Salient Features of Monomeric Alpha-Synuclein Revealed by NMR Spectroscopy
Abstract
1. Introduction
2. Pre-structured Motifs (PreSMos) in IDPs
3. Inconsistency
4. The Effect of Environmental Conditions on αS Conformation
4.1. Protein Concentration
4.2. pH
4.3. Temperature
4.4. Buffer and Ionic Strength
4.5. Lipid Membranes
5. Summary
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Maroteaux, L.; Campanelli, J.; Scheller, R. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [PubMed]
- Iwai, A.; Masliah, E.; Yoshimoto, M.; Ge, N.; Flanagan, L.; De Silva, H.R.; Kittel, A.; Saitoh, T. The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 1995, 14, 467–475. [Google Scholar] [PubMed]
- Burré, J. The synaptic function of α-synuclein. J. Parkinsons Dis. 2015, 5, 699–713. [Google Scholar] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar]
- Singleton, A.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R. α-Synuclein locus triplication causes Parkinson’s disease. Science 2003, 302, 841. [Google Scholar]
- Chartier-Harlin, M.-C.; Kachergus, J.; Roumier, C.; Mouroux, V.; Douay, X.; Lincoln, S.; Levecque, C.; Larvor, L.; Andrieux, J.; Hulihan, M. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 2004, 364, 1167–1169. [Google Scholar]
- Flagmeier, P.; Meisl, G.; Vendruscolo, M.; Knowles, T.P.; Dobson, C.M.; Buell, A.K.; Galvagnion, C. Mutations associated with familial Parkinson’s disease alter the initiation and amplification steps of α-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2016, 113, 10328–10333. [Google Scholar]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature. 1997, 388, 839–840. [Google Scholar]
- Braak, H.; Del Tredici, K.; Rüb, U.; De Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar]
- Kirschner, D.A.; Abraham, C.; Selkoe, D.J. X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer disease indicates cross-beta conformation. Proc. Natl. Acad. Sci. USA 1986, 83, 503–507. [Google Scholar]
- Serpell, L.C.; Berriman, J.; Jakes, R.; Goedert, M.; Crowther, R.A. Fiber diffraction of synthetic α-synuclein filaments shows amyloid-like cross-β conformation. Proc. Natl. Acad. Sci. USA 2000, 97, 4897–4902. [Google Scholar] [PubMed]
- Tuttle, M.D.; Comellas, G.; Nieuwkoop, A.J.; Covell, D.J.; Berthold, D.A.; Kloepper, K.D.; Courtney, J.M.; Kim, J.K.; Barclay, A.M.; Kendall, A. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 2016, 23, 409. [Google Scholar] [PubMed]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Fibrils formed in vitro from α-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000, 39, 2552–2563. [Google Scholar] [PubMed]
- El-Agnaf, O.M.A.; Jakes, R.; Curran, M.D.; Wallace, A. Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of α-synuclein protein implicated in Parkinson’s disease. FEBS Lett. 1998, 440, 67–70. [Google Scholar] [PubMed]
- Chen, S.W.; Drakulic, S.; Deas, E.; Ouberai, M.; Aprile, F.A.; Arranz, R.; Ness, S.; Roodveldt, C.; Guilliams, T.; De-Genst, E.J. Structural characterization of toxic oligomers that are kinetically trapped during α-synuclein fibril formation. Proc. Natl. Acad. Sci. USA 2015, 112, E1994–E2003. [Google Scholar] [PubMed]
- Varela, J.A.; Rodrigues, M.; De, S.; Flagmeier, P.; Gandhi, S.; Dobson, C.M.; Klenerman, D.; Lee, S.F. Optical structural analysis of individual α-synuclein oligomers. Angew. Chem. 2018, 57, 4886–4890. [Google Scholar]
- Fusco, G.; Pape, T.; Stephens, A.D.; Mahou, P.; Costa, A.R.; Kaminski, C.F.; Schierle, G.S.K.; Vendruscolo, M.; Veglia, G.; Dobson, C.M. Structural basis of synaptic vesicle assembly promoted by α-synuclein. Nat. Commun. 2016, 7, 12563. [Google Scholar]
- Fusco, G.; Chen, S.W.; Williamson, P.T.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N. Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar]
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.-I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.-H. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, aah3374. [Google Scholar]
- Ferreira, D.G.; Temido-Ferreira, M.; Miranda, H.V.; Batalha, V.L.; Coelho, J.E.; Szegö, É.M.; Marques-Morgado, I.; Vaz, S.H.; Rhee, J.S.; Schmitz, M. α-synuclein interacts with PrP C to induce cognitive impairment through mGluR5 and NMDAR2B. Nat. Neurosci. 2017, 20, 1569. [Google Scholar]
- Whiten, D.R.; Cox, D.; Horrocks, M.H.; Taylor, C.G.; De, S.; Flagmeier, P.; Tosatto, L.; Kumita, J.R.; Ecroyd, H.; Dobson, C.M. Single-molecule characterization of the interactions between extracellular chaperones and toxic α-synuclein oligomers. Cell Rep. 2018, 23, 3492–3500. [Google Scholar] [PubMed]
- Weinreb, P.H.; Zhen, W.; Poon, A.W.; Conway, K.A.; Lansbury, P.T. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996, 35, 13709–13715. [Google Scholar] [PubMed]
- Lee, H.; Mok, K.H.; Muhandiram, R.; Park, K.-H.; Suk, J.-E.; Kim, D.-H.; Chang, J.; Sung, Y.C.; Choi, K.Y.; Han, K.-H. Local structural elements in the mostly unstructured transcriptional activation domain of human p53. J. Biol. Chem. 2000, 275, 29426–29432. [Google Scholar] [PubMed]
- Lee, S.-H.; Kim, D.-H.; Han, J.; Cha, E.-J.; Lim, J.-E.; Cho, Y.-J.; Lee, C.; Han, K.-H. Understanding pre-structured motifs (PreSMos) in intrinsically unfolded proteins. Curr. Protein Pept. Sci. 2012, 13, 34–54. [Google Scholar]
- Kim, D.-H.; Han, K.-H. PreSMo target-binding signatures in intrinsically disordered proteins. Mol. Cells 2018, 41, 889–899. [Google Scholar]
- Uéda, K.; Fukushima, H.; Masliah, E.; Xia, Y.; Iwai, A.; Yoshimoto, M.; Otero, D.A.; Kondo, J.; Ihara, Y.; Saitoh, T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 11282–11286. [Google Scholar]
- Bodner, C.R.; Dobson, C.M.; Bax, A. Multiple tight phospholipid-binding modes of α-synuclein revealed by solution NMR spectroscopy. J. Mol. Biol. 2009, 390, 775–790. [Google Scholar]
- Eliezer, D.; Kutluay, E.; Bussell, R.; Browne, G. Conformational properties of α-synuclein in its free and lipid-associated states. J. Mol. Biol. 2001, 307, 1061–1073. [Google Scholar]
- EI-Agnaf, O.M.A.; Irvine, G.B. Aggregation and neurotoxicity of α-synuclein and related peptides. Biochem. Soc. Trans. 2002, 30, 559–565. [Google Scholar]
- Uversky, V.N.; Li, J.; Fink, A.L. Evidence for a partially folded intermediate in α-synuclein fibril formation. J. Biol. Chem. 2001, 276, 10737–10744. [Google Scholar]
- Uversky, V.N.; Fink, A.L. Conformational constraints for amyloid fibrillation: The importance of being unfolded. Biochim. Biophys. Acta 2004, 1698, 131–153. [Google Scholar]
- Wetzel, R. For protein misassembly, it’s the “I” decade. Cell. 1996, 86, 699–702. [Google Scholar] [PubMed]
- Li, J.; Uversky, V.N.; Fink, A.L. Conformational behavior of human α-synuclein is modulated by familial Parkinson’s disease point mutations A30P and A53T. Neurotoxicology 2002, 23, 553–567. [Google Scholar] [PubMed]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998, 4, 1318–1320. [Google Scholar] [PubMed]
- Wu, K.-P.; Kim, S.; Fela, D.A.; Baum, J. Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: implication for aggregation. J. Mol. Biol. 2008, 378, 1104–1115. [Google Scholar]
- Esteban-Martín, S.; Fenwick, R.B.; Salvatella, X. Refinement of ensembles describing unstructured proteins using NMR residual dipolar couplings. J. Am. Chem. Soc. 2010, 132, 4626–4632. [Google Scholar]
- Jensen, M.R.; Salmon, L.c.; Nodet, G.; Blackledge, M. Defining conformational ensembles of intrinsically disordered and partially folded proteins directly from chemical shifts. J. Am. Chem. Soc. 2010, 132, 1270–1272. [Google Scholar]
- Fisher, C.K.; Stultz, C.M. Constructing ensembles for intrinsically disordered proteins. Curr. Opin. Struct. Biol. 2011, 21, 426–431. [Google Scholar]
- Barbar, E. NMR characterization of partially folded and unfolded conformational ensembles of proteins. Biopolymers 1999, 51, 191–207. [Google Scholar]
- Lindorff-Larsen, K.; Kristjansdottir, S.; Teilum, K.; Fieber, W.; Dobson, C.M.; Poulsen, F.M.; Vendruscolo, M. Determination of an ensemble of structures representing the denatured state of the bovine acyl-coenzyme a binding protein. J. Am. Chem. Soc. 2004, 126, 3291–3299. [Google Scholar]
- Kristjansdottir, S.; Lindorff-Larsen, K.; Fieber, W.; Dobson, C.M.; Vendruscolo, M.; Poulsen, F.M. Formation of native and non-native interactions in ensembles of denatured ACBP molecules from paramagnetic relaxation enhancement studies. J. Mol. Biol. 2005, 347, 1053–1062. [Google Scholar] [PubMed]
- Marsh, J.A.; Forman-Kay, J.D. Structure and disorder in an unfolded state under nondenaturing conditions from ensemble models consistent with a large number of experimental restraints. J. Mol. Biol. 2009, 391, 359–374. [Google Scholar] [PubMed]
- Huang, J.-r.; Grzesiek, S. Ensemble calculations of unstructured proteins constrained by RDC and PRE data: a case study of urea-denatured ubiquitin. J. Am. Chem. Soc. 2010, 132, 694–705. [Google Scholar] [PubMed]
- Bernado, P.; Blanchard, L.; Timmins, P.; Marion, D.; Ruigrok, R.W.; Blackledge, M. A structural model for unfolded proteins from residual dipolar couplings and small-angle x-ray scattering. Proc. Natl. Acad. Sci. USA 2005, 102, 17002–17007. [Google Scholar] [PubMed]
- Marsh, J.A.; Baker, J.M.; Tollinger, M.; Forman-Kay, J.D. Calculation of residual dipolar couplings from disordered state ensembles using local alignment. J. Am. Chem. Soc. 2008, 130, 7804–7805. [Google Scholar]
- Dedmon, M.M.; Lindorff-Larsen, K.; Christodoulou, J.; Vendruscolo, M.; Dobson, C.M. Mapping long-range interactions in α-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J. Am. Chem. Soc. 2005, 127, 476–477. [Google Scholar]
- Ganguly, D.; Chen, J. Structural interpretation of paramagnetic relaxation enhancement-derived distances for disordered protein states. J. Mol. Biol. 2009, 390, 467–477. [Google Scholar]
- Bernadó, P.; Mylonas, E.; Petoukhov, M.V.; Blackledge, M.; Svergun, D.I. Structural characterization of flexible proteins using small-angle X-ray scattering. J. Am. Chem. Soc. 2007, 129, 5656–5664. [Google Scholar]
- Mukrasch, M.D.; Bibow, S.; Korukottu, J.; Jeganathan, S.; Biernat, J.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 2009, 7, e1000034. [Google Scholar]
- Kim, D.-H.; Han, K.-H. Transient secondary structures as general target-binding motifs in intrinsically disordered proteins. Int. J. Mol. Sci. 2018, 19, 3614. [Google Scholar]
- Kussie, P.H.; Gorina, S.; Marechal, V.; Elenbaas, B.; Moreau, J.; Levine, A.J.; Pavletich, N.P. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 1996, 274, 948–953. [Google Scholar] [PubMed]
- Kim, D.-H.; Lee, C.; Cho, Y.-J.; Lee, S.-H.; Cha, E.-J.; Lim, J.-E.; Sabo, T.M.; Griesinger, C.; Lee, D.; Han, K.-H. A pre-structured helix in the intrinsically disordered 4EBP1. Mol. BioSyst. 2015, 11, 366–369. [Google Scholar] [PubMed]
- Lukhele, S.; Bah, A.; Lin, H.; Sonenberg, N.; Forman-Kay, J.D. Interaction of the eukaryotic initiation factor 4E with 4E-BP2 at a dynamic bipartite interface. Structure. 2013, 21, 2186–2196. [Google Scholar] [PubMed]
- Kim, D.-H.; Lee, S.-H.; Nam, K.H.; Chi, S.-W.; Chang, I.; Han, K.-H. Multiple hTAF(II)31-binding motifs in the intrinsically unfolded transcriptional activation domain of VP16. BMB Rep. 2009, 42, 411–417. [Google Scholar] [PubMed]
- Jonker, H.R.; Wechselberger, R.W.; Boelens, R.; Folkers, G.E.; Kaptein, R. Structural properties of the promiscuous VP16 activation domain. Biochemistry 2005, 44, 827–839. [Google Scholar] [PubMed]
- Wüthrich, K. NMR of Proteins and Nucleic Acids; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Gagné, S.M.; Tsuda, S.; Li, M.X.; Chandra, M.; Smillie, L.B.; Sykes, B.D. Quantification of the calcium-induced secondary structural changes in the regulatory domain of troponin-C. Protein Sci. 1994, 3, 1961–1974. [Google Scholar] [PubMed]
- Maltsev, A.S.; Grishaev, A.; Bax, A. Monomeric α-synuclein binds congo red micelles in a disordered manner. Biochemistry 2012, 51, 631–642. [Google Scholar]
- Bertoncini, C.W.; Jung, Y.-S.; Fernandez, C.O.; Hoyer, W.; Griesinger, C.; Jovin, T.M.; Zweckstetter, M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 1430–1435. [Google Scholar]
- Theillet, F.-X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; van Rossum, M.; Goldfarb, D.; et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 2016, 530, 45–50. [Google Scholar]
- Lee, C.; Kim, D.-H.; Lee, S.-H.; Su, J.; Han, K.-H. Structural investigation on the intrinsically disordered N-terminal region of HPV16 E7 protein. BMB Rep. 2016, 49, 431–436. [Google Scholar]
- Kim, D.-H.; Wright, A.; Han, K.-H. An NMR study on the intrinsically disordered core transactivation domain of human glucocorticoid receptor. BMB Rep. 2017, 50, 522–527. [Google Scholar] [PubMed]
- Markley, J.L.; Bax, A.; Arata, Y.; Hilbers, C.W.; Kaptein, R.; Sykes, B.D.; Wright, P.E.; Wüthrich, K. Recommendations for the presentation of NMR structures of proteins and nucleic acids. Eur. J. Biochem. 1998, 256, 1–15. [Google Scholar] [PubMed]
- Marsh, J.A.; Singh, V.K.; Jia, Z.; Forman-Kay, J.D. Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: Implications for fibrillation. Protein Sci. 2006, 15, 2795–2804. [Google Scholar]
- Camilloni, C.; De Simone, A.; Vranken, W.F.; Vendruscolo, M. Determination of secondary structure populations in disordered states of proteins using nuclear magnetic resonance chemical shifts. Biochemistry 2012, 51, 2224–2231. [Google Scholar] [PubMed]
- Mantsyzov, A.B.; Shen, Y.; Lee, J.H.; Hummer, G.; Bax, A. MERA: a webserver for evaluating backbone torsion angle distributions in dynamic and disordered proteins from NMR data. J. Biomol. NMR 2015, 63, 85–95. [Google Scholar]
- Feldman, H.J.; Hogue, C.W.V. A fast method to sample real protein conformational space. Proteins 2000, 39, 112–131. [Google Scholar]
- Ozenne, V.; Bauer, F.; Salmon, L.; Huang, J.-r.; Jensen, M.R.; Segard, S.; Bernadó, P.; Charavay, C.; Blackledge, M. Flexible-meccano: a tool for the generation of explicit ensemble descriptions of intrinsically disordered proteins and their associated experimental observables. Bioinformatics 2012, 28, 1463–1470. [Google Scholar]
- Krzeminski, M.; Marsh, J.A.; Neale, C.; Choy, W.-Y.; Forman-Kay, J.D. Characterization of disordered proteins with ENSEMBLE. Bioinformatics 2012, 29, 398–399. [Google Scholar]
- Nodet, G.; Salmon, L.c.; Ozenne, V.; Meier, S.; Jensen, M.R.; Blackledge, M. Quantitative description of backbone conformational sampling of unfolded proteins at amino acid resolution from NMR residual dipolar couplings. J. Am. Chem. Soc. 2009, 131, 17908–17918. [Google Scholar]
- Ytreberg, F.M.; Borcherds, W.; Wu, H.; Daughdrill, G.W. Using chemical shifts to generate structural ensembles for intrinsically disordered proteins with converged distributions of secondary structure. Intrinsically Disord. Proteins 2015, 3, e984565. [Google Scholar]
- Nielsen, J.T.; Mulder, F.A. POTENCI: prediction of temperature, neighbor and pH-corrected chemical shifts for intrinsically disordered proteins. J. Biomol. NMR 2018, 70, 141–165. [Google Scholar] [PubMed]
- Chandra, S.; Chen, X.; Rizo, J.; Jahn, R.; Südhof, T.C. A broken α-helix in folded α-synuclein. J. Biol. Chem. 2003, 278, 15313–15318. [Google Scholar] [PubMed]
- Bermel, W.; Bertini, I.; Felli, I.C.; Lee, Y.-M.; Luchinat, C.; Pierattelli, R. Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J. Am. Chem. Soc. 2006, 128, 3918–3919. [Google Scholar] [PubMed]
- Wu, K.-P.; Weinstock, D.S.; Narayanan, C.; Levy, R.M.; Baum, J. Structural reorganization of α-synuclein at low pH observed by NMR and REMD simulations. J. Mol. Biol. 2009, 391, 784–796. [Google Scholar]
- Cho, M.-K.; Nodet, G.; Kim, H.-Y.; Jensen, M.R.; Bernado, P.; Fernandez, C.O.; Becker, S.; Blackledge, M.; Zweckstetter, M. Structural characterization of α-synuclein in an aggregation prone state. Protein Sci. 2009, 18, 1840–1846. [Google Scholar]
- Bodner, C.R.; Maltsev, A.S.; Dobson, C.M.; Bax, A. Differential phospholipid binding of α-synuclein variants implicated in Parkinson’s disease revealed by solution NMR spectroscopy. Biochemistry 2010, 49, 862–871. [Google Scholar]
- Maltsev, A.S.; Ying, J.; Bax, A. Impact of N-terminal acetylation of α-synuclein on its random coil and lipid binding properties. Biochemistry 2012, 51, 5004–5013. [Google Scholar]
- Waudby, C.A.; Camilloni, C.; Fitzpatrick, A.W.; Cabrita, L.D.; Dobson, C.M.; Vendruscolo, M.; Christodoulou, J. In-cell NMR characterization of the secondary structure populations of a disordered conformation of α-synuclein within E. coli cells. PloS one 2013, 8, e72286. [Google Scholar]
- Okazaki, H.; Ohori, Y.; Komoto, M.; Lee, Y.-H.; Goto, Y.; Tochio, N.; Nishimura, C. Remaining structures at the N-and C-terminal regions of alpha-synuclein accurately elucidated by amide-proton exchange NMR with fitting. FEBS Lett. 2013, 587, 3709–3714. [Google Scholar]
- Mantsyzov, A.B.; Maltsev, A.S.; Ying, J.; Shen, Y.; Hummer, G.; Bax, A. A maximum entropy approach to the study of residue-specific backbone angle distributions in α-synuclein, an intrinsically disordered protein. Protein Sci. 2014, 23, 1275–1290. [Google Scholar]
- Tavassoly, O.; Nokhrin, S.; Dmitriev, O.Y.; Lee, J.S. Cu (II) and dopamine bind to α-synuclein and cause large conformational changes. FEBS J. 2014, 281, 2738–2753. [Google Scholar] [PubMed]
- Schwalbe, M.; Ozenne, V.; Bibow, S.; Jaremko, M.; Jaremko, L.; Gajda, M.; Jensen, M.R.; Biernat, J.; Becker, S.; Mandelkow, E. Predictive atomic resolution descriptions of intrinsically disordered hTau40 and α-synuclein in solution from NMR and small angle scattering. Structure 2014, 22, 238–249. [Google Scholar] [PubMed]
- Porcari, R.; Proukakis, C.; Waudby, C.A.; Bolognesi, B.; Mangione, P.P.; Paton, J.F.; Mullin, S.; Cabrita, L.D.; Penco, A.; Relini, A. The H50Q mutation induces a 10-fold decrease in the solubility of alpha-synuclein. Journal of biological chemistry. J. Biol. Chem. 2015, 290, 2395–2404. [Google Scholar] [PubMed]
- Lin, W.; Insley, T.; Tuttle, M.D.; Zhu, L.; Berthold, D.A.; Král, P.; Rienstra, C.M.; Murphy, C.J. Control of protein orientation on gold nanoparticles. J. Phys. Chem. C 2015, 119, 21035–21043. [Google Scholar]
- Rezaeian, N.; Shirvanizadeh, N.; Mohammadi, S.; Nikkhah, M.; Arab, S.S. The inhibitory effects of biomimetically designed peptides on α-synuclein aggregation. Arch. Biochem. Biophys. 2017, 634, 96–106. [Google Scholar]
- Rezaei-Ghaleh, N.; Parigi, G.; Soranno, A.; Holla, A.; Becker, S.; Schuler, B.; Luchinat, C.; Zweckstetter, M. Local and Global Dynamics in Intrinsically Disordered Synuclein. Angew. Chem. 2018, 57, 15262–15266. [Google Scholar]
- Murrali, M.G.; Schiavina, M.; Sainati, V.; Bermel, W.; Pierattelli, R.; Felli, I.C. 13C APSY-NMR for sequential assignment of intrinsically disordered proteins. J. Biomol. NMR 2018, 70, 167–175. [Google Scholar]
- Lee, J.H.; Li, F.; Grishaev, A.; Bax, A. Quantitative residue-specific protein backbone torsion angle dynamics from concerted measurement of 3J couplings. J. Am. Chem. Soc. 2015, 137, 1432–1435. [Google Scholar]
- De Simone, A.; Cavalli, A.; Hsu, S.-T.D.; Vranken, W.; Vendruscolo, M. Accurate random coil chemical shifts from an analysis of loop regions in native states of proteins. J. Am. Chem. Soc. 2009, 131, 16332–16333. [Google Scholar]
- Rance, M.; Sørensen, O.; Bodenhausen, G.; Wagner, G.; Ernst, R.; Wüthrich, K. Improved spectral resolution in COSY 1H NMR spectra of proteins via double quantum filtering. Biochem. Biophys. Res. Commun. 1983, 117, 479–485. [Google Scholar]
- Rance, M.; Wagner, G.; Sørensen, O.; Wüthrich, K.; Ernst, R. Application of ω1-decoupled 2D correlation spectra to the study of proteins. J. Magn. Reson. 1984, 59, 250–261. [Google Scholar]
- Wagner, G.; Braun, W.; Havel, T.F.; Schaumann, T.; Gō, N.; Wüthrich, K. Protein structures in solution by nuclear magnetic resonance and distance geometry: the polypeptide fold of the basic pancreatic trypsin inhibitor determined using two different algorithms, DISGEO and DISMAN. J. Mol. Biol. 1987, 196, 611–639. [Google Scholar] [PubMed]
- Carr, C.M.; Kim, P.S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 1993, 73, 823–832. [Google Scholar]
- Jiang, Z.; de Messieres, M.; Lee, J.C. Membrane remodeling by α-synuclein and effects on amyloid formation. J. Am. Chem. Soc. 2013, 135, 15970–15973. [Google Scholar] [PubMed]
- Wang, L.; Das, U.; Scott, D.A.; Tang, Y.; McLean, P.J.; Roy, S. α-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr. Biol. 2014, 24, 2319–2326. [Google Scholar] [PubMed]
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M.-Y. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000, 20, 3214–3220. [Google Scholar]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar]
- Zhu, M.; Fink, A.L. Lipid binding inhibits α-synuclein fibril formation. J. Biol. Chem. 2003, 278, 16873–16877. [Google Scholar]
- Galvagnion, C.; Brown, J.W.; Ouberai, M.M.; Flagmeier, P.; Vendruscolo, M.; Buell, A.K.; Sparr, E.; Dobson, C.M. Chemical properties of lipids strongly affect the kinetics of the membrane-induced aggregation of α-synuclein. Proc. Natl. Acad. Sci. USA 2016, 113, 7065–7070. [Google Scholar]
- Davidson, W.S.; Jonas, A.; Clayton, D.F.; George, J.M. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998, 273, 9443–9449. [Google Scholar]
- Rhoades, E.; Ramlall, T.F.; Webb, W.W.; Eliezer, D. Quantification of α-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys. J. 2006, 90, 4692–4700. [Google Scholar] [PubMed]
- Ulmer, T.S.; Bax, A.; Cole, N.B.; Nussbaum, R.L. Structure and dynamics of micelle-bound human α-synuclein. J. Biol. Chem. 2005, 280, 9595–9603. [Google Scholar] [PubMed]
- Lee, J.H.; Ying, J.; Bax, A. Nuclear magnetic resonance observation of α-synuclein membrane interaction by monitoring the acetylation reactivity of its lysine side chains. Biochemistry 2016, 55, 4949–4959. [Google Scholar] [PubMed]
- Radhakrishnan, I.; Pérez-Alvarado, G.C.; Parker, D.; Dyson, H.J.; Montminy, M.R.; Wright, P.E. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator: coactivator interactions. Cell 1997, 91, 741–752. [Google Scholar]
- Marnett, L.J. Decoding endocannabinoid signaling. Nat. Chem. Biol. 2009, 5, 8–9. [Google Scholar]
- Russo, L.; Giller, K.; Pfitzner, E.; Griesinger, C.; Becker, S. Insight into the molecular recognition mechanism of the coactivator NCoA1 by STAT6. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z. BioMagResBank. Nucleic Acids Res. 2007, 36, D402–D408. [Google Scholar]
- Selenko, P. Quo vadis biomolecular NMR spectroscopy? Int J. Mol. Sci. 2019, 20, 1278. [Google Scholar]
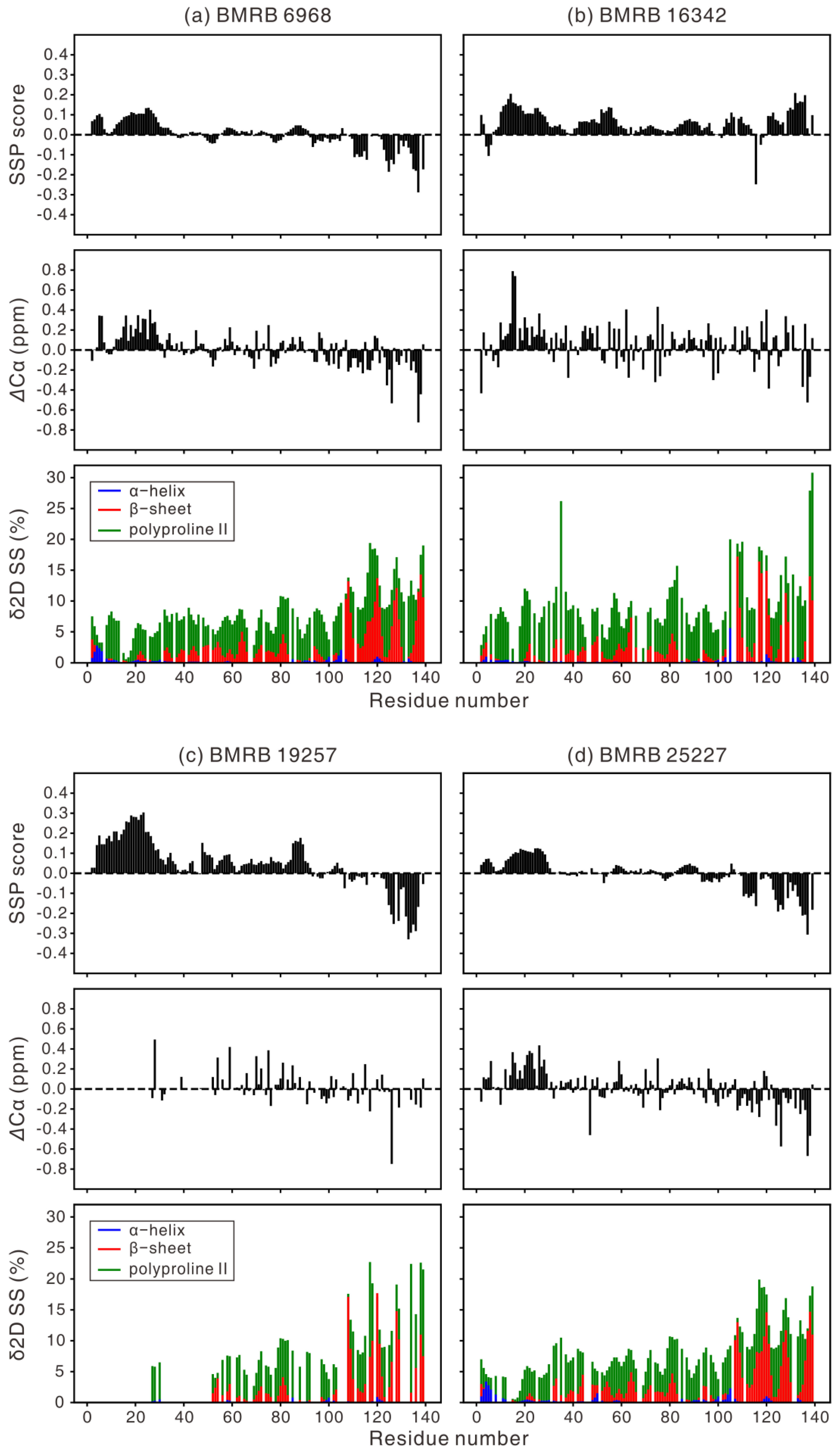
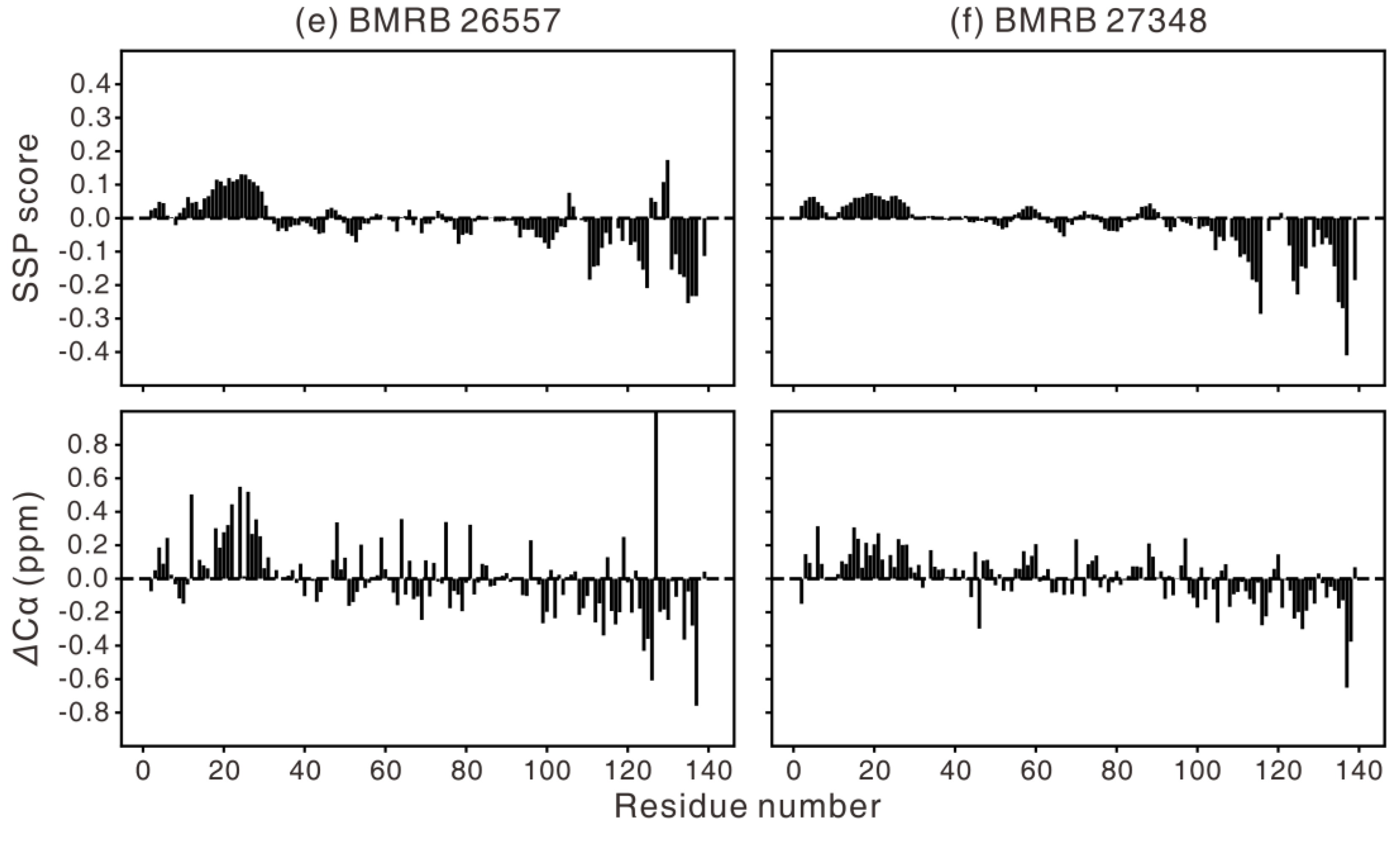
| Year | Sample Condition | BMRB | ref |
|---|---|---|---|
| 2001 | ~100 μM αS, 100 mM NaCl, 10 mM Na2HPO4, pH 7.4, 283 K | [28] | |
| 2003 | 0.3 mM αS, 20 mM sodium phosphate, 50 mM SDS, pH 7.4, 298 K | 5744 d | [73] |
| 2006 | 1 mM αS, 20 mM phosphate, 0.5 mM EDTA, 200 mM NaCl, 10% D2O, pH 6.5, 285.5 K | 6968 | [74] |
| 2008 | 0.2 mM αS, PBS buffer, pH 7.4, 263 K | [35] | |
| 2009 | 0.65 mM αS, 10 mM phosphate, 140 mM NaCl, pH 2.5, 10% D2O, 288 K | [75] | |
| 2009 | 0.3 mM αS, 20 mM NaOAc, 100 mM NaCl, 10% D2O, pH 3.0 & pH 7.4, 288 K | 16342 | [76] |
| 2009 | 0.6 mM αS, 20mM Na2HPO4 (pH 6.0), 6% D2O, 0,02% NaN3, in phospholipids, 293 K | [27] | |
| 2010 | 0.6 mM wild-type αS, mutants (A30P, E46K, A53T), 20 mM Na2HPO4, pH 6.0, 6% D2O, 0.02% NaN3, in phospholipids, 293 K | [77] | |
| 2012 | 0.1 mM αS, 5 mM dioxane, 20 mM sodium phosphate buffer, pH 6, in phospholipids, 288 K | [78] | |
| 2013 a | 1.7 mM αS, 10% D2O, 90% H2O, pH 6.2, 277 K | 19257 | [79] |
| 2013 b | - mM αS, 20 mM Tris-HCl, pH 7, 100 mM NaCl, 10% D2O, 288 K | [80] | |
| 2014 | 0.35 mM αS, 20 mM sodium phosphate, pH 6, 288 K | [81] | |
| 2014 | 50 μM αS, NaCl/sodium phosphate buffer, 5% glycerol, 288 K | [82] | |
| 2014 | 0.3 mM αS, 20 mM NaOAc, 100 mM NaCl, 10% D2O, pH 3.0 & pH 7, 288 K | [83] | |
| 2015 | 0.5 mM/0.7 mM wild-type/H50Q αS, 10 mM sodium phosphate, pH 7.5, 100 mM NaCl, 5% D2O, 0.01% NaN3, 0.001% DSS, 283 K | 25227 | [84] |
| 2015 | ~0.43 mM (6.6mg/mL) αS, 20 mM HEPES, 10% D2O, pH 7.0, 277 K | 26557 | [85] |
| 2016 a | 0.4 mM αS, 20 mM sodium phosphate, 150 mM NaCl, pH 7.0, 283 K | [60] | |
| 2017 c | 75 μM αS, PBS buffer, 0.02% NaN3, pH 7.4, 310 K | [86] | |
| 2018 | ~1 mM αS, pH 5.0, 10% D2O, 298 K | [87] | |
| 2018 | 1 mM αS, 20 mM phosphate, 200 mM NaCl, 0.5 mM EDTA, pH 6.5, 285.5 K, 295 K, 305 K, 315 K | 27348 | [88] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Lee, J.; Mok, K.H.; Lee, J.H.; Han, K.-H. Salient Features of Monomeric Alpha-Synuclein Revealed by NMR Spectroscopy. Biomolecules 2020, 10, 428. https://doi.org/10.3390/biom10030428
Kim D-H, Lee J, Mok KH, Lee JH, Han K-H. Salient Features of Monomeric Alpha-Synuclein Revealed by NMR Spectroscopy. Biomolecules. 2020; 10(3):428. https://doi.org/10.3390/biom10030428
Chicago/Turabian StyleKim, Do-Hyoung, Jongchan Lee, K. H. Mok, Jung Ho Lee, and Kyou-Hoon Han. 2020. "Salient Features of Monomeric Alpha-Synuclein Revealed by NMR Spectroscopy" Biomolecules 10, no. 3: 428. https://doi.org/10.3390/biom10030428
APA StyleKim, D.-H., Lee, J., Mok, K. H., Lee, J. H., & Han, K.-H. (2020). Salient Features of Monomeric Alpha-Synuclein Revealed by NMR Spectroscopy. Biomolecules, 10(3), 428. https://doi.org/10.3390/biom10030428





