17,18] |
| Leptospira interrogans (Leptospirosis) | 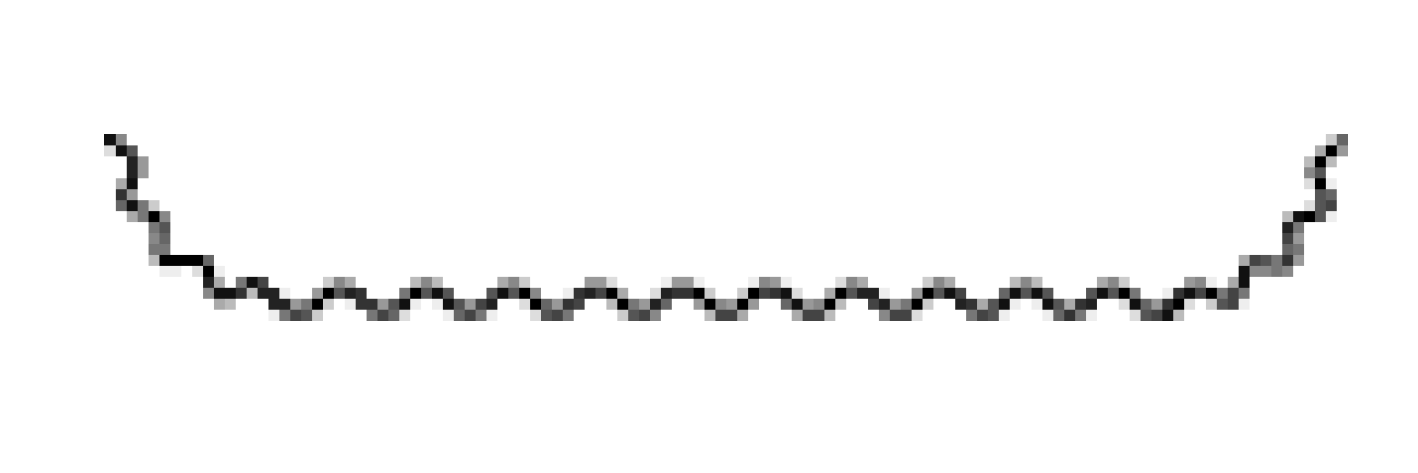 | Right-handed helix | ~20 μm | ~0.15 μm | ~0.7 μm | 2 | Coiled shape | No | FlaA1,2 FlaB1,2 FcpA, FcpB | [4,10,15,19,20,21,22,23] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S. Spirochete Flagella and Motility. Biomolecules 2020, 10, 550. https://doi.org/10.3390/biom10040550
Nakamura S. Spirochete Flagella and Motility. Biomolecules. 2020; 10(4):550. https://doi.org/10.3390/biom10040550
Chicago/Turabian StyleNakamura, Shuichi. 2020. "Spirochete Flagella and Motility" Biomolecules 10, no. 4: 550. https://doi.org/10.3390/biom10040550
APA StyleNakamura, S. (2020). Spirochete Flagella and Motility. Biomolecules, 10(4), 550. https://doi.org/10.3390/biom10040550





