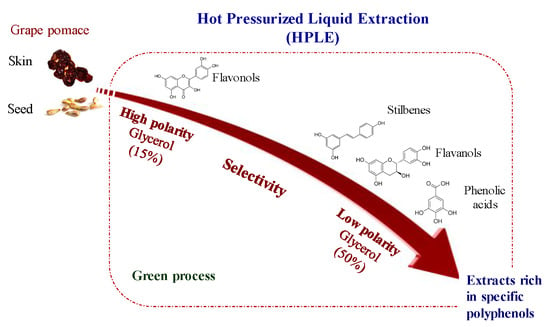
Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Pomace
2.2. Chemicals and Analytic Reagents
2.3. Hot Pressurized Liquid Extraction (HPLE) of Carménère Pomace
2.4. Quantification of Specific Polyphenols
2.5. Statistical Analysis
2.6. Computational Chemistry Calculations
3. Results and Discussions
3.1. Use of Glycerol as Alternative Co-Solvent in Hot Pressurized Liquid Extraction
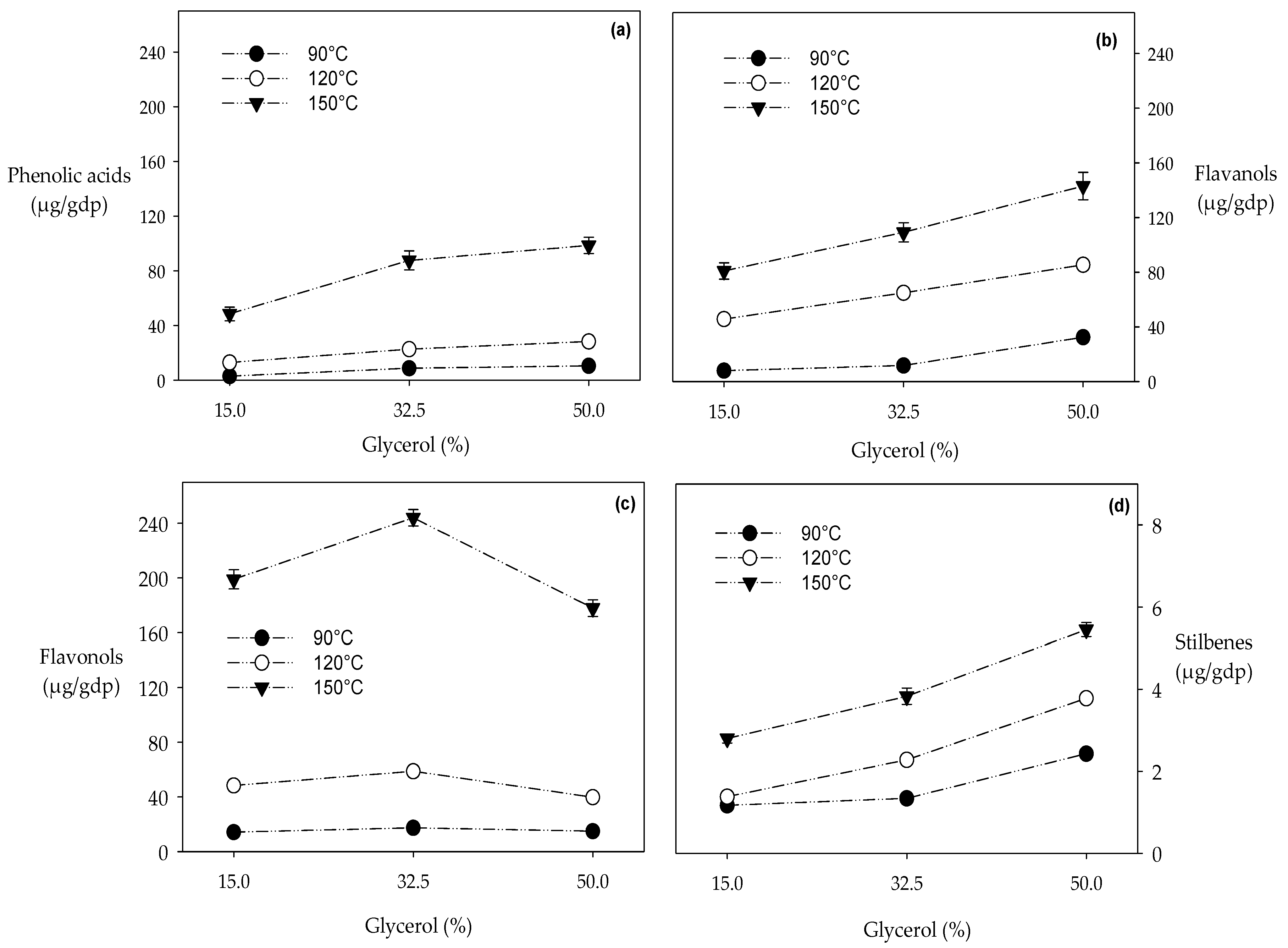
3.1.1. Phenolic Acids Extraction
3.1.2. Flavanols Extraction
3.1.3. Flavonols Extraction
3.1.4. Resveratrol Extraction
3.2. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ODEPA. Informe Ejecutivo Producción de Vinos. Available online: https://www.odepa.gob.cl/publicaciones/boletines/boletin-del-vino-agosto-de-2019 (accessed on 20 August 2019).
- Fernandez, K.; Kennedy, J.A.; Agosin, E. Characterization of Vitis vinifera L. Cv. Carménère Grape and Wine Proanthocyanidins. J. Agric. Food Chem. 2007, 55, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Polyphenols of Carménère Grapes. Mini-Reviews Org. Chem. 2017, 14, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Terracone, C. Varietal Differences among the Phenolic Profiles and Antioxidant Activities of Seven Table Grape Cultivars Grown in the South of Italy Based on Chemometrics. J. Agric. Food Chem. 2011, 59, 9815–9826. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2012, 48, 221–237. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhaes, L.; Barreiros, L.; Queiroz, J.; Cunha, L. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crop. Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, A.; López-Solís, R. Precipitation of low molecular weight phenolic compounds of grape seeds cv. Carménère (Vitis vinifera L.) by whole saliva. Eur. Food Res. Technol. 2010, 232, 113–121. [Google Scholar] [CrossRef]
- Valls, J.; Agnolet, S.; Haas, F.; Struffi, I.; Ciesa, F.; Robatscher, P.; Oberhuber, M. Valorization of Lagrein grape pomace as a source of phenolic compounds: Analysis of the contents of anthocyanins, flavanols and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 2211–2224. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.; Cabezudo, I.; Da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Grand View Research Polyphenols Market Size, Share & Trends Analysis Report By Product (Grape Seed, Green Tea, Cocoa), By Application (Beverages, Food, Feed, Dietary Supplements, Cosmetics), And Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/polyphenols-market-analysis (accessed on 10 July 2019).
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Koteswari, L.L.; Kumari, S.; Kumar, A.B.; Malla, R.R. A comparative anticancer study on procyanidin C1 against receptor positive and receptor negative breast cancer. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Hour, T.-C.; Liang, Y.-C.; Chu, I.-S.; Lin, J.-K. Inhibition of Eleven Mutagens by Various Tea Extracts, (−)Epigallocatechin-3-gallate, Gallic Acid and Caffeine. Food Chem. Toxicol. 1999, 37, 569–579. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.; Jena, B. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Kores, K.; Lesnik, S.; Bren, U.; Janez, D.; Konc, J. Discovery of Novel Potential Human Targets of Resveratrol by Inverse Molecular Docking. J. Chem. Inf. Model. 2019, 59, 2467–2478. [Google Scholar] [CrossRef]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, M.; Gottfried, C.; Lin, H.-Y.; et al. What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol. PLOS ONE 2011, 6, e19881. [Google Scholar] [CrossRef]
- Delmas, M.; Lançon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef]
- Deus, C.; Serafim, T.; Magalhaes-Novais, S.; Vilaça, A.; Moreira, A.C.; Sardão, V.A.; Cardoso, S.; Oliveira, P.J. Sirtuin 1-dependent resveratrol cytotoxicity and pro-differentiation activity on breast cancer cells. Arch. Toxicol. 2016, 91, 1261–1278. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R.B. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Dayangaç-Erden, D.; Bora, G.; Ayhan, P.; Kocaefe, C.; Dalkara, S.; Yelekci, K.; Demir, A.S.; Erdem-Yurter, H. Histone Deacetylase, Inhibition Activity and Molecular Docking of (e)-Resveratrol: Its Therapeutic Potential in Spinal Muscular Atrophy. Chem. Biol. Drug Des. 2009, 73, 355–364. [Google Scholar]
- Bhandari, R.; Kuhad, A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem. Int. 2017, 103, 8–23. [Google Scholar] [CrossRef]
- Li, F.; Gong, Q.; Dong, H.; Shi, J. Resveratrol, a Neuroprotective Supplement for Alzheimer’s Disease. Curr. Pharm. Des. 2012, 18, 27–33. [Google Scholar] [CrossRef] [PubMed]
- A Kennedy, J. Grape and wine phenolics: Observations and recent findings. Ciencia e investigación agraria 2008, 35, 77–90. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, N.; Li, L.; Zhang, Y.; Yu, L.; Jiang, L.; Sui, X. Valorization of Soy Whey Wastewater: How Epigallocatechin-3-gallate Regulates Protein Precipitation. ACS Sustain. Chem. Eng. 2019, 7, 15504–15513. [Google Scholar] [CrossRef]
- Kasprzak, K.; Oniszczuk, T.; Wójtowicz, A.; Waksmundzka-Hajnos, M.; Olech, M.; Nowak, R.; Polak, R.; Oniszczuk, A. Phenolic Acid Content and Antioxidant Properties of Extruded Corn Snacks Enriched with Kale. J. Anal. Methods Chem. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Alva-Ensastegui, J.C.; Palomar-Pardavé, M.; Romo, M.A.R.; Ramírez-Silva, M. Quercetin spectrofluorometric quantification in aqueous media using different surfactants as fluorescence promoters. RSC Adv. 2018, 8, 10980–10986. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-Sanjosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2016, 16, 3–22. [Google Scholar] [CrossRef]
- Braidot, E.; Zancani, M.; Petrussa, E.; Peresson, C.; Bertolini, A.; Patui, S.; Macrì, F.; Vianello, A. Transport and accumulation of flavonoids in grapevine (Vitis vinifera L.). Plant Signal. Behav. 2008, 3, 626–632. [Google Scholar] [CrossRef]
- Gao, Y.; Zietsman, A.; Vivier, M.A.; Moore, J. Deconstructing Wine Grape Cell Walls with Enzymes During Winemaking: New Insights from Glycan Microarray Technology. Molecules 2019, 24, 165. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of Antioxidant Phenolics from Agri-Food Waste Biomass Using a Newly Designed Glycerol-Based Natural Low-Transition Temperature Mixture: A Comparison with Conventional Eco-Friendly Solvents. Recycling 2016, 1, 194. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Jambrak, A.R.; Granato, D.; Montesano, D.; Kovačević, D.B. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Bosso, A.; Guaita, M.; Petrozziello, M. Influence of solvents on the composition of condensed tannins in grape pomace seed extracts. Food Chem. 2016, 207, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, A.; Grigorakis, S.; Makris, D.P. Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep. Purif. Technol. 2014, 128, 89–95. [Google Scholar] [CrossRef]
- Makris, D.P.; Passalidi, V.; Kallithraka, S.; Mourtzinos, I. Optimization of polyphenol extraction from red grape pomace using aqueous glycerol/tartaric acid mixtures and response surface methodology. Prep. Biochem. Biotechnol. 2016, 46, 176–182. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Tan, H.; Aziz, A.A.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391. [Google Scholar] [CrossRef]
- Jessop, P.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Picó, Y. Pressurized Liquid Extraction of Organic Contaminants in Environmental and Food Samples. In Comprehensive Analytical Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2017; Volume 76, pp. 83–110. [Google Scholar]
- Vergara-Salinas, J.; Vergara, M.; Altamirano, C.; Gonzalez, A.; Pérez-Correa, J.; Pérez-Correa, J.R. Characterization of pressurized hot water extracts of grape pomace: Chemical and biological antioxidant activity. Food Chem. 2015, 171, 62–69. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Bulnes, P.; Zúñiga, M.C.; Pérez-Jiménez, J.; Torres, J.L.; Mateos-Martín, M.L.; Agosin, E.; Pérez-Correa, J.R. Effect of Pressurized Hot Water Extraction on Antioxidants from Grape Pomace before and after Enological Fermentation. J. Agric. Food Chem. 2013, 61, 6929–6936. [Google Scholar] [CrossRef]
- Tangkhavanich, B.; Kobayashi, T.; Adachi, S. Effects of repeated treatment on the properties of rice stem extract using subcritical water, ethanol, and their mixture. J. Ind. Eng. Chem. 2014, 20, 2610–2614. [Google Scholar] [CrossRef]
- Monrad, J.K.; Howard, L.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical Solvent Extraction of Anthocyanins from Dried Red Grape Pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Otero-Pareja, M.J.; Cardoso, L.C.; Ponce, M.T.F.; Mantell, C.; De La Ossa, E.J.M. Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef] [PubMed]
- Monrad, J.K.; Howard, L.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical Solvent Extraction of Procyanidins from Dried Red Grape Pomace†. J. Agric. Food Chem. 2010, 58, 4014–4021. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.L.; Pedreschi, F.; Iglesias-Rebolledo, N.; Pérez-Correa, J.R. Impact of an integrated process of hot pressurised liquid extraction-macroporous resin purification over the polyphenols, hydroxymethylfurfural and reducing sugars content of Vitis vinifera ‘Carménère’ pomace extracts. Int. J. Food Sci. Technol. 2017, 53, 1072–1078. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Martínez-Cifuentes, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.; Pérez-Correa, J.R. The Impact of Temperature and Ethanol Concentration on the Global Recovery of Specific Polyphenols in an Integrated HPLE/RP Process on Carménère Pomace Extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef]
- Shahi, A.; Arunan, E. Hydrogen bonding, halogen bonding and lithium bonding: An atoms in molecules and natural bond orbital perspective towards conservation of total bond order, inter- and intra-molecular bonding. Phys. Chem. Chem. Phys. 2014, 16, 22935–22952. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cifuentes, M.; Weiss-López, B.E.; Santos, L.S.; Araya-Maturana, R. Intramolecular Hydrogen Bond in Biologically Active o-Carbonyl Hydroquinones. Molecules 2014, 19, 9354–9368. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cifuentes, M.; Cardona, W.; Saitz-Barría, C.; Weiss-López, B.E.; Araya-Maturana, R. A Study about Regioisomeric Hydroquinones with Multiple Intramolecular Hydrogen Bonding. Molecules 2017, 22, 593. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Zhang, J.; Wang, Q.; Wang, F.; Qiao, Y.; Zhang, Y. Rapid determination of ten polyphenols in Kudiezi injection using ultra-performance liquid chromatography-tandem mass spectrometry in multiple reaction monitoring mode. Anal. Methods 2012, 4, 4230. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, G.E.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, UK, 2009. [Google Scholar]
- García-Marino, M.; Rivas-Gonzalo, J.-C.; Ibáñez, E.; García-Moreno, C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal. Chim. Acta 2006, 563, 44–50. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine (NIH). Flavanols Chemical Structure. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ (accessed on 18 August 2019).
- Wijngaard, H.H.; Brunton, N. The Optimization of Extraction of Antioxidants from Apple Pomace by Pressurized Liquids. J. Agric. Food Chem. 2009, 57, 10625–10631. [Google Scholar] [CrossRef]
- Cheigh, C.-I.; Yoo, S.-Y.; Ko, M.-J.; Chang, P.-S.; Chung, M.-S. Extraction characteristics of subcritical water depending on the number of hydroxyl group in flavonols. Food Chem. 2015, 168, 21–26. [Google Scholar] [CrossRef]
- Furlan, V.; Bren, U. Protective Effects of [6]-Gingerol Against Chemical Carcinogens: Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 695. [Google Scholar] [CrossRef]
- Hostnik, G.; Gladovic, M.; Bren, U. Tannin Basic Building Blocks as Potential Scavengers of Chemical. Carcinogens: A Computational Study. J. Nat. Prod. 2019, 82, 3279–3287. [Google Scholar] [CrossRef]
- Xu, J.; Deng, G.; Wang, Y.-T.; Guo, H.-Y.; Kalhor, P.; Yu, Z.-W. Local Acid Strength of Solutions and Its Quantitative Evaluation Using Excess Infrared Nitrile Probes. J. Phys. Chem. Lett. 2020, 11, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cifuentes, M.; Monroy-Cárdenas, M.; Millas-Vargas, J.P.; Weiss-López, B.E.; Araya-Maturana, R. Assessing Parameter Suitability for the Strength Evaluation of Intramolecular Resonance Assisted Hydrogen Bonding in o-Carbonyl Hydroquinones. Molecules 2019, 24, 280. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, J.L.; Sanli, N.; Fonrodona, G.; Barrón, D.; Özkan, G.; Barbosa, J. Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile–water media. Anal. Chimica Acta 2003, 484, 253–264. [Google Scholar] [CrossRef]
- Jabbari, M. Solvent dependence of protonation equilibria for gallic acid in water and different acetonitrile–water cosolvent systems. J. Mol. Liq. 2015, 208, 5–10. [Google Scholar] [CrossRef]

| Description | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 90 °C | 120 °C | 150 °C | |||||||
| Glycerol (%) | Glycerol (%) | Glycerol (%) | |||||||
| 15% | 32.5% | 50% | 15% | 32.5% | 50% | 15% | 32.5% | 50% | |
| Acids (µg/gdp) | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV |
| Gallic | 1.99a 0.06 | 4.53b 0.09 | 5.54b 0.08 | 7.42a 0.11 | 15.35b 0.09 | 19.69c 0.11 | 37.48a 0.11 | 67.86b 0.08 | 70.84c 0.10 |
| Chlorogenic | 0.88a 0.07 | 0.93a 0.08 | 0.45b 0.06 | 1.03 a 0.04 | 0.85a 0.05 | 0.17a 0.09 | 2.87a 0.12 | 3.04a 0.11 | 2.25a 0.07 |
| Vanillic | ND | 2.37 a 0.03 | 3.31a 0.07 | 2.91a 0.06 | 4.37a 0.08 | 7.40b 0.02 | 5.14a 0.04 | 12.75b 0.09 | 19.15c 0.05 |
| Caffeic | ND | 0.81a 0.10 | 0.93a 0.11 | 0.91a 0.10 | 1.14a 0.10 | 0.68a 0.12 | 1.28a 0.09 | 3.39b 0.09 | 5.32c 0.07 |
| Ferulic | ND | ND | 0.14 0.08 | 0.56a 0.03 | 0.86a 0.06 | 0.29a 0.09 | 0.65a 0.05 | 0.52a 0.11 | 0.97a 0.13 |
| Σ: | 2.87 a 0.08 | 8.64 b 0.08 | 10.37b 0.07 | 12.83a 0.08 | 22.57b 0.08 | 28.21c 0.08 | 48.42a 0.09 | 87.56b 0.10 | 98.53c 0.09 |
| Flavanols (µg/gdp) | |||||||||
| Catechin | 1.79a 0.06 | 3.63b 0.06 | 12.14c 0.09 | 9.25a 0.09 | 15.19b 0.11 | 25.20c 0.09 | 22.43a 0.05 | 29.06b 0.08 | 39.26c 0.11 |
| Epicatechin | 1.71a 0.09 | 3.21b 0.08 | 8.50c 0.07 | 10.35a 0.06 | 14.75b 0.08 | 16.50b 0.11 | 13.15 0.05 | 22.17 0.04 | 19.92 0.09 |
| Epigallocatechin | 4.44a 0.05 | 4.85a 0.04 | 11.75b 0.06 | 26.11a 0.10 | 34.96b 0.08 | 43.72c 0.12 | 45.34a 0.11 | 57.94b 0.12 | 83.85c 0.10 |
| Σ: | 7.94 a 0.07 | 11.70b 0.06 | 32. 39c 0.07 | 45.71a 0.09 | 64.90b 0.09 | 85.42c 0.11 | 80.92a 0.06 | 109.17b 0.08 | 143.03c 0.10 |
| Flavonols (µg/gdp) | |||||||||
| Quercetin | 12.28a 0.04 | 14.53b 0.06 | 13.46a,b 0.07 | 39.77a 0.10 | 47.26b 0.09 | 35.53c 0.11 | 184.35a 0.06 | 257.60b 0.12 | 166.95c 0.11 |
| Kaempherol | 1.93a 0.06 | 2.79a 0.06 | 1.41a 0.03 | 8.58a 0.09 | 11.36b 0.13 | 4.24c 0.06 | 14.58a 0.09 | 26.36b 0.13 | 11.24c 0.06 |
| Σ: | 14.21a 0.05 | 17.32b 0.06 | 14.87a 0.04 | 48.35a 0.09 | 58.62b 0.11 | 39.77c 0.08 | 198.93a 0.08 | 243.96b 0.13 | 177.89c 0.08 |
| Stilbenes (µg/gdp) | |||||||||
| Resveratrol | 1.17a 0.08 | 2.34a 0.06 | 2.43a 0.04 | 1.38a 0.06 | 2.18a,b 0.08 | 3.78b 0.09 | 2.80a 0.08 | 3.83a 0.09 | 5.45b 0.06 |
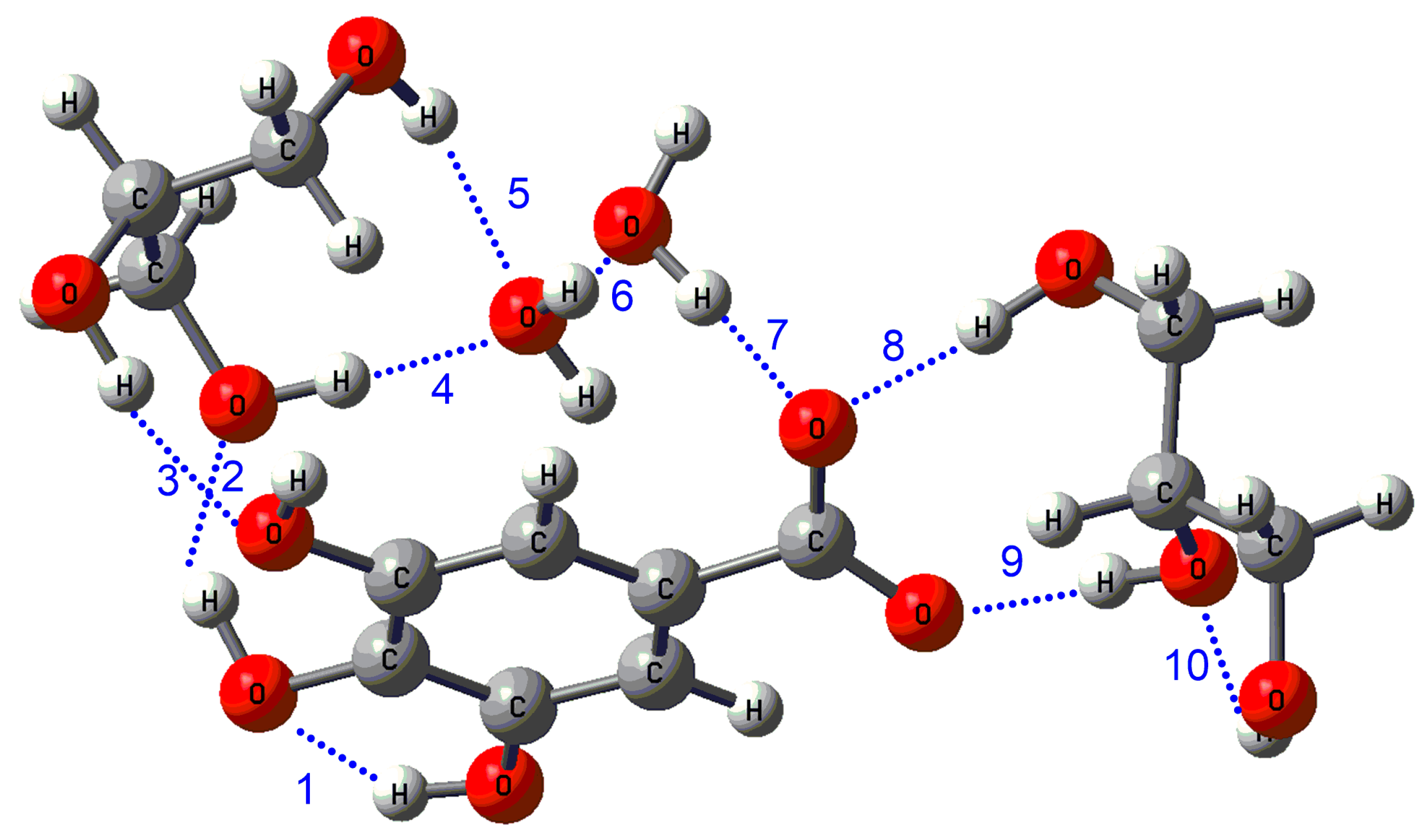
| Hydrogen Bond Distances (Å) | Hydrogen Bond ΔEij(2) (kcal) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | 5 | 6 | 7 | 8 | 9 | 10 | 5 | 6 | 7 | 8 | 9 | 10 | ∑ |
| A | 1.59 | 1.81 | 1.76 | − | − | − | 38.73 | 13.90 | 17.57 | − | − | − | 70.20 |
| B | 1.60 | 1.84 | 1.72 | − | − | − | 36.77 | 11.95 | 22.75 | − | − | − | 71.47 |
| C | 1.63 | 1.84 | 1.79 | 1.88 | − | − | 33.76 | 12.03 | 18.07 | 9.54 | − | − | 73.40 |
| D | 1.55 | 2.07 | 1.74 | 2.07 | 1.89 | 2.12 | 44.58 | 3.46 | 20.31 | 2.44 | 5.76 | 2.95 | 79.50 |
| Hydrogen Bond (HB) | Distances (Å) | ΔEij(2) (kcal) |
|---|---|---|
| 1 | 2.11 | 2.48 |
| 2 | 1.84 | 11.55 |
| 3 | 2.04 | 5.24 |
| 4 | 1.79 | 17.94 |
| 5 | 2.04 | 3.52 |
| 6 | 1.70 | 24.10 |
| 7 | 1.64 | 23.54 |
| 8 | 1.78 | 16.17 |
| 9 | 1.77 | 20.43 |
| 10 | 2.10 | 2.96 |
| ∑=127.93 |
| Solvent Mixture | ∑Total (kcal) | ∑carboxylate group (kcal) |
|---|---|---|
| water-methanol | 74.21 | 42.88 |
| water-ethanol | 70.88 | 42.66 |
| water-ethylene glycol | 85.84 | 43.33 |
| water-glycerol | 127.93 | 60.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. https://doi.org/10.3390/biom10030474
Huamán-Castilla NL, Mariotti-Celis MS, Martínez-Cifuentes M, Pérez-Correa JR. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules. 2020; 10(3):474. https://doi.org/10.3390/biom10030474
Chicago/Turabian StyleHuamán-Castilla, Nils Leander, María Salomé Mariotti-Celis, Maximiliano Martínez-Cifuentes, and José Ricardo Pérez-Correa. 2020. "Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations" Biomolecules 10, no. 3: 474. https://doi.org/10.3390/biom10030474
APA StyleHuamán-Castilla, N. L., Mariotti-Celis, M. S., Martínez-Cifuentes, M., & Pérez-Correa, J. R. (2020). Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules, 10(3), 474. https://doi.org/10.3390/biom10030474