The TGFβ Family in Human Placental Development at the Fetal-Maternal Interface
Abstract
1. Human Fetal-Maternal Interface
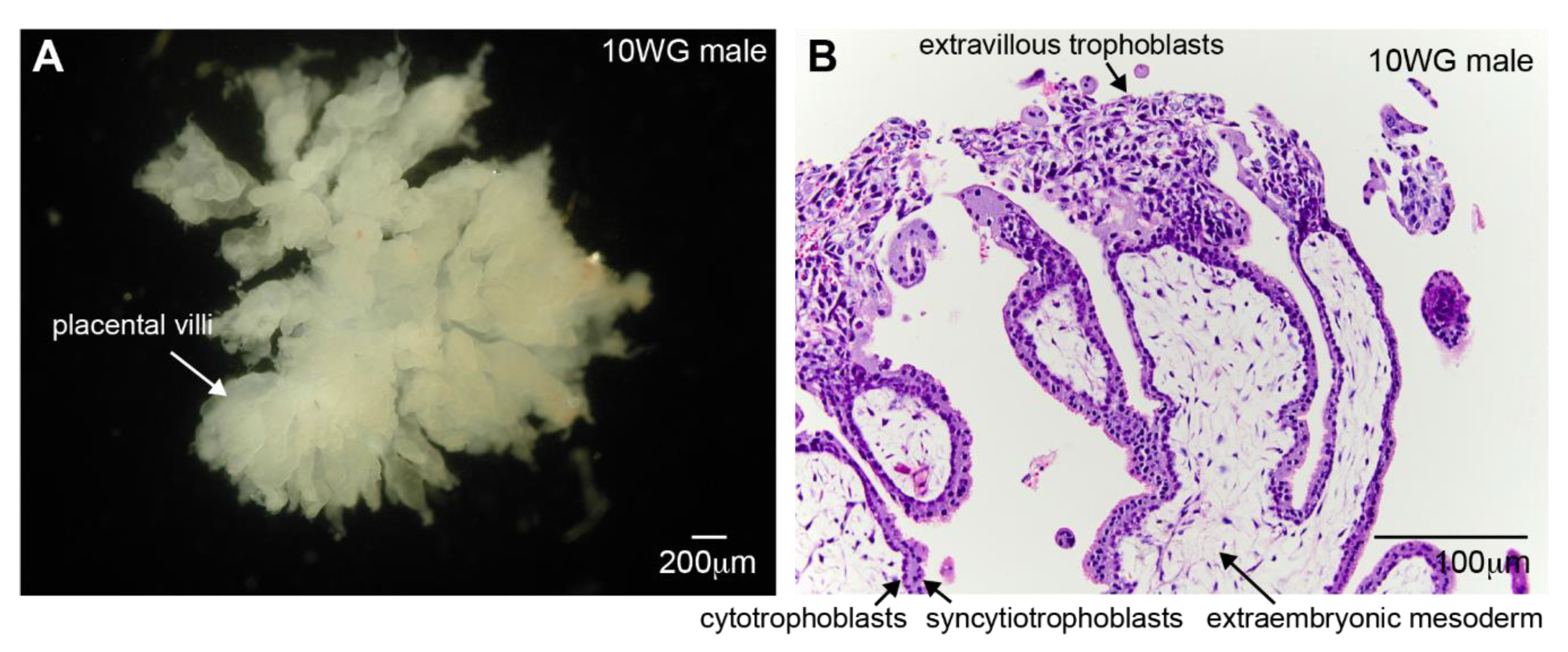
1.1. Placental Development
1.2. The Uterus During Human Development
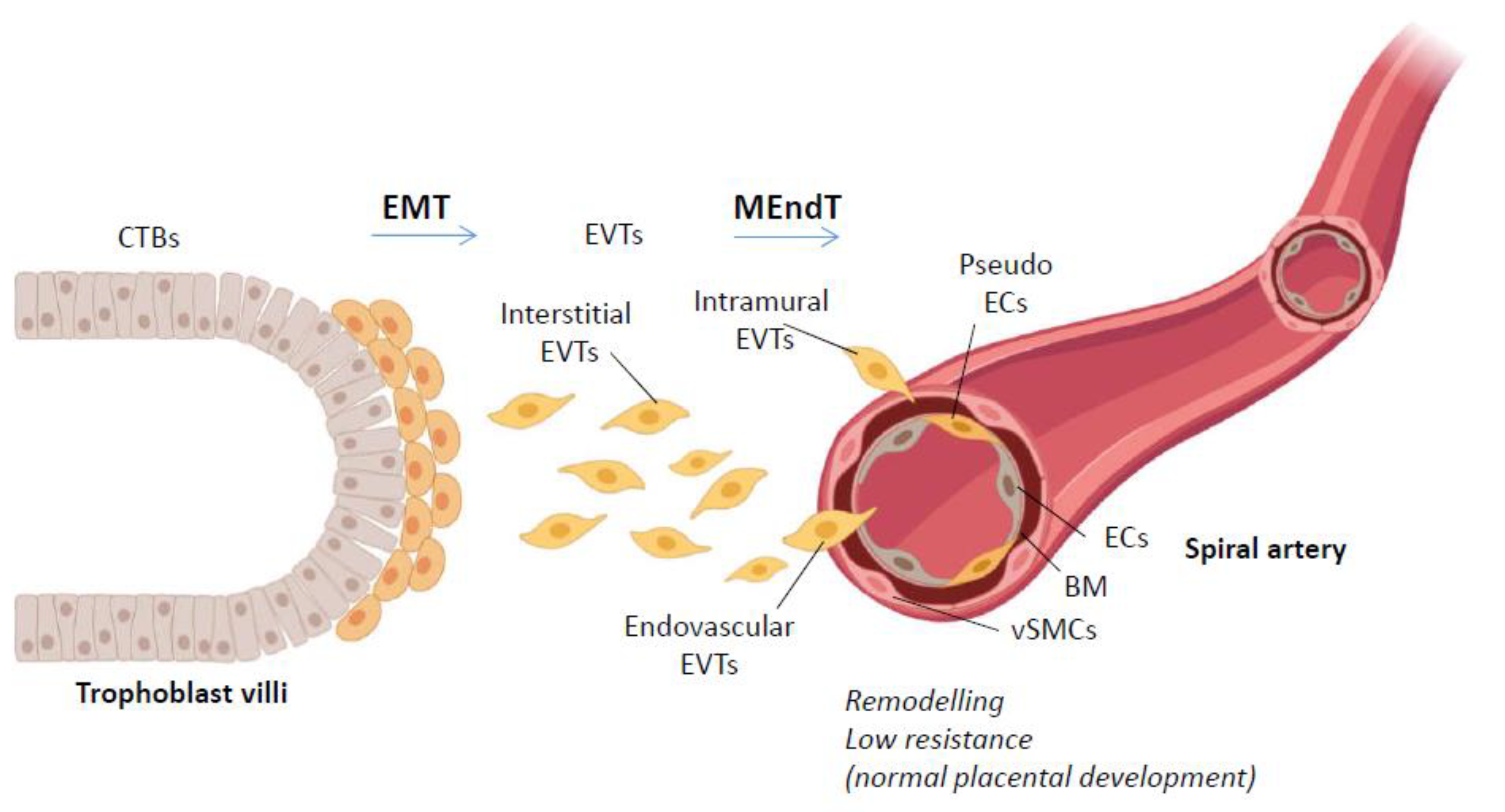
2. Trophoblast Invasion
2.1. EMT in Trophoblast Invasion
2.2. MEndT in Trophoblast Pseudovasculogenesis
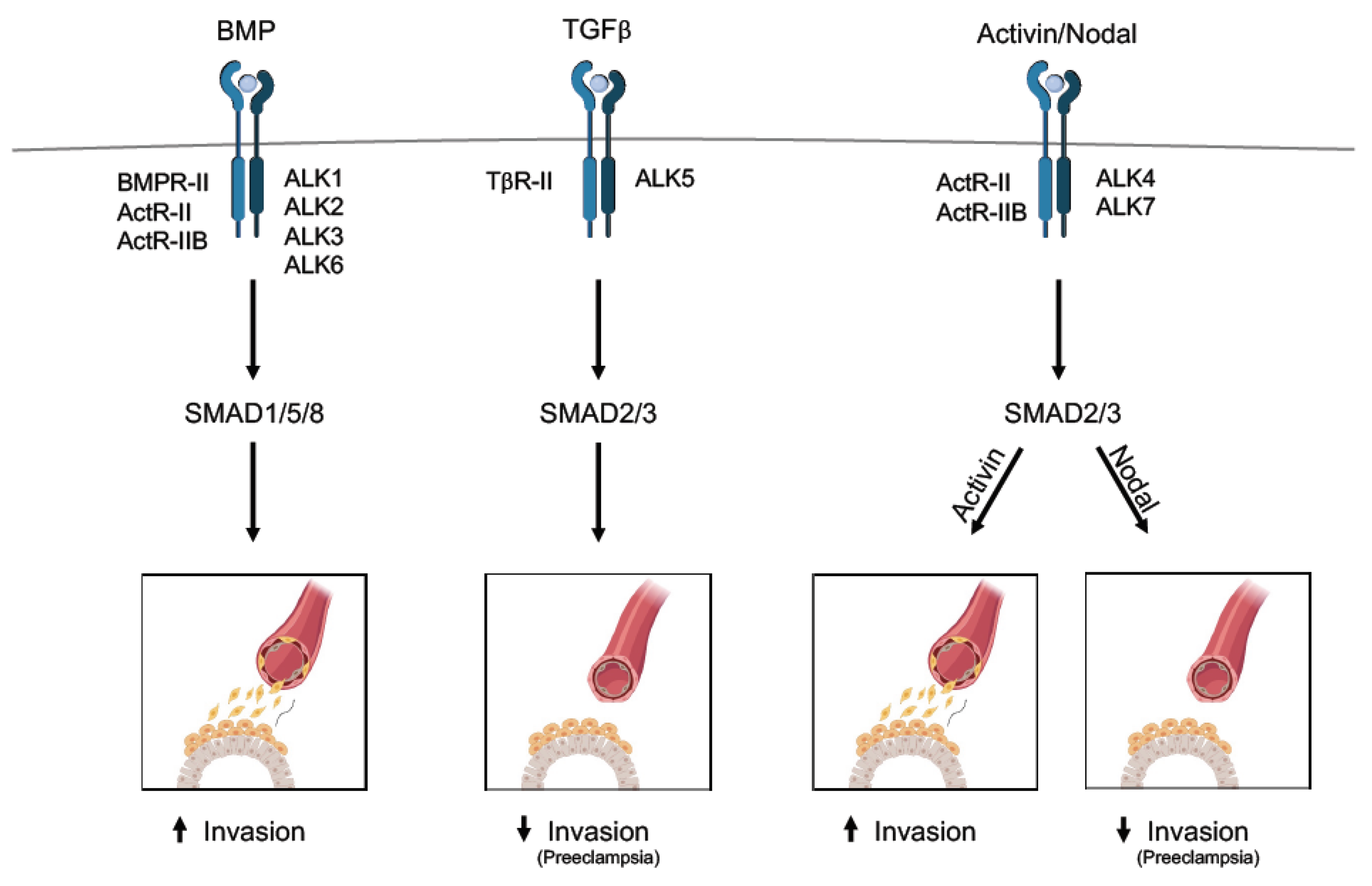
3. The TGFβ Family in Human Placental Development
3.1. TGFβ Cascade
3.2. Activin and Nodal Cascade
3.3. BMP Cascade
3.4. TGFβ Family-Associated Factors
4. Preeclampsia
5. Stem Cells for Human Placental Development and Organoids
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyd, J.D.; Hamilton, W.J. Development and structure of the human placenta from the end of the 3rd month of gestation. J. Obstet. Gynaecol. Br. Commonw. 1967, 74, 161–226. [Google Scholar] [CrossRef] [PubMed]
- Diczfalusy, E. Fetoplacental hormones and human gestation. Basic Life Sci. 1974, 4, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.S.; Karumanchi, S.A. Angiogenic factors in preeclampsia and related disorders. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Matzuk, M.M. Reproductive tract function and dysfunction in women. Nat. Rev. Endocrinol. 2011, 7, 517–525. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Highet, A.R.; Buckberry, S.; Mayne, B.T.; Khoda, S.M.; Bianco-Miotto, T.; Roberts, C.T. First trimester trophoblasts forming endothelial-like tubes In Vitro emulate a ‘blood vessel development’ gene expression profile. Gene Expr. Patterns 2016, 21, 103–110. [Google Scholar] [CrossRef]
- Burton, G.J.; Scioscia, M.; Rademacher, T.W. Endometrial secretions: Creating a stimulatory microenvironment within the human early placenta and implications for the aetiopathogenesis of preeclampsia. J. Reprod. Immunol. 2011, 89, 118–125. [Google Scholar] [CrossRef]
- Marshall, S.A.; Hannan, N.J.; Jelinic, M.; Nguyen, T.P.H.; Girling, J.E.; Parry, L.J. Animal models of preeclampsia: Translational failings and why. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R499–R508. [Google Scholar] [CrossRef]
- Crosley, E.J.; Elliot, M.G.; Christians, J.K.; Crespi, B.J. Placental invasion, preeclampsia risk and adaptive molecular evolution at the origin of the great apes: Evidence from genome-wide analyses. Placenta 2013, 34, 127–132. [Google Scholar] [CrossRef]
- Pijnenborg, R.; D’Hooghe, T.; Vercruysse, L.; Bambra, C. Evaluation of trophoblast invasion in placental bed biopsies of the baboon, with immunohistochemical localisation of cytokeratin, fibronectin, and laminin. J. Med. Primatol. 1996, 25, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Comparative studies of placentation and immunology in non-human primates suggest a scenario for the evolution of deep trophoblast invasion and an explanation for human pregnancy disorders. Reproduction 2011, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Knofler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polanski, K.; Goncalves, A.; et al. Single-Cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.H. Mechanisms of TGFbeta-induced epithelial-mesenchymal transition. J. Clin. Med. 2016, 5, 63. [Google Scholar] [CrossRef]
- Tsubakihara, Y.; Moustakas, A. Epithelial-Mesenchymal transition and metastasis under the control of transforming growth factor beta. Int. J. Mol. Sci. 2018, 19, 3672. [Google Scholar] [CrossRef]
- Davies, J.E.; Pollheimer, J.; Yong, H.E.; Kokkinos, M.I.; Kalionis, B.; Knofler, M.; Murthi, P. Epithelial-Mesenchymal transition during extravillous trophoblast differentiation. Cell Adhes. Migr. 2016, 10, 310–321. [Google Scholar] [CrossRef]
- Staun-Ram, E.; Goldman, S.; Shalev, E. p53 mediates epidermal growth factor (EGF) induction of MMP-2 transcription and trophoblast invasion. Placenta 2009, 30, 1029–1036. [Google Scholar] [CrossRef]
- LaMarca, H.L.; Ott, C.M.; Honer Zu Bentrup, K.; Leblanc, C.L.; Pierson, D.L.; Nelson, A.B.; Scandurro, A.B.; Whitley, G.S.; Nickerson, C.A.; Morris, C.A. Three-Dimensional growth of extravillous cytotrophoblasts promotes differentiation and invasion. Placenta 2005, 26, 709–720. [Google Scholar] [CrossRef]
- Cai, L.; Xiong, X.; Kong, X.; Xie, J. The role of the lysyl oxidases in tissue repair and remodeling: A concise review. Tissue Eng. Regen. Med. 2017, 14, 15–30. [Google Scholar] [CrossRef]
- Canesin, G.; Cuevas, E.P.; Santos, V.; Lopez-Menendez, C.; Moreno-Bueno, G.; Huang, Y.; Csiszar, K.; Portillo, F.; Peinado, H.; Lyden, D.; et al. Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: Novel partners in E-cadherin repression and early metastasis colonization. Oncogene 2015, 34, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Jia, Y.; Zhou, X.; Xie, D.; Huang, X.; Jia, L.; Zhou, Q.; Zheng, Q.; Zhou, X.; Wang, K.; et al. Downregulation of lysyl oxidase and lysyl oxidase-like protein 2 suppressed the migration and invasion of trophoblasts by activating the TGF-beta/collagen pathway in preeclampsia. Exp. Mol. Med. 2019, 51, 20. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; Ten Dijke, P. TGF-beta signaling in control of cardiovascular function. Cold Spring Harb. Perspect. Biol. 2017, 10. [Google Scholar] [CrossRef]
- Ubil, E.; Duan, J.; Pillai, I.C.; Rosa-Garrido, M.; Wu, Y.; Bargiacchi, F.; Lu, Y.; Stanbouly, S.; Huang, J.; Rojas, M.; et al. Mesenchymal-Endothelial transition contributes to cardiac neovascularization. Nature 2014, 514, 585–590. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Dixon, G.; Robertson, W.B.; Brosens, I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1, 3–19. [Google Scholar] [CrossRef]
- He, N.; van Iperen, L.; de Jong, D.; Szuhai, K.; Helmerhorst, F.M.; van der Westerlaken, L.A.; Chuva de Sousa Lopes, S.M. Human extravillous trophoblasts penetrate decidual veins and lymphatics before remodeling spiral arteries during early pregnancy. PLoS ONE 2017, 12, e0169849. [Google Scholar] [CrossRef]
- Moser, G.; Windsperger, K.; Pollheimer, J.; de Sousa Lopes, S.C.; Huppertz, B. Human trophoblast invasion: New and unexpected routes and functions. Histochem. Cell Biol. 2018, 150, 361–370. [Google Scholar] [CrossRef]
- Lala, P.K.; Nandi, P. Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: The role of decorin. Cell Adhes. Migr. 2016, 10, 111–125. [Google Scholar] [CrossRef]
- Smith, S.D.; Dunk, C.E.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 2009, 174, 1959–1971. [Google Scholar] [CrossRef]
- James, J.L.; Cartwright, J.E.; Whitley, G.S.; Greenhill, D.R.; Hoppe, A. The regulation of trophoblast migration across endothelial cells by low shear stress: Consequences for vascular remodelling in pregnancy. Cardiovasc. Res. 2012, 93, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Whitley, G.S.; Cartwright, J.E. Cellular and molecular regulation of spiral artery remodelling: Lessons from the cardiovascular field. Placenta 2010, 31, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.K.; Keogh, R.J.; Wareing, M.; Baker, P.N.; Cartwright, J.E.; Aplin, J.D.; Whitley, G.S. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am. J. Pathol. 2006, 169, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Hod, T.; Cerdeira, A.S.; Karumanchi, S.A. Molecular mechanisms of preeclampsia. Cold Spring Harb. Perspect. Med. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Li, Q. Transforming growth factor beta signaling in uterine development and function. J. Anim. Sci. Biotechnol. 2014, 5, 52. [Google Scholar] [CrossRef]
- Jones, R.L.; Stoikos, C.; Findlay, J.K.; Salamonsen, L.A. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction 2006, 132, 217–232. [Google Scholar] [CrossRef]
- Huang, Z.; Li, S.; Fan, W.; Ma, Q. Transforming growth factor beta1 promotes invasion of human JEG-3 trophoblast cells via TGF-beta/Smad3 signaling pathway. Oncotarget 2017, 8, 33560–33570. [Google Scholar] [CrossRef]
- Cheng, J.C.; Yi, Y.; Chang, H.M.; Leung, P.C.K. TGF-beta1 up-regulates cadherin-11 expression through snail: A potential mechanism for human trophoblast cell differentiation. Cell Signal. 2018, 43, 55–61. [Google Scholar] [CrossRef]
- Lash, G.E.; Otun, H.A.; Innes, B.A.; Bulmer, J.N.; Searle, R.F.; Robson, S.C. Inhibition of trophoblast cell invasion by TGFB1, 2, and 3 is associated with a decrease in active proteases. Biol. Reprod. 2005, 73, 374–381. [Google Scholar] [CrossRef]
- Graham, C.H. Effect of transforming growth factor-beta on the plasminogen activator system in cultured first trimester human cytotrophoblasts. Placenta 1997, 18, 137–143. [Google Scholar] [CrossRef]
- Caniggia, I.; Lye, S.J.; Cross, J.C. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology 1997, 138, 3976–3986. [Google Scholar] [CrossRef] [PubMed]
- Bearfield, C.; Jauniaux, E.; Groome, N.; Sargent, I.L.; Muttukrishna, S. The secretion and effect of inhibin A, activin A and follistatin on first-trimester trophoblasts In Vitro. Eur. J. Endocrinol. 2005, 152, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.T.; Soloveva, V.; Tzeng, S.J.; Lowe, L.A.; Pfendler, K.C.; Iannaccone, P.M.; Kuehn, M.R.; Linzer, D.I. Nodal regulates trophoblast differentiation and placental development. Dev. Biol. 2001, 236, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Xu, G.; Wu, Y.; Yang, B.; Lala, P.K.; Peng, C. Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J. Biol. Chem. 2004, 279, 31277–31286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Chang, H.M.; Zhu, H.; Klausen, C.; Li, Y.; Leung, P.C.K. Bone morphogenetic protein 2 promotes human trophoblast cell invasion by inducing activin A production. Endocrinology 2018, 159, 2815–2825. [Google Scholar] [CrossRef]
- Zhao, H.J.; Klausen, C.; Zhu, H.; Chang, H.M.; Li, Y.; Leung, P.C.K. Bone morphogenetic protein 2 promotes human trophoblast cell invasion and endothelial-like tube formation through ID1-mediated upregulation of IGF binding protein-3. FASEB J. 2020, 34, 3151–3164. [Google Scholar] [CrossRef]
- Richter, A.; Valdimarsdottir, L.; Hrafnkelsdottir, H.E.; Runarsson, J.F.; Omarsdottir, A.R.; Ward-van Oostwaard, D.; Mummery, C.; Valdimarsdottir, G. BMP4 promotes EMT and mesodermal commitment in human embryonic stem cells via SLUG and MSX2. Stem Cells 2014, 32, 636–648. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Q.; Lu, J.; Yang, G.; Tian, N.; Wang, X.; Tan, Y.; Tan, D. MSX2 induces trophoblast invasion in human placenta. PLoS ONE 2016, 11, e0153656. [Google Scholar] [CrossRef]
- Soncin, F.; Khater, M.; To, C.; Pizzo, D.; Farah, O.; Wakeland, A.; Arul Nambi Rajan, K.; Nelson, K.K.; Chang, C.W.; Moretto-Zita, M.; et al. Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development 2018, 145. [Google Scholar] [CrossRef]
- Heldin, C.H.; Miyazono, K.; ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Feng, X.H. TGF-beta receptor signaling. Biochim. Biophys. Acta 1997, 1333, F105–F150. [Google Scholar] [PubMed]
- Massague, J. TGF-beta signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Luo, S.; Wang, Y.; Zhuang, L.; Chen, Y.; Peng, C. Smads in human trophoblast cells: Expression, regulation and role in TGF-beta-induced transcriptional activity. Mol. Cell. Endocrinol. 2001, 175, 111–121. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Choi, Y.L.; Shin, Y.K.; Ahn, G.H.; Kim, K.H.; Kim, W.J.; Lee, H.C.; Kim, S.H. Expression of TGF-beta signaling proteins in normal placenta and gestational trophoblastic disease. Histol. Histopathol. 2007, 22, 227–234. [Google Scholar] [CrossRef]
- Caniggia, I.; Grisaru-Gravnosky, S.; Kuliszewsky, M.; Post, M.; Lye, S.J. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J. Clin. Investig. 1999, 103, 1641–1650. [Google Scholar] [CrossRef]
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Rosendahl, A.; Sideras, P.; ten Dijke, P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002, 21, 1743–1753. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, J.C.; Klausen, C.; Leung, P.C.K. TGF-beta1 inhibits human trophoblast cell invasion by upregulating cyclooxygenase-2. Placenta 2018, 68, 44–51. [Google Scholar] [CrossRef]
- Brkic, J.; Dunk, C.; O’Brien, J.; Fu, G.; Nadeem, L.; Wang, Y.L.; Rosman, D.; Salem, M.; Shynlova, O.; Yougbare, I.; et al. MicroRNA-218-5p promotes endovascular trophoblast differentiation and spiral artery remodeling. Mol. Ther. 2018, 26, 2189–2205. [Google Scholar] [CrossRef]
- Mylonas, I.; Schiessl, B.; Jeschke, U.; Vogl, J.; Makrigiannakis, A.; Kuhn, C.; Schulze, S.; Kainer, F.; Friese, K. Expression of inhibin/activin subunits alpha (-α), betaA (-βA), and betaB (-βB) in placental tissue of normal, preeclamptic, and HELLP pregnancies. Endocr. Pathol. 2006, 17, 19–33. [Google Scholar] [CrossRef]
- Li, Y.; Klausen, C.; Cheng, J.C.; Zhu, H.; Leung, P.C. Activin A, B, and AB increase human trophoblast cell invasion by up-regulating N-cadherin. J. Clin. Endocrinol. Metab. 2014, 99, E2216–E2225. [Google Scholar] [CrossRef][Green Version]
- Nadeem, L.; Munir, S.; Fu, G.; Dunk, C.; Baczyk, D.; Caniggia, I.; Lye, S.; Peng, C. Nodal signals through activin receptor-like kinase 7 to inhibit trophoblast migration and invasion: Implication in the pathogenesis of preeclampsia. Am. J. Pathol. 2011, 178, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Kato, M.; Nakao, A.; Imamura, T.; ten Dijke, P.; Heldin, C.H.; Kawabata, M.; Shimada, S.; Miyazono, K. Identification of receptors and Smad proteins involved in activin signalling in a human epidermal keratinocyte cell line. Genes Cells 1998, 3, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Pardali, E.; van der Horst, G.; Cheung, H.; van den Hoogen, C.; van der Pluijm, G.; Ten Dijke, P. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 2010, 29, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Byfield, S.D.; Roberts, A.B. Lateral signaling enhances TGF-beta response complexity. Trends Cell Biol. 2004, 14, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Dennler, S.; Huet, S.; Gauthier, J.M. A short amino-acid sequence in MH1 domain is responsible for functional differences between Smad2 and Smad3. Oncogene 1999, 18, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Pardali, E.; Sanchez-Duffhues, G.; ten Dijke, P. BMP signaling in vascular diseases. FEBS Lett. 2012, 586, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Vinuesa, A.; Abdelilah-Seyfried, S.; Knaus, P.; Zwijsen, A.; Bailly, S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 2016, 27, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Pera, M.F.; Andrade, J.; Houssami, S.; Reubinoff, B.; Trounson, A.; Stanley, E.G.; Ward-van Oostwaard, D.; Mummery, C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J. Cell Sci. 2004, 117, 1269–1280. [Google Scholar] [CrossRef]
- Valdimarsdottir, G.; Goumans, M.J.; Rosendahl, A.; Brugman, M.; Itoh, S.; Lebrin, F.; Sideras, P.; ten Dijke, P. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation 2002, 106, 2263–2270. [Google Scholar] [CrossRef]
- Wetendorf, M.; DeMayo, F.J. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol. Cell. Endocrinol. 2012, 357, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Urness, L.D.; Sorensen, L.K.; Li, D.Y. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat. Genet. 2000, 26, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; Mummery, C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int. J. Dev. Biol. 2000, 44, 253–265. [Google Scholar] [PubMed]
- Soncin, F.; Natale, D.; Parast, M.M. Signaling pathways in mouse and human trophoblast differentiation: A comparative review. Cell. Mol. Life Sci. 2015, 72, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Larrivee, B.; Prahst, C.; Gordon, E.; del Toro, R.; Mathivet, T.; Duarte, A.; Simons, M.; Eichmann, A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev. Cell 2012, 22, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Lebrin, F.; Srun, S.; Raymond, K.; Martin, S.; van den Brink, S.; Freitas, C.; Breant, C.; Mathivet, T.; Larrivee, B.; Thomas, J.L.; et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat. Med. 2010, 16, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; Zwijsen, A.; Ten Dijke, P.; Bailly, S. Bone Morphogenetic proteins in vascular homeostasis and disease. Cold Spring Harb. Perspect. Biol. 2017, 10. [Google Scholar] [CrossRef]
- Zhao, H.J.; Klausen, C.; Li, Y.; Zhu, H.; Wang, Y.L.; Leung, P.C.K. Bone morphogenetic protein 2 promotes human trophoblast cell invasion by upregulating N-cadherin via non-canonical SMAD2/3 signaling. Cell Death Dis. 2018, 9, 174. [Google Scholar] [CrossRef]
- Ramachandran, A.; Vizan, P.; Das, D.; Chakravarty, P.; Vogt, J.; Rogers, K.W.; Muller, P.; Hinck, A.P.; Sapkota, G.P.; Hill, C.S. TGF-beta uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. eLife 2018, 7. [Google Scholar] [CrossRef]
- David, L.; Mallet, C.; Mazerbourg, S.; Feige, J.J.; Bailly, S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 2007, 109, 1953–1961. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ohga, N.; Morishita, Y.; Hida, K.; Miyazono, K.; Watabe, T. BMP-9 induces proliferation of multiple types of endothelial cells In Vitro and In Vivo. J. Cell Sci. 2010, 123, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- van Meeteren, L.A.; Thorikay, M.; Bergqvist, S.; Pardali, E.; Gallo Stampino, C.; Hu-Lowe, D.; Goumans, M.J.; Ten Dijke, P. An anti-human ALK1 antibody attenuates BMP9 induced ALK1 signaling and interferes with endothelial cell sprouting. J. Biol. Chem. 2012, 287, 18551–18561. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.L.; Xu, G.; Sotov, V.; Letarte, M. Review: The enigmatic role of endoglin in the placenta. Placenta 2014, 35 (Suppl. S93–S99). [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Sorensen, L.K.; Brooke, B.S.; Urness, L.D.; Davis, E.C.; Taylor, D.G.; Boak, B.B.; Wendel, D.P. Defective angiogenesis in mice lacking endoglin. Science 1999, 284, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Muhl, L.; Burmakin, M.; Wang, Y.; Duchez, A.C.; Betsholtz, C.; Arthur, H.M.; Jakobsson, L. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nat. Cell Biol. 2017, 19, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Kotani, T.; Shibata, K.; Matsumura, H.; Tsuda, H.; Sumigama, S.; Yamamoto, E.; Iwase, A.; Senga, T.; Kikkawa, F. The loss of endoglin promotes the invasion of extravillous trophoblasts. Endocrinology 2011, 152, 4386–4394. [Google Scholar] [CrossRef]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell 2018, 22, 50–63 e56. [Google Scholar] [CrossRef]
- Xue, W.C.; Feng, H.C.; Chan, K.Y.; Chiu, P.M.; Ngan, H.Y.; Khoo, U.S.; Tsao, S.W.; Chan, K.W.; Cheung, A.N. Id helix-loop-helix proteins are differentially expressed in gestational trophoblastic disease. Histopathology 2005, 47, 303–309. [Google Scholar] [CrossRef]
- Janatpour, M.J.; McMaster, M.T.; Genbacev, O.; Zhou, Y.; Dong, J.; Cross, J.C.; Israel, M.A.; Fisher, S.J. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development 2000, 127, 549–558. [Google Scholar]
- Selesniemi, K.; Albers, R.E.; Brown, T.L. Id2 mediates differentiation of labyrinthine placental progenitor cell line, SM10. Stem Cells Dev. 2016, 25, 959–974. [Google Scholar] [CrossRef]
- Nichol, D.; Stuhlmann, H. EGFL7: A unique angiogenic signaling factor in vascular development and disease. Blood 2012, 119, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, I.; Stankovic, N.D.; Bicker, F.; Meister, J.; Braun, H.; Awwad, K.; Baumgart, J.; Simon, K.; Thal, S.C.; Patra, C.; et al. EGFL7 ligates alphavbeta3 integrin to enhance vessel formation. Blood 2013, 121, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, C.; Uphaus, T.; Broux, B.; Gowing, E.; Paterka, M.; Michel, L.; Dudvarski Stankovic, N.; Bicker, F.; Lemaitre, F.; Prat, A.; et al. EGFL7 reduces CNS inflammation in mouse. Nat. Commun. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Meister, J.; Schmidt, M.H. miR-126 and miR-126*: New players in cancer. Sci. World J. 2010, 10, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Lacko, L.A.; Massimiani, M.; Sones, J.L.; Hurtado, R.; Salvi, S.; Ferrazzani, S.; Davisson, R.L.; Campagnolo, L.; Stuhlmann, H. Novel expression of EGFL7 in placental trophoblast and endothelial cells and its implication in preeclampsia. Mech. Dev. 2014, 133, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Nichol, D.; Shawber, C.; Fitch, M.J.; Bambino, K.; Sharma, A.; Kitajewski, J.; Stuhlmann, H. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7. Blood 2010, 116, 6133–6143. [Google Scholar] [CrossRef]
- Rodriguez, A.; Griffiths-Jones, S.; Ashurst, J.L.; Bradley, A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004, 14, 1902–1910. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef]
- Parker, L.H.; Schmidt, M.; Jin, S.W.; Gray, A.M.; Beis, D.; Pham, T.; Frantz, G.; Palmieri, S.; Hillan, K.; Stainier, D.Y.; et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature 2004, 428, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Lacko, L.A.; Hurtado, R.; Hinds, S.; Poulos, M.G.; Butler, J.M.; Stuhlmann, H. Altered feto-placental vascularization, feto-placental malperfusion and fetal growth restriction in mice with Egfl7 loss of function. Development 2017, 144, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Lelievre, E.; Hinek, A.; Lupu, F.; Buquet, C.; Soncin, F.; Mattot, V. VE-statin/egfl7 regulates vascular elastogenesis by interacting with lysyl oxidases. EMBO J. 2008, 27, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Junus, K.; Centlow, M.; Wikstrom, A.K.; Larsson, I.; Hansson, S.R.; Olovsson, M. Gene expression profiling of placentae from women with early-and late-onset pre-eclampsia: Down-Regulation of the angiogenesis-related genes ACVRL1 and EGFL7 in early-onset disease. Mol. Hum. Reprod. 2012, 18, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Alexdottir, M.S.; Magnus, S.H.; Richter, T.R.; Morikawa, M.; Zwijsen, A.; Valdimarsdottir, G. EGFL7 mediates BMP9-induced sprouting angiogenesis of endothelial cells derived from human embryonic stem cells. Stem Cell Rep. 2019, 12, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Star, G.P.; Giovinazzo, M.; Langleben, D. Bone morphogenic protein-9 stimulates endothelin-1 release from human pulmonary microvascular endothelial cells: A potential mechanism for elevated ET-1 levels in pulmonary arterial hypertension. Microvasc. Res. 2010, 80, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Paradis, A.; Zhang, L. Role of endothelin in uteroplacental circulation and fetal vascular function. Curr. Vasc. Pharmacol. 2013, 11, 594–605. [Google Scholar] [CrossRef]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Thornton, J.G.; Onwude, J.L. Convulsions in pregnancy in related gorillas. Am. J. Obstet. Gynecol. 1992, 167, 240–241. [Google Scholar] [CrossRef]
- Makris, A.; Yeung, K.R.; Lim, S.M.; Sunderland, N.; Heffernan, S.; Thompson, J.F.; Iliopoulos, J.; Killingsworth, M.C.; Yong, J.; Xu, B.; et al. Placental growth factor reduces blood pressure in a uteroplacental ischemia model of preeclampsia in nonhuman primates. Hypertension 2016, 67, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Hawinkels, L.J.; Kuiper, P.; Wiercinska, E.; Verspaget, H.W.; Liu, Z.; Pardali, E.; Sier, C.F.; ten Dijke, P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010, 70, 4141–4150. [Google Scholar] [CrossRef] [PubMed]
- Kaitu’u-Lino, T.J.; Palmer, K.R.; Whitehead, C.L.; Williams, E.; Lappas, M.; Tong, S. MMP-14 is expressed in preeclamptic placentas and mediates release of soluble endoglin. Am. J. Pathol. 2012, 180, 888–894. [Google Scholar] [CrossRef]
- Castonguay, R.; Werner, E.D.; Matthews, R.G.; Presman, E.; Mulivor, A.W.; Solban, N.; Sako, D.; Pearsall, R.S.; Underwood, K.W.; Seehra, J.; et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J. Biol. Chem. 2011, 286, 30034–30046. [Google Scholar] [CrossRef]
- Perucci, L.O.; Gomes, K.B.; Freitas, L.G.; Godoi, L.C.; Alpoim, P.N.; Pinheiro, M.B.; Miranda, A.S.; Teixeira, A.L.; Dusse, L.M.; Sousa, L.P. Soluble endoglin, transforming growth factor-Beta 1 and soluble tumor necrosis factor alpha receptors in different clinical manifestations of preeclampsia. PLoS ONE 2014, 9, e97632. [Google Scholar] [CrossRef]
- Lawera, A.; Tong, Z.; Thorikay, M.; Redgrave, R.E.; Cai, J.; van Dinther, M.; Morrell, N.W.; Afink, G.B.; Charnock-Jones, D.S.; Arthur, H.M.; et al. Role of soluble endoglin in BMP9 signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 17800–17808. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Small, H.Y.; Currie, G.; Delles, C. Systematic review of micro-RNA expression in pre-eclampsia identifies a number of common pathways associated with the disease. PLoS ONE 2016, 11, e0160808. [Google Scholar] [CrossRef]
- Lyall, F.; Simpson, H.; Bulmer, J.N.; Barber, A.; Robson, S.C. Transforming growth factor-beta expression in human placenta and placental bed in third trimester normal pregnancy, preeclampsia, and fetal growth restriction. Am. J. Pathol. 2001, 159, 1827–1838. [Google Scholar] [CrossRef]
- Wu, K.; Liu, F.; Wu, W.; Chen, Y.; Zhang, W. Bioinformatics approach reveals the critical role of TGF-beta signaling pathway in pre-eclampsia development. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 130–138. [Google Scholar] [CrossRef]
- Alahari, S.; Garcia, J.; Post, M.; Caniggia, I. The von Hippel Lindau tumour suppressor gene is a novel target of E2F4-mediated transcriptional repression in preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3298–3308. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Schneider-Kolsky, M.; Dole, A.; Wallace, E.M. Activin A and activin receptors in gestational tissue from preeclamptic pregnancies. J. Endocrinol. 2001, 171, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Silver, H.M.; Lambert-Messerlian, G.M.; Reis, F.M.; Diblasio, A.M.; Petraglia, F.; Canick, J.A. Mechanism of increased maternal serum total activin a and inhibin a in preeclampsia. J. Soc. Gynecol. Investig. 2002, 9, 308–312. [Google Scholar] [CrossRef]
- Bersinger, N.A.; Smarason, A.K.; Muttukrishna, S.; Groome, N.P.; Redman, C.W. Women with preeclampsia have increased serum levels of pregnancy-associated plasma protein A (PAPP-A), inhibin A, activin A and soluble E-selectin. Hypertens. Pregnancy 2003, 22, 45–55. [Google Scholar] [CrossRef]
- Thulluru, H.K.; Michel, O.J.; Oudejans, C.B.; van Dijk, M. ACVR2A promoter polymorphism rs1424954 in the Activin-A signaling pathway in trophoblasts. Placenta 2015, 36, 345–349. [Google Scholar] [CrossRef]
- Blakeley, P.; Fogarty, N.M.; del Valle, I.; Wamaitha, S.E.; Hu, T.X.; Elder, K.; Snell, P.; Christie, L.; Robson, P.; Niakan, K.K. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 2015, 142, 3151–3165. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Edsgard, D.; Reinius, B.; Deng, Q.; Panula, S.P.; Codeluppi, S.; Plaza Reyes, A.; Linnarsson, S.; Sandberg, R.; Lanner, F. Single-Cell RNA-Seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell 2016, 165, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Wamaitha, S.E.; Niakan, K.K. Human pre-gastrulation development. Curr. Top. Dev. Biol. 2018, 128, 295–338. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, R.; Yuan, P.; Ren, Y.; Mao, Y.; Li, R.; Lian, Y.; Li, J.; Wen, L.; Yan, L.; et al. Reconstituting the transcriptome and DNA methylome landscapes of human implantation. Nature 2019, 572, 660–664. [Google Scholar] [CrossRef]
- Pijuan-Sala, B.; Griffiths, J.A.; Guibentif, C.; Hiscock, T.W.; Jawaid, W.; Calero-Nieto, F.J.; Mulas, C.; Ibarra-Soria, X.; Tyser, R.C.V.; Ho, D.L.L.; et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 2019, 566, 490–495. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M.; Ezashi, T.; Sheridan, M.A.; Yang, Y. Specification of trophoblast from embryonic stem cells exposed to BMP4. Biol. Reprod. 2018, 99, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, B.; Xu, J.; Wang, J.; Wu, J.; Shi, C.; Xu, Y.; Dong, J.; Wang, C.; Lai, W.; et al. Derivation of Pluripotent stem cells with In Vivo embryonic and extraembryonic potency. Cell 2017, 169, 243–257 e225. [Google Scholar] [CrossRef] [PubMed]
- Kime, C.; Kiyonari, H.; Ohtsuka, S.; Kohbayashi, E.; Asahi, M.; Yamanaka, S.; Takahashi, M.; Tomoda, K. Induced 2C expression and implantation-competent blastocyst-like cysts from primed pluripotent stem cells. Stem Cell Rep. 2019, 13, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Orendi, K.; Gauster, M.; Moser, G.; Meiri, H.; Huppertz, B. The choriocarcinoma cell line BeWo: Syncytial fusion and expression of syncytium-specific proteins. Reproduction 2010, 140, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.; Shawky, S.A.; Aryasomayajula, A.; Green, M.A.; Ewart, T.; Selvaganapathy, P.R.; Raha, S. Extracellular matrix surface regulates self-assembly of three-dimensional placental trophoblast spheroids. PLoS ONE 2018, 13, e0199632. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.; Wahed, M.; Shawky, S.A.; Dvorkin-Gheva, A.; Raha, S. Transcriptomic and functional analyses of 3D placental extravillous trophoblast spheroids. Sci. Rep. 2019, 9, 12607. [Google Scholar] [CrossRef]
- Wang, H.; Pilla, F.; Anderson, S.; Martinez-Escribano, S.; Herrer, I.; Moreno-Moya, J.M.; Musti, S.; Bocca, S.; Oehninger, S.; Horcajadas, J.A. A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Mol. Hum. Reprod. 2012, 18, 33–43. [Google Scholar] [CrossRef]
- Wang, H.; Bocca, S.; Anderson, S.; Yu, L.; Rhavi, B.S.; Horcajadas, J.; Oehninger, S. Sex steroids regulate epithelial-stromal cell cross talk and trophoblast attachment invasion in a three-dimensional human endometrial culture system. Tissue Eng. Part C Methods 2013, 19, 676–687. [Google Scholar] [CrossRef]
- Bentin-Ley, U.; Pedersen, B.; Lindenberg, S.; Larsen, J.F.; Hamberger, L.; Horn, T. Isolation and culture of human endometrial cells in a three-dimensional culture system. J. Reprod. Fertil. 1994, 101, 327–332. [Google Scholar] [CrossRef]
- Arnold, J.T.; Kaufman, D.G.; Seppala, M.; Lessey, B.A. Endometrial stromal cells regulate epithelial cell growth In Vitro: A new co-culture model. Hum. Reprod. 2001, 16, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Choi, D.S.; Ryu, H.S.; Kwon, H.C.; Joo, H.; Min, C.K. A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer Lett. 2003, 195, 185–192. [Google Scholar] [CrossRef]
- Kaushik, G.; Ponnusamy, M.P.; Batra, S.K. Concise review: Current status of three-dimensional organoids as preclinical models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef]
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U.; Ellinger, A.; Burkard, T.R.; Fiala, C.; Pollheimer, J.; et al. Self-Renewing Trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 2018, 11, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-Term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-Derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Masse, S.; Kim, J.; Reis, L.; et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016, 15, 669–678. [Google Scholar] [CrossRef]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef]

| TGFβ FACTOR | ROLE | CELL LINE/SYSTEM | REFERNCES |
|---|---|---|---|
| TGFβ1 |
|
| [38] [39] [40] [41] |
| TGFβ2 |
|
| [40] |
| TGFβ3 |
|
| [40] |
| ACTIVIN A |
|
| [42] [43] |
| NODAL |
|
| [44] [45] [45] |
| BMP2 |
|
| [46] [47] |
| BMP4 |
|
| [48,49] |
| ENG |
|
| [50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuva de Sousa Lopes, S.M.; Alexdottir, M.S.; Valdimarsdottir, G. The TGFβ Family in Human Placental Development at the Fetal-Maternal Interface. Biomolecules 2020, 10, 453. https://doi.org/10.3390/biom10030453
Chuva de Sousa Lopes SM, Alexdottir MS, Valdimarsdottir G. The TGFβ Family in Human Placental Development at the Fetal-Maternal Interface. Biomolecules. 2020; 10(3):453. https://doi.org/10.3390/biom10030453
Chicago/Turabian StyleChuva de Sousa Lopes, Susana M., Marta S. Alexdottir, and Gudrun Valdimarsdottir. 2020. "The TGFβ Family in Human Placental Development at the Fetal-Maternal Interface" Biomolecules 10, no. 3: 453. https://doi.org/10.3390/biom10030453
APA StyleChuva de Sousa Lopes, S. M., Alexdottir, M. S., & Valdimarsdottir, G. (2020). The TGFβ Family in Human Placental Development at the Fetal-Maternal Interface. Biomolecules, 10(3), 453. https://doi.org/10.3390/biom10030453





